Comparative Oncology: Management of Hepatic Neoplasia in Humans and Dogs
Abstract
:Simple Summary
Abstract
1. Introduction
2. Diagnostics
3. Tumor Sampling
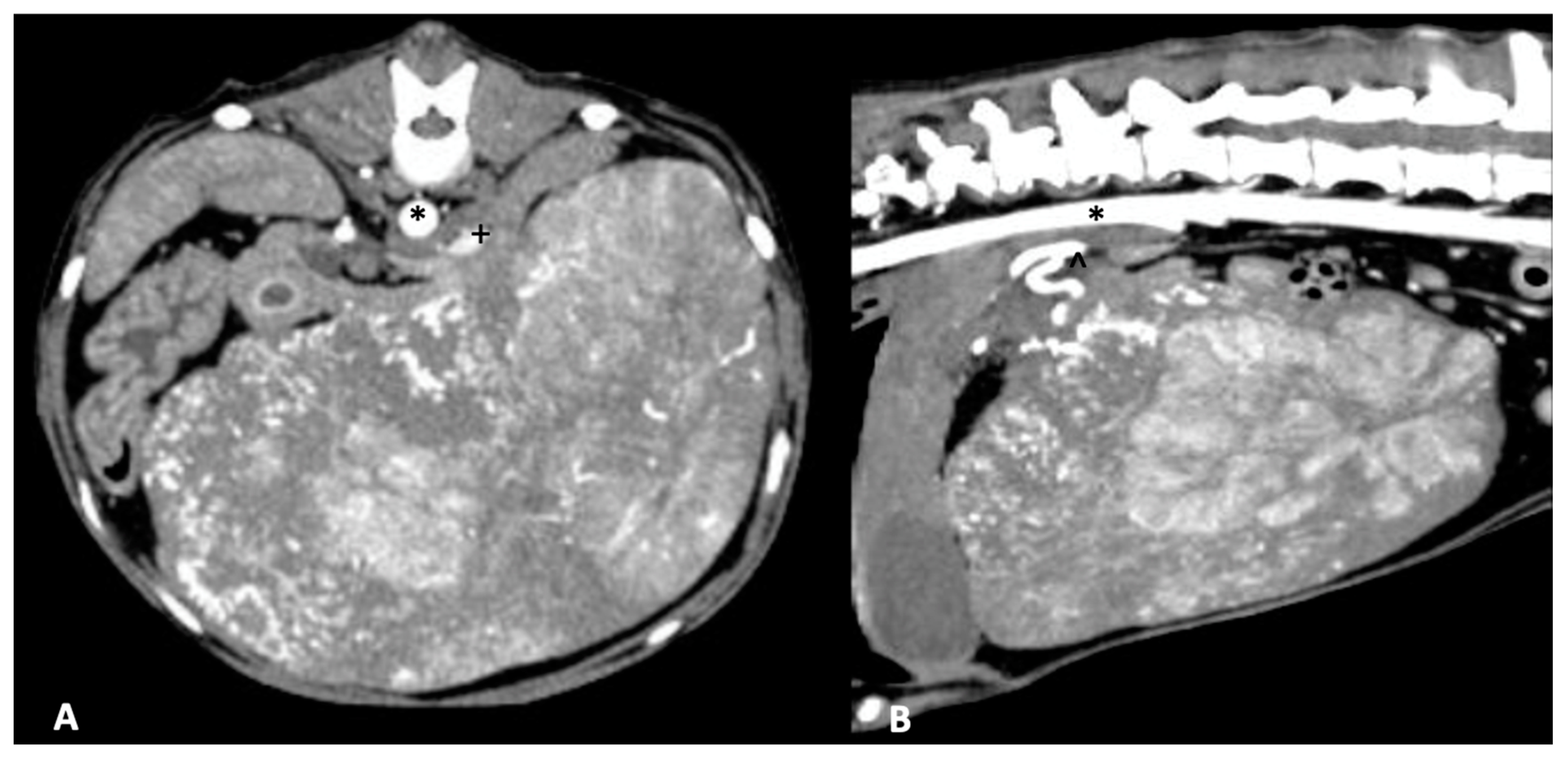
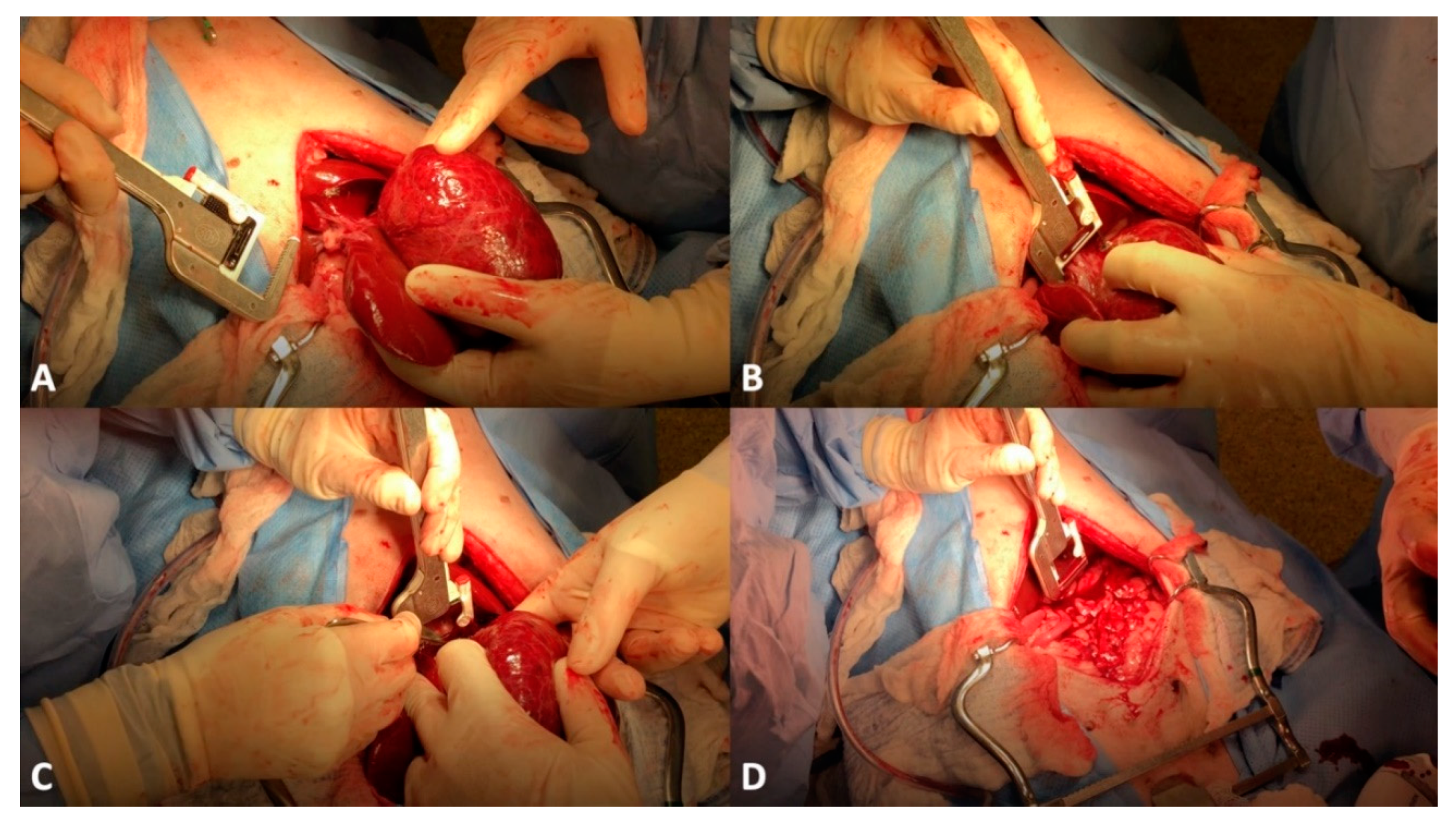
4. Surgical Treatment of Hepatic Neoplasia
5. Interventional Management of Hepatic Neoplasia
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Patnaik, A.K.; Hurvitz, A.I.; Lieberman, P.H. Canine hepatic neoplasms: A clinicopathologic study. Vet Pathol. 1980, 17, 553–564. [Google Scholar] [CrossRef]
- Hammer, A.S.; Sikkema, D.A. Hepatic neoplasia in the dog and cat. Vet. Clin. N. Am. Small Anim. Pract. 1995, 25, 419–435. [Google Scholar] [CrossRef]
- Weiss, D.J.; Moritz, A. Liver cytology. Vet. Clin. N. Am. Small Anim. Pract. 2002, 32, 1267–1291. [Google Scholar] [CrossRef]
- van Sprundel, R.G.; van den Ingh, T.S.; Guscetti, F.; Kershaw, O.; Kanemoto, H.; van Gils, H.M.; Rothuizen, J.; Roskams, T.; Spee, B. Classification of primary hepatic tumours in the dog. Vet. J. 2013, 197, 596–606. [Google Scholar] [CrossRef]
- Patnaik, A.K.; Hurvitz, A.I.; Lieberman, P.H.; Johnson, G.F. Canine hepatocellular carcinoma. Vet. Pathol. 1981, 18, 427–438. [Google Scholar] [CrossRef]
- Kinsey, J.R.; Gilson, S.D.; Hauptman, J.; Mehler, S.J.; May, L.R. Factors associated with long-term survival in dogs undergoing liver lobectomy as treatment for liver tumors. Can. Vet. J. 2015, 56, 598–604. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, J.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Sia, D.; Villanueva, A.; Friedman, S.L.; Llovet, J.M. Liver Cancer Cell of Origin, Molecular Class, and Effects on Patient Prognosis. Gastroenterology 2017, 152, 745–761. [Google Scholar] [CrossRef]
- Kew, M.C. Epidemiology of chronic hepatitis B virus infection, hepatocellular carcinoma, and hepatitis B virus-induced hepatocellular carcinoma. Pathol. Biol. 2010, 58, 273–277. [Google Scholar] [CrossRef]
- Hussain, S.P.; Schwank, J.; Staib, F.; Wang, X.W.; Harris, C.C. TP53 mutations and hepatocellular carcinoma: Insights into the etiology and pathogenesis of liver cancer. Oncogene 2007, 26, 2166–2176. [Google Scholar] [CrossRef] [Green Version]
- Diakoudi, G.; Capozza, P.; Lanave, G.; Pellegrini, F.; Di Martino, B.; Elia, G.; Decaro, N.; Camero, M.; Ghergo, P.; Stasi, F.; et al. A novel hepadnavirus in domestic dogs. Sci. Rep. 2022, 12, 2864. [Google Scholar] [CrossRef]
- Pesavento, P.A.; Jackson, K.; Hampson, T.S.T.T.B.; Munday, J.S.; Barrs, V.R.; Beatty, J.A. A Novel Hepadnavirus is Associated with Chronic Hepatitis and Hepatocellular Carcinoma in Cats. Viruses 2019, 11, 969. [Google Scholar] [CrossRef]
- Liptak, J.M.; Dernell, W.S.; Monnet, E.; Powers, B.E.; Bachand, A.M.; Kenney, J.G.; Withrow, S.J. Massive hepatocellular carcinoma in dogs: 48 cases (1992–2002). J. Am. Vet. Med. Assoc. 2004, 15, 1225–1230. [Google Scholar] [CrossRef]
- Vatnikov, Y.; Vilkovysky, I.; Kulikov, E.; Popova, I.; Khairova, N.; Gazin, A.; Zharov, A.; Lukina, D. Size of canine hepatocellular carcinoma as an adverse prognostic factor for surgery. J. Adv. Vet. Anim. Res. 2020, 7, 127–132. [Google Scholar] [CrossRef]
- Adigun, O.O.; Yarrarapu, S.N.S.; Khetarpal, S. Alpha Fetoprotein. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Benson, A.B.; D’Angelica, M.I.; Abbott, D.E.; Anaya, D.A.; Anders, R.; Are, C.; Bachini, M.; Borad, M.; Brown, D.; Burgoyne, A.; et al. Hepatobiliary Cancers, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2021, 19, 541–565. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Fujita, M.; Kitao, S.; Ashida, Y.; Nishizono, K.; Tsuchiya, R.; Shida, T.; Kobayashi, K. Serum alpha-fetoprotein values in dogs with various hepatic diseases. J. Vet. Med. Sci. 1999, 61, 657–659. [Google Scholar] [CrossRef]
