Effect of Dickkopf-1 (Dkk-1) and SP600125, a JNK Inhibitor, on Wnt Signaling in Canine Prostate Cancer Growth and Bone Metastases
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Dkk-1 ELISA
2.3. Immunohistochemistry
2.4. Activator Protein-1 (AP-1) Reporter Transfection
2.5. Cell Proliferation Assay
2.6. Wound Healing Assay
2.7. JNK Inhibitor (SP600125) Treatment In Vitro
2.8. RNA Extraction and Quantitative RT-PCR
2.9. Subcutaneous Injection and JNK Inhibitor (SP600125) Treatment
2.10. Intratibial Injection and JNK Inhibitor (SP600125) Treatment
2.11. Bioluminescent Imaging
2.12. Faxitron Imaging
2.13. Histopathological Studies
2.14. Statistical Analysis
3. Results
3.1. Dkk-1 Secretion
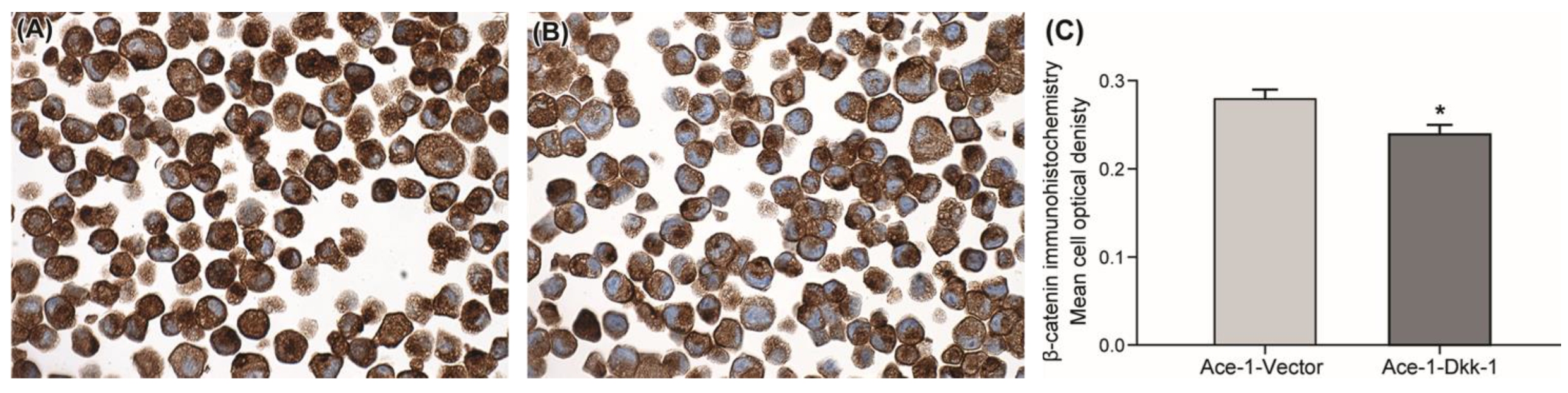
3.2. Immunohistochemistry