- Lowseth, L.A.; Gillett, N.A.; Chang, I.Y.; Muggenburg, B.A.; Boecker, B.B. Detection of serum alpha-fetoprotein in dogs with hepatic tumors. J. Am. Vet. Med. Assoc. 1991, 199, 735–741. [Google Scholar]
- Zini, E.; Glaus, T.M.; Minuto, F.; Arvigo, M.; Hauser, B.; Reusch, C.E. Paraneoplastic hypoglycemia due to an insulin-like growth factor type-II secreting hepatocellular carcinoma in a dog. J. Vet. Intern. Med. 2007, 21, 193–195. [Google Scholar] [CrossRef]
- Garla, V.; Sonani, H.; Palabindala, V.; Gomez-Sanchez, C.; Subauste, J.; Lien, L.F. Non-islet cell hypoglycemia: Case series and review of the literature. Front. Endocrinol. 2019, 10, 316. [Google Scholar] [CrossRef]
- Cooper, E.S.; Wellman, M.L.; Carsillo, M.E. Hyperalbuminemia associated with hepatocellular carcinoma in a dog. Vet. Clin. Pathol. 2009, 38, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Nizam, R.; Ahmed, F. Hyperthyroxinemia and elevated lipids as paraneoplastic phenomena in hepatocellular carcinoma. A case report. J. Clin. Gastroenterol. 1995, 21, 246–248. [Google Scholar] [CrossRef] [PubMed]
- Woolcock, A.D.; Keenan, A.; Cheung, C.; Christian, J.A.; Moore, G.E. Thrombocytosis in 715 dogs (2011–2015). J. Vet. Intern. Med. 2017, 31, 1691–1699. [Google Scholar] [CrossRef] [PubMed]
- Neel, J.A.; Snyder, L.; Grindem, C.B. Thrombocytosis: A retrospective study of 165 dogs. Vet. Clin. Pathol. 2012, 41, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Carr, B.I.; Guerra, V. Thrombocytosis and hepatocellular carcinoma. Dig. Dis. Sci. 2013, 58, 1790–1796. [Google Scholar] [CrossRef]
- Hwang, S.J.; Luo, J.C.; Li, C.P.; Chu, C.W.; Wu, J.C.; Lai, C.R.; Chiang, J.H.; Chau, G.Y.; Lui, W.L.; Lee, C.C.; et al. Thrombocytosis: A paraneoplastic syndrome in patients with hepatocellular carcinoma. World J. Gastroenterol. 2004, 10, 2472–2477. [Google Scholar] [CrossRef]
- Ghobrial, M.W.; George, J.; Mannam, S.; Henien, S.R. Severe hypercalcemia as an initial presenting manifestation of hepatocellular carcinoma. Can. J. Gastroenterol. 2002, 16, 607–609. [Google Scholar] [CrossRef]
- Abe, Y.; Makiyama, H.; Fujita, Y.; Tachibana, Y.; Kamada, G.; Uebayashi, M. Severe hypercalcemia associated with hepatocellular carcinoma secreting intact parathyroid hormone: A case report. Intern. Med. 2011, 50, 329–333. [Google Scholar] [CrossRef]
- Koyama, Y.; Ishijima, H.; Ishibashi, A.; Katsuya, T.; Ishizaka, H.; Aoki, J.; Endo, K. Intact PTH-producing hepatocellular carcinoma treated by transcatheter arterial embolization. Abdom. Imaging 1999, 24, 144–146. [Google Scholar] [CrossRef]
- Chang, P.E.; Ong, W.C.; Lui, H.F.; Tan, C.K. Epidemiology and prognosis of paraneoplastic syndromes in hepatocellular carcinoma. ISRN Oncol. 2013, 2013, 684026. [Google Scholar] [CrossRef]
- Guillot, M.; Danjou, M.A.; Alexander, K.; Bédard, C.; Desnoyers, M.; Beauregard, G.; Del Castillo, J.R.E. Can sonographic findings predict the results of liver aspirates in dogs with suspected liver disease? Vet. Radiol. Ultrasound 2009, 50, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Huaijantug, S.; Yatmark, P.; Phophug, P.; Worapakdee, M.; Phutrakul, A.; Julapanthong, P.; Chuaychoo, K. Quantitative ultrasound elastography and serum ferritin level in dogs with liver tumors. J. Adv. Vet. Anim. Res. 2020, 7, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Banzato, T.; Rubini, G.; Orlandi, R.; Bargellini, P.; Bonsembiante, F.; Zotti, A. Contrast-enhanced ultrasound features of hepatocellular carcinoma in dogs. Vet. Rec. 2020, 186, 187. [Google Scholar] [CrossRef]
- Nakamura, K.; Takagi, S.; Sasaki, N.; Bandula Kumara, W.R.; Murakami, M.; Ohta, H.; Yamasaki, M.; Takiguchi, M. Contrast-enhanced ultrasonography for characterization of canine focal liver lesions. Vet. Radiol. Ultrasound 2010, 51, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Nolsøe, C.P.; Barr, R.G.; Berzigotti, A.; Burns, P.N.; Cantisani, V.; Chammas, M.C.; Chaubal, N.; Choi, B.I.; Clevert, D.A. Guidelines and good clinical practice recommendations for contrast-enhanced ultrasound (CEUS) in the liver-Update 2020 WFUMB in cooperation with EFSUMB, AFSUMB, AIUM, and FLAUS. Ultrasound Med. Biol. 2020, 46, 2579–2604. [Google Scholar] [CrossRef]
- Schellhaas, B.; Görtz, R.S.; Pfeifer, L.; Kielisch, C.; Neurath, M.F.; Strobel, D. Diagnostic accuracy of contrast-enhanced ultrasound for the differential diagnosis of hepatocellular carcinoma: ESCULAP versus CEUS-LI-RADS. Eur. J. Gastroenterol. Hepatol. 2017, 29, 1036–1044. [Google Scholar] [CrossRef]
- Fukushima, K.; Kanemoto, H.; Ohno, K.; Takahashi, M.; Nakashima, K.; Fujino, Y.; Uchida, K.; Fujiwara, R.; Nishimura, R.; Tsujimoto, H. CT characteristics of primary hepatic mass lesions in dogs. Vet. Radiol. Ultrasound 2012, 53, 252–257. [Google Scholar] [CrossRef]
- Kutara, K.; Seki, M.; Ishikawa, C.; Sakai, M.; Kagawa, Y.; Iida, G.; Ishigaki, K.; Teshima, K.; Edamura, K.; Nakayama, T.; et al. Triple-phase helical computed tomography in dogs with hepatic masses. Vet. Radiol. Ultrasound 2014, 55, 7–15. [Google Scholar] [CrossRef]
- Burti, S.; Zotti, A.; Bonsembiante, F.; Contiero, B.; Banzato, T. Diagnostic accuracy of delayed phase post contrast computed tomographic images in the diagnosis of focal liver lesions in dogs: 69 cases. Front. Vet. Sci. 2021, 8, 611556. [Google Scholar] [CrossRef]
- Burti, S.; Zotti, A.; Contiero, B.; Banzato, T. Computed tomography features for differentiating malignant and benign focal liver lesions in dogs: A meta-analysis. Vet. J. 2021, 278, 105773. [Google Scholar] [CrossRef] [PubMed]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef]
- Di Martino, M.; De Filippis, G.; De Santis, A.; Geiger, D.; Del Monte, M.; Lombardo, C.V.; Rossi, M.; Corradini, S.G.; Mennini, G.; Catalano, C. Hepatocellular carcinoma in cirrhotic patients: Prospective comparison of US, CT and MR imaging. Eur. Radiol. 2013, 23, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Marrero, J.A.; Ahn, J.; Rajender Reddy, K. ACG clinical guideline: The diagnosis and management of focal liver lesions. Am. J. Gastroenterol. 2014, 109, 1328–1347. [Google Scholar] [CrossRef]
- Nadarevic, T.; Giljaca, V.; Colli, A.; Fraquelli, M.; Casazza, G.; Miletic, D.; Stimac, D. Computed tomography for the diagnosis of hepatocellular carcinoma in chronic advanced liver disease. Cochrane Database Syst. Rev. 2019, 2019, CD013362. [Google Scholar] [CrossRef]
- Clifford, C.A.; Pretorius, E.S.; Weisse, C.; Sorenmo, K.U.; Drobatz, K.J.; Siegelman, E.S.; Solomon, J.A. Magnetic resonance imaging of focal splenic and hepatic lesions in the dog. J. Vet. Intern. Med. 2004, 18, 330–338. [Google Scholar] [CrossRef]
- Borusewicz, P.; Stańczyk, E.; Kubiak, K.; Spużak, J.; Glińska-Suchocka, K.; Jankowski, M.; Slawuta, P.; Kubiak-Nowak, D.; Podgorski, P. Magnetic resonance imaging of liver tumors using gadoxetic acid (Gd-EOB-DTPA)—Pilot study. BMC Vet. Res. 2019, 15, 293. [Google Scholar] [CrossRef]
- Yonetomi, D.; Kadosawa, T.; Miyoshi, K.; Nakao, Y.; Homma, E.; Hanazono, K.; Yamada, E.; Nakamura, K.; Ljiri, A.; Minegishi, N. Contrast agent Gd-EOB-DTPA (EOB·Primovist®) for low-field magnetic resonance imaging of canine focal liver lesions. Vet. Radiol. Ultrasound 2012, 53, 371–380. [Google Scholar] [CrossRef]
- Masserdotti, C.; Drigo, M. Retrospective study of cytologic features of well-differentiated hepatocellular carcinoma in dogs. Vet. Clin. Pathol. 2012, 41, 382–390. [Google Scholar] [CrossRef]
- Roth, L. Comparison of liver cytology and biopsy diagnoses in dogs and cats: 56 cases. Vet. Clin. Pathol. 2001, 30, 35–38. [Google Scholar] [CrossRef]
- Wang, H.; Yu, H.; Qian, Y.W.; Cao, Z.Y.; Wu, M.C.; Cong, W.M. Impact of surgical margin on the prognosis of early hepatocellular carcinoma (≤5 cm): A propensity score matching analysis. Front. Med. 2020, 7, 139. [Google Scholar] [CrossRef]
- Moyer, J.; Lopez, D.J.; Balkman, C.E.; Sumner, J.P. Factors associated with survival in dogs with a histopathological diagnosis of hepatocellular carcinoma: 94 cases (2007–2018). Open Vet. J. 2021, 11, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Bahr, K.L.; Sharkey, L.C.; Murakami, T.; Feeney, D.A. Accuracy of US-guided FNA of focal liver lesions in dogs: 140 cases (2005-2008). J. Am. Anim. Hosp. Assoc. 2013, 49, 190–196. [Google Scholar] [CrossRef]
- Bigge, L.A.; Brown, D.J.; Penninck, D.G. Correlation between coagulation profile findings and bleeding complications after ultrasound-guided biopsies: 434 cases (1993–1996). J. Am. Anim. Hosp. Assoc. 2001, 37, 228–233. [Google Scholar] [CrossRef]
- Reece, J.; Pavlick, M.; Penninck, D.G.; Webster, C.R.L. Hemorrhage and complications associated with percutaneous ultrasound guided liver biopsy in dogs. J. Vet. Intern. Med. 2020, 34, 2398–2404. [Google Scholar] [CrossRef]
- Cole, T.L.; Center, S.A.; Flood, S.N.; Rowland, P.H.; Valentine, B.A.; Warner, K.L.; Erb, H.N. Diagnostic comparison of needle and wedge biopsy specimens of the liver in dogs and cats. J. Am. Vet. Med. Assoc. 2002, 220, 1483–1490. [Google Scholar] [CrossRef]
- Kemp, S.D.; Zimmerman, K.L.; Panciera, D.L.; Monroe, W.E.; Leib, M.S.; Lanz, O.I. A comparison of liver sampling techniques in dogs. J. Vet. Intern. Med. 2015, 29, 51–57. [Google Scholar] [CrossRef]
- Kimbrell, T.L.; Milovancev, M.; Olsen, R.; Löhr, C.V. Comparison of diagnostic accuracy of laparoscopic 3 mm and 5 mm cup biopsies to wedge biopsies of canine livers. J. Vet. Intern. Med. 2018, 32, 701–706. [Google Scholar] [CrossRef]
- Fernandez, N.; Del-Pozo, J.; Shaw, D.; Marques, A.I.C. Comparison of two minimally invasive techniques for liver biopsy collection in dogs. J. Small Anim. Pract. 2017, 58, 555–561. [Google Scholar] [CrossRef]
- McDevitt, H.L.; Mayhew, P.D.; Giuffrida, M.A.; Brown, D.C.; Culp, W.T.; Runge, J.J. Short-term clinical outcome of laparoscopic liver biopsy in dogs: 106 cases (2003–2013). J. Am. Vet. Med. Assoc. 2016, 248, 83–90. [Google Scholar] [CrossRef]
- Petre, S.L.; McClaran, J.K.; Bergman, P.J.; Monette, S. Safety and efficacy of laparoscopic hepatic biopsy in dogs: 80 cases (2004–2009). J. Am. Vet. Med. Assoc. 2012, 240, 181–185. [Google Scholar] [CrossRef]
- Vasanjee, S.C.; Bubenik, L.J.; Hosgood, G.; Bauer, R. Evaluation of hemorrhage, sample size, and collateral damage for five hepatic biopsy methods in dogs. Vet. Surg. 2006, 35, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Flatmark, A.; Rö, J.S.; Jakobsen, A.; Enge, I.; Skrede, S. Regeneration and metabolic changes following extensive liver resection in dogs. Scand. J. Gastroenterol. 1973, 8, 609–613. [Google Scholar] [CrossRef]
- Francavilla, A.; Porter, K.A.; Benichou, J.; Jones, A.F.; Starzl, T.E. Liver regeneration in dogs: Morphologic and chemical changes. J. Surg. Res. 1978, 25, 409–419. [Google Scholar] [CrossRef]
- Vauthey, J.N.; Chaoui, A.; Do, K.A.; Bilimoria, M.M.; Fenstermacher, M.J.; Charnsangavej, C.; Hicks, M.; Alsfasser, G.; Lauwers, G.; Hawkins, I.F.; et al. Standardized measurement of the future liver remnant prior to extended liver resection: Methodology and clinical associations. Surgery 2000, 127, 512–519. [Google Scholar] [CrossRef]
- Avritscher, R.; Duke, E.; Madoff, D.C. Portal vein embolization: Rationale, outcomes, controversies and future directions. Expert Rev. Gastroenterol. Hepatol. 2010, 4, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Kubota, K.; Makuuchi, M.; Kusaka, K.; Kobayashi, T.; Miki, K.; Hasegawa, K.; Harihara, Y.; Takayama, T. Measurement of liver volume and hepatic functional reserve as a guide to decision-making in resectional surgery for hepatic tumors. Hepatology 1997, 26, 1176–1181. [Google Scholar] [CrossRef]
- Huang, J.Y.; Yang, W.Z.; Li, J.J.; Jiang, N.; Zheng, Q.B. Portal vein embolization induces compensatory hypertrophy of remnant liver. World J. Gastroenterol. 2006, 12, 408–414. [Google Scholar] [CrossRef]
- Saraswat, V.A.; Pandey, G.; Shetty, S. Treatment algorithms for managing hepatocellular carcinoma. J. Clin. Exp. Hepatol. 2014, 4, 80–89. [Google Scholar] [CrossRef]
- O’Leary, C.; Mahler, M.; Soulen, M.C. Curative-intent therapies in localized hepatocellular carcinoma. Curr. Treat. Options Oncol. 2020, 21, 31. [Google Scholar] [CrossRef]
- Marconato, L.; Sabattini, S.; Marisi, G.; Rossi, F.; Leone, V.F.; Casadei-Gardini, A. Sorafenib for the treatment of unresectable hepatocellular carcinoma: Preliminary toxicity and activity data in dogs. Cancers 2020, 12, 1272. [Google Scholar] [CrossRef]
- da Silva, P.H.S.; de Souza, M.C.C.; de oliveira Cavalcanti, E.B.; Fonseca, V.B.; Silveira, T.L.; Flecher, M.C.; Cassali, G.D.; dos Santos Horta, R. Metronomic chemotherapy for advanced diffuse hepatocellular carcinoma in a dog. Braz. J. Vet. Pathol. 2021, 14, 199–205. [Google Scholar] [CrossRef]
- Elpiner, A.K.; Brodsky, E.M.; Hazzah, T.N.; Post, G.S. Single-agent gemcitabine chemotherapy in dogs with hepatocellular carcinomas. Vet. Comp. Oncol. 2011, 9, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Su, G.L.; Altayar, O.; O’Shea, R.; Shah, R.; Estfan, B.; Wenzell, C.; Sultan, S.; Falck-Ytter, Y. AGA Clinical Practice Guideline on Systemic Therapy for Hepatocellular Carcinoma. Gastroenterology 2022, 162, 920–934. [Google Scholar] [CrossRef]
- Vogel, A.; Martinelli, E. ESMO Guidelines Committee. Electronic address: [email protected]; ESMO Guidelines Committee. Updated treatment recommendations for hepatocellular carcinoma (HCC) from the ESMO Clinical Practice Guidelines. Ann. Oncol. 2021, 32, 801–805. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network (2022). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Guideline Hepatobiliary Cancers, V.1.2022. Available online: https://www.nccn.org/guidelines/recently-published-guidelines (accessed on 12 May 2022).
- Suzuki, H.; Iwamoto, H.; Nakano, M.; Nakamura, T.; Masuda, A.; Sakaue, T.; Tanaka, T.; Nakano, D.; Kuromatsu, R.; Niizeki, T. Efficacy and tolerability of Sorafenib plus metronomic chemotherapy S-1 for advanced hepatocellular carcinoma in preclinical and clinical assessments. Transl. Oncol. 2021, 14, 101201. [Google Scholar] [CrossRef] [PubMed]
- Covey, J.L.; Degner, D.A.; Jackson, A.H.; Hofeling, A.D.; Walshaw, R. Hilar liver resection in dogs. Vet. Surg. 2009, 38, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.D.; Bellenger, C.R.; Lewis, D.T.; Latter, M.R. Hepatic lobectomy in the dog. A comparison of stapling and ligation techniques. Vet. Surg. 1990, 19, 221–225. [Google Scholar] [CrossRef]
- Risselada, M.; Ellison, G.W.; Bacon, N.J.; Polyak, M.M.; van Gilder, J.; Kirkby, K.; Kim, S.E. Comparison of 5 surgical techniques for partial liver lobectomy in the dog for intraoperative blood loss and surgical time. Vet. Surg. 2010, 39, 856–862. [Google Scholar] [CrossRef]
- May, L.R.; Mehler, S.J. Complications of hepatic surgery in companion animals. Vet. Clin. N. Am. Small Anim. Pract. 2011, 41, 935–948. [Google Scholar] [CrossRef]
- Linden, D.S.; Liptak, J.M.; Vinayak, A.; Cappelle, K.; Hoffman, C.; Fan, S.; Smiley, W.; Matz, B.M. Outcomes and prognostic variables associated with central division hepatic lobectomies: 61 dogs. Vet. Surg. 2019, 48, 309–314. [Google Scholar] [CrossRef]
- Michael, A.E.; Case, J.B.; Massari, F.; Giuffrida, M.A.; Mayhew, P.D.; Carvajal, J.L.; Regier, P.J.; Runge, J.J.; Singh, A. Feasibility of laparoscopic liver lobectomy in dogs. Vet. Surg. 2021, 50 (Suppl. 1), O89–O98. [Google Scholar] [CrossRef] [PubMed]
- Vilkovyskiy, I.F.; Vatnikov, Y.A.; Kulikov, E.V.; Sotnikova, E.D.; Yagnikov, S.A.; Seleznev, S.B.; Krotova, E.A.; Byakhova, V.M.; Grishin, V.N.; Avdotin, V.P. Influence of hepatic neoplasia on life expectancy in dogs. Vet. World. 2020, 13, 413–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuyama, A.; Takagi, S.; Hosoya, K.; Kagawa, Y.; Nakamura, K.; Deguchi, T.; Takiguchi, M. Impact of surgical margins on survival of 37 dogs with massive hepatocellular carcinoma. N. Z. Vet. J. 2017, 65, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Shimada, M.; Hashizume, M.; Maehara, S.; Tsujita, E.; Rikimaru, T.; Yamashita, Y.; Tanaka, S.; Adachi, E.; Sugimachi, K. Laparoscopic hepatectomy for hepatocellular carcinoma. Surg. Endosc. 2001, 15, 541–544. [Google Scholar] [CrossRef]
- Schneider, P.D. Preoperative assessment of liver function. Surg. Clin. N. Am. 2004, 84, 355–373. [Google Scholar] [CrossRef]
- Lisotti, A.; Azzaroli, F.; Buonfiglioli, F.; Montagnani, M.; Cecinato, P.; Turco, L.; Calvanese, C.; Simoni, P.; Guardigli, M.; Arena, R.; et al. Indocyanine green retention test as a noninvasive marker of portal hypertension and esophageal varices in compensated liver cirrhosis. Hepatology 2014, 59, 643–650. [Google Scholar] [CrossRef]
- Jarnagin, W.; Chapman, W.C.; Curley, S.; D’Angelica, M.; Rosen, C.; Dixon, E.; Nagorney, D. American Hepato-Pancreato-Biliary Association; Society of Surgical Oncology; Society for Surgery of the Alimentary Tract. Surgical treatment of hepatocellular carcinoma: Expert consensus statement. HPB 2010, 12, 302–310. [Google Scholar] [CrossRef]
- Dimick, J.B.; Cowan, J.A., Jr.; Knol, J.A.; Upchurch, G.R., Jr. Hepatic resection in the United States: Indications, outcomes, and hospital procedural volumes from a nationally representative database. Arch. Surg. 2003, 138, 185–191. [Google Scholar] [CrossRef]
- Shi, M.; Guo, R.P.; Lin, X.J.; Zhang, Y.Q.; Chen, M.S.; Zhang, C.Q.; Lau, W.Y.; Li, J.Q. Partial hepatectomy with wide versus narrow resection margin for solitary hepatocellular carcinoma: A prospective randomized trial. Ann. Surg. 2007, 245, 36–43. [Google Scholar] [CrossRef]
- Liu, R.; Wakabayashi, G.; Kim, H.J.; Choi, G.H.; Yiengpruksawan, A.; Fong, Y.; He, J.; Boggi, U.; Troisi, R.I.; Efanov, M.; et al. International consensus statement on robotic hepatectomy surgery in 2018. World J. Gastroenterol. 2019, 25, 1432–1444. [Google Scholar] [CrossRef]
- Song, J.E.; Kim, D.Y. Conventional vs drug-eluting beads transarterial chemoembolization for hepatocellular carcinoma. World J. Hepatol. 2017, 9, 808–814. [Google Scholar] [CrossRef]
- Lewandowski, R.J.; Gabr, A.; Abouchaleh, N.; Ali, R.; Al Asadi, A.; Mora, R.A.; Kulik, L.; Ganger, D.; Dsai, K.; Thorngurg, B.; et al. Radiation segmentectomy: Potential curative therapy for early hepatocellular carcinoma. Radiology 2018, 287, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Kim, H.J.; Won, J.Y.; Kim, D.Y.; Han, K.H.; Jung, I.; Seong, J. Retrospective analysis of stereotactic body radiation therapy efficacy over radiofrequency ablation for hepatocellular carcinoma. Radiother. Oncol. 2019, 131, 81–87. [Google Scholar] [CrossRef]
- Yang, E.; Kubicek, L.; Pavletic, M.M. Use of imaging-guided intensity-modulated stereotactic body radiation therapy to treat a well-differentiated hepatocellular carcinoma in a dog. J. Am. Vet. Med. Assoc. 2021, 259, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Ito, Y.; Kawabe, M.; Iwasaki, R.; Sakai, H.; Murakami, M.; Maruo, K. Three-dimensional conformal radiation therapy for inoperable massive hepatocellular carcinoma in six dogs. J. Small Anim. Pract. 2015, 56, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Culp, W.T.N.; Johnson, E.G.; Palm, C.A.; Burton, J.H.; Rebhun, R.B.; Rodriguez, C.O.; Kent, M.S.; Glaiberman, C.B. Use of percutaneous microwave ablation in the treatment of retroperitoneal neoplasia in three dogs. J. Am. Vet. Med. Assoc. 2021, 259, 1171–1177. [Google Scholar] [CrossRef] [PubMed]
- Weisse, C. Veterinary interventional oncology: From concept to clinic. Vet. J. 2015, 205, 198–203. [Google Scholar] [CrossRef]
- von Scheel, J.; Golde, G. Pharmacokinetics of intra-arterial tumour therapy. An experimental study. Arch. Otorhinolaryngol. 1984, 239, 153–161. [Google Scholar] [CrossRef]
- Wu, B.; Zhou, J.; Ling, G.; Zhu, D.; Long, Q. CalliSpheres drug-eluting beads versus lipiodol transarterial chemoembolization in the treatment of hepatocellular carcinoma: A short-term efficacy and safety study. World J. Surg. Oncol. 2018, 16, 69. [Google Scholar] [CrossRef]
- Li, J.; Wang, N.; Shi, C.; Liu, Q.; Song, J.; Ye, X. Short-term efficacy and safety of callispheres drug-loaded microsphere embolization in primary hepatocellular carcinoma. J. Cancer Res. Ther. 2021, 17, 733–739. [Google Scholar] [CrossRef]
- Varela, M.; Real, M.I.; Burrel, M.; Forner, A.; Sala, M.; Brunet, M.; Ayuso, C.; Castells, L.; Montana, X.; Llovet, J.M.; et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: Efficacy and doxorubicin pharmacokinetics. J. Hepatol. 2007, 46, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Weisse, C.; Berent, A.; Soulenl, M.C. Comparison of serum doxorubicin levels following different DEB chemoembolization techniques as well as systemic administration in the same canine patients with naturally occurring hepatocellular carcinoma. J. Vasc. Interv. Radiol. 2013, 24, S143. [Google Scholar] [CrossRef]
- Nakasumi, K.; Yamamoto, N.; Takami, T.; Itoh, H.; Itamoto, K.; Horikirizono, H.; Iseri, T.; Nakaichi, M.; Nemoto, Y.; Sunahara, H.; et al. Effect of drug-eluting bead transarterial chemoembolization loaded with cisplatin on normal dogs. J. Vet. Med. Sci. 2022, 84, 114–120. [Google Scholar] [CrossRef]
- Rogatko, C.P.; Weisse, C.; Schwarz, T.; Berent, A.C.; Diniz, M.A. Drug-eluting bead chemoembolization for the treatment of nonresectable hepatic carcinoma in dogs: A prospective clinical trial. J. Vet. Intern. Med. 2021, 35, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Weisse, C.; Clifford, C.A.; Holt, D.; Solomon, J.A. Percutaneous arterial embolization and chemoembolization for treatment of benign and malignant tumors in three dogs and a goat. J. Am. Vet. Med. Assoc. 2002, 221, 1430–1436. [Google Scholar] [CrossRef]
- Cave, T.A.; Johnson, V.; Beths, T.; Edwards, R.; Argyle, D.J. Treatment of unresectable hepatocellular adenoma in dogs with transarterial iodized oil and chemotherapy with and without an embolic agent: A report of two cases. Vet. Comp. Oncol. 2003, 1, 191–199. [Google Scholar] [CrossRef]
- Culp, W.T.N.; Johnson, E.G.; Giuffrida, M.A.; Rebhun, R.B.; Burton, J.H.; Kent, M.S. Use of a Novel Drug-Eluting Embolic Microsphere in the Treatment of Nonresectable Liver Neoplasia in Dogs. In Proceedings of the Veterinary Interventional Radiology and Interventional Endoscopy Society Meeting, Lake Tahoe, CA, USA, 2 May 2019. [Google Scholar]
- Mason, M.C.; Massarweh, N.N.; Salami, A.; Sultenfuss, M.A.; Anaya, D.A. Post-embolization syndrome as an early predictor of overall survival after transarterial chemoembolization for hepatocellular carcinoma. HPB 2015, 17, 1137–1144. [Google Scholar] [CrossRef]
- Tu, J.; Jia, Z.; Ying, X.; Zhang, D.; Li, S.; Tian, F.; Jiang, G. The incidence and outcome of major complication following conventional TAE/TACE for hepatocellular carcinoma. Medicine 2016, 95, 5606. [Google Scholar] [CrossRef] [PubMed]
- McWilliams, J.P.; Yamamoto, S.; Raman, S.S.; Loh, C.T.; Lee, E.W.; Liu, D.M.; Kee, S.T. Percutaneous ablation of hepatocellular carcinoma: Current status. J. Vasc. Interv. Radiol. 2010, 21 (Suppl. 8), S204–S213. [Google Scholar] [CrossRef]
- Lubner, M.G.; Brace, C.L.; Hinshaw, J.L.; Lee, F.T., Jr. Microwave tumor ablation: Mechanism of action, clinical results, and devices. J. Vasc. Interv. Radiol. 2010, 21 (Suppl. 8), S192–S203. [Google Scholar] [CrossRef]
- Yang, T.; Case, J.B.; Boston, S.; Dark, M.J.; Toskich, B. Microwave ablation for treatment of hepatic neoplasia in five dogs. J. Am. Vet. Med. Assoc. 2017, 250, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Mazzaccari, K.; Boston, S.E.; Toskich, B.B.; Bowles, K.; Case, J.B. Video-assisted microwave ablation for the treatment of a metastatic lung lesion in a dog with appendicular osteosarcoma and hypertrophic osteopathy. Vet. Surg. 2017, 46, 1161–1165. [Google Scholar] [CrossRef] [PubMed]
- Pollard, R.E.; Long, C.D.; Nelson, R.W.; Hornof, W.J.; Feldman, E.C. Percutaneous ultrasonographically guided radiofrequency heat ablation for treatment of primary hyperparathyroidism in dogs. J. Am. Vet. Med. Assoc. 2001, 218, 1106–1110. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Gao, J.; Liu, Y.; Li, T.; Feng, K.; Ma, K.; Dong, J.; Li, X.; Wang, S.; Bie, P. Determining a minimal safe distance to prevent thermal injury to intrahepatic bile ducts in radiofrequency ablation of the liver: A study in dogs. Int. J. Hyperth. 2012, 28, 210–217. [Google Scholar] [CrossRef]
- Ahmed, M.; Liu, Z.; Afzal, K.S.; Weeks, D.; Lobo, S.M.; Kruskal, J.B.; Lenkinski, R.E.; Goldberg, S.N. Radiofrequency ablation: Effect of surrounding tissue composition on coagulation necrosis in a canine tumor model. Radiology 2004, 230, 761–767. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gibson, E.A.; Goldman, R.E.; Culp, W.T.N. Comparative Oncology: Management of Hepatic Neoplasia in Humans and Dogs. Vet. Sci. 2022, 9, 489. https://doi.org/10.3390/vetsci9090489
Gibson EA, Goldman RE, Culp WTN. Comparative Oncology: Management of Hepatic Neoplasia in Humans and Dogs. Veterinary Sciences. 2022; 9(9):489. https://doi.org/10.3390/vetsci9090489
Chicago/Turabian StyleGibson, Erin A., Roger E. Goldman, and William T. N. Culp. 2022. "Comparative Oncology: Management of Hepatic Neoplasia in Humans and Dogs" Veterinary Sciences 9, no. 9: 489. https://doi.org/10.3390/vetsci9090489