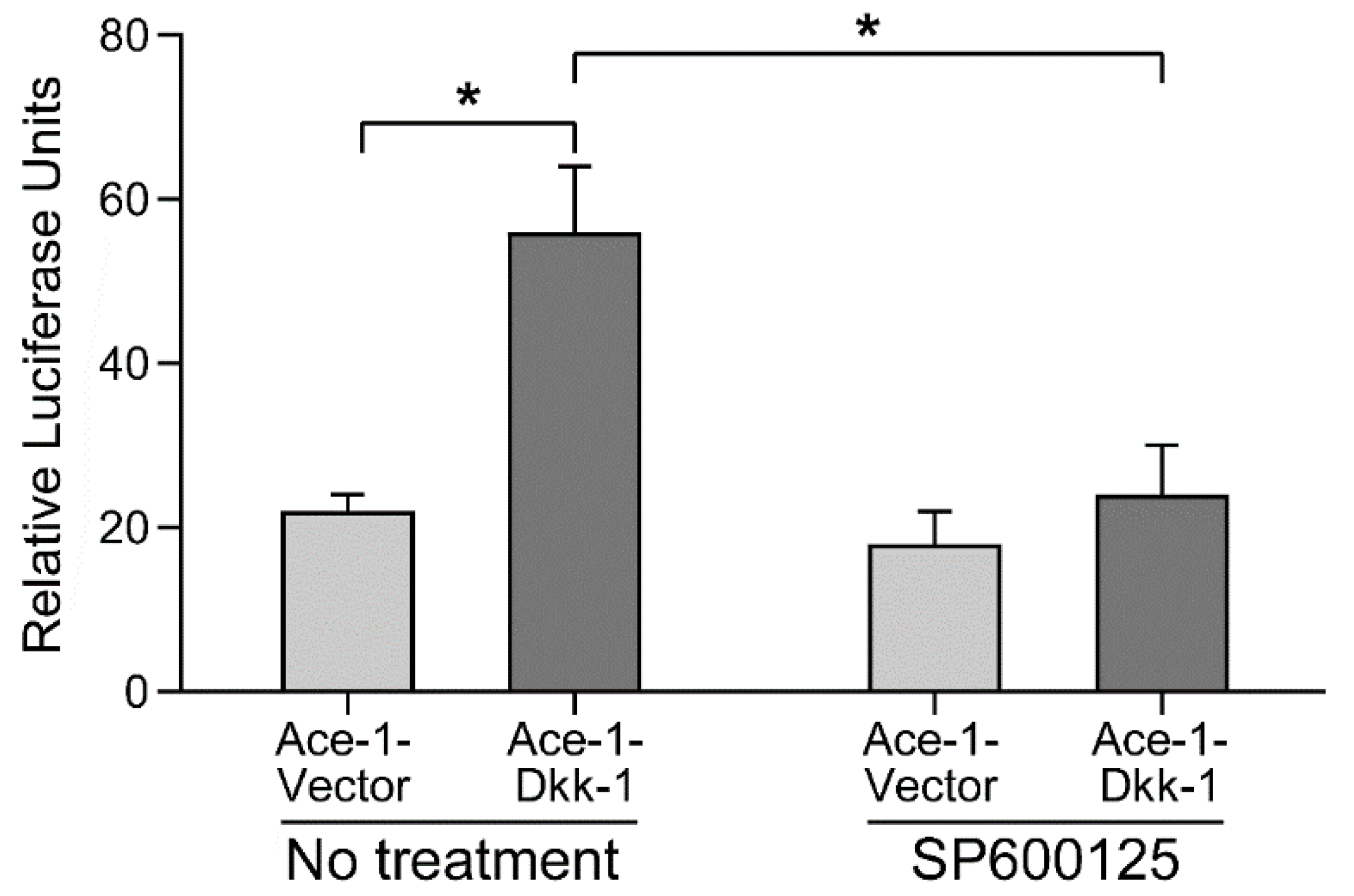
3.3. AP-1 Reporter Activity
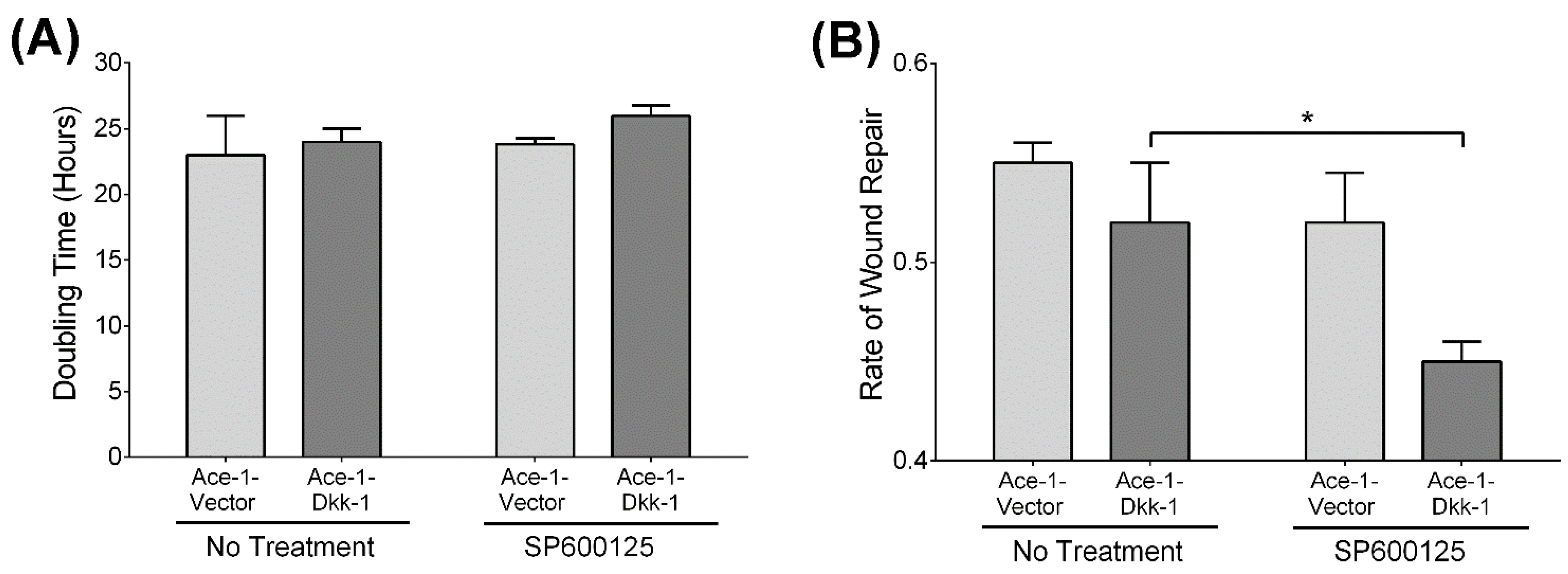
3.4. Cell Proliferation Assay
3.5. Wound Healing Assay
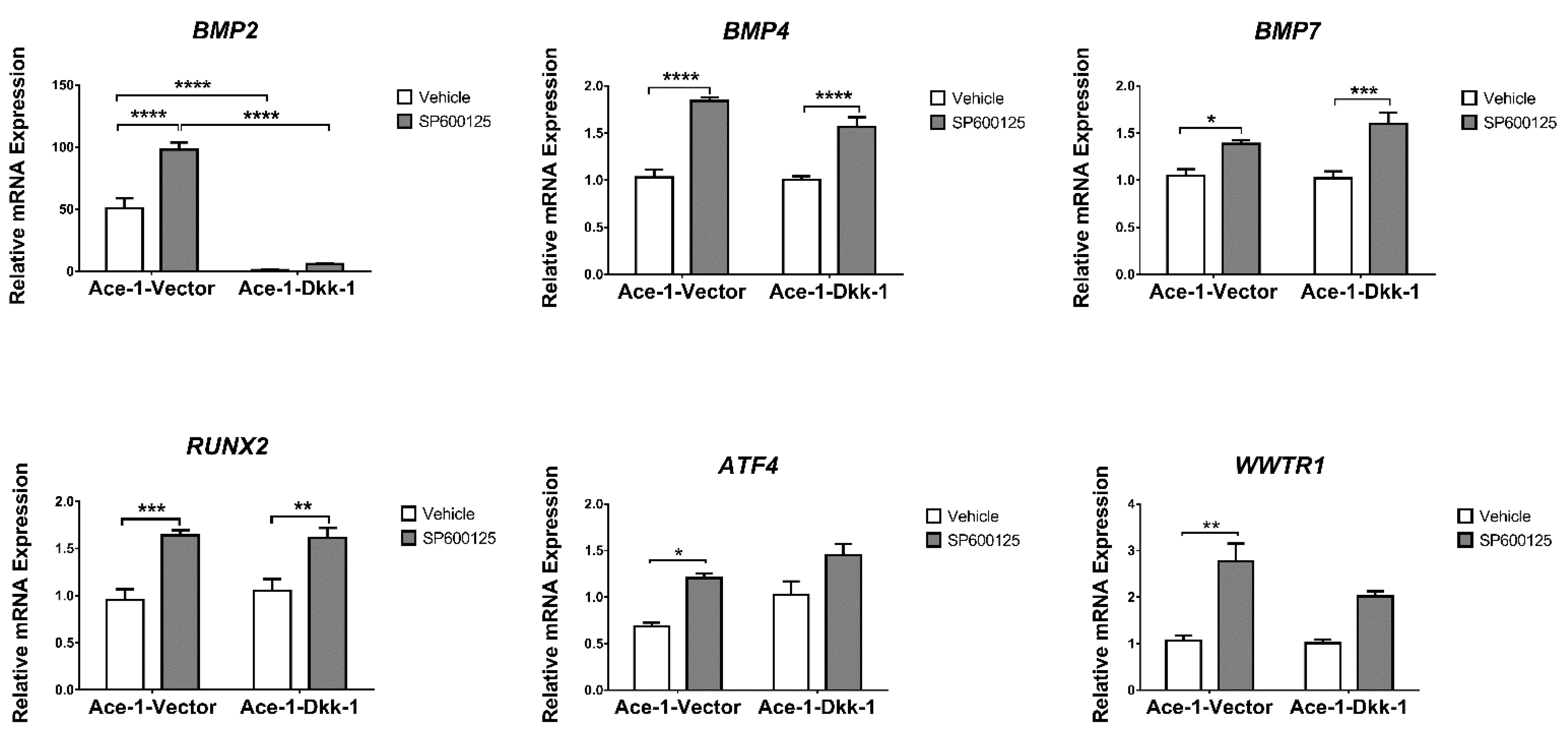
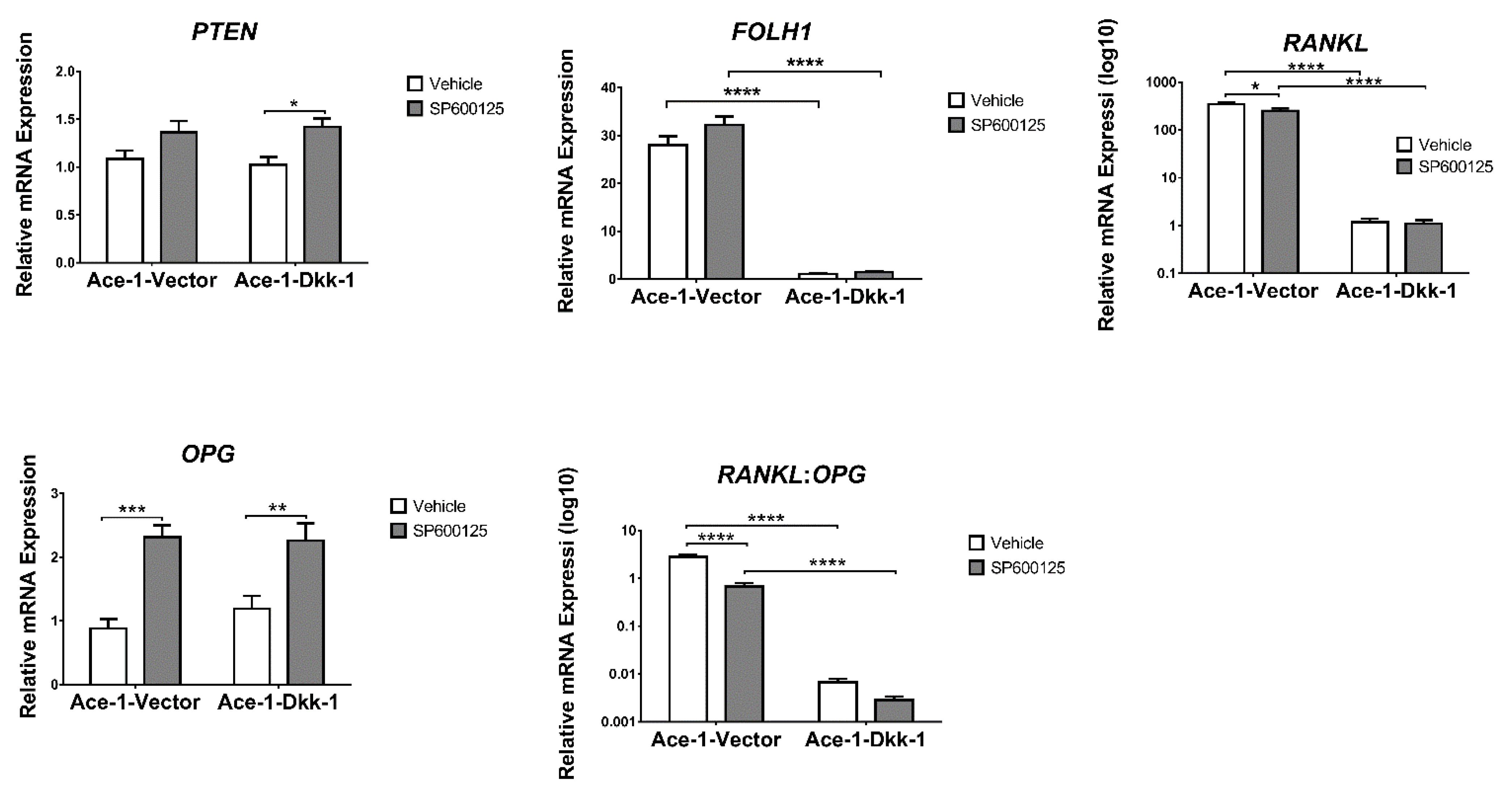
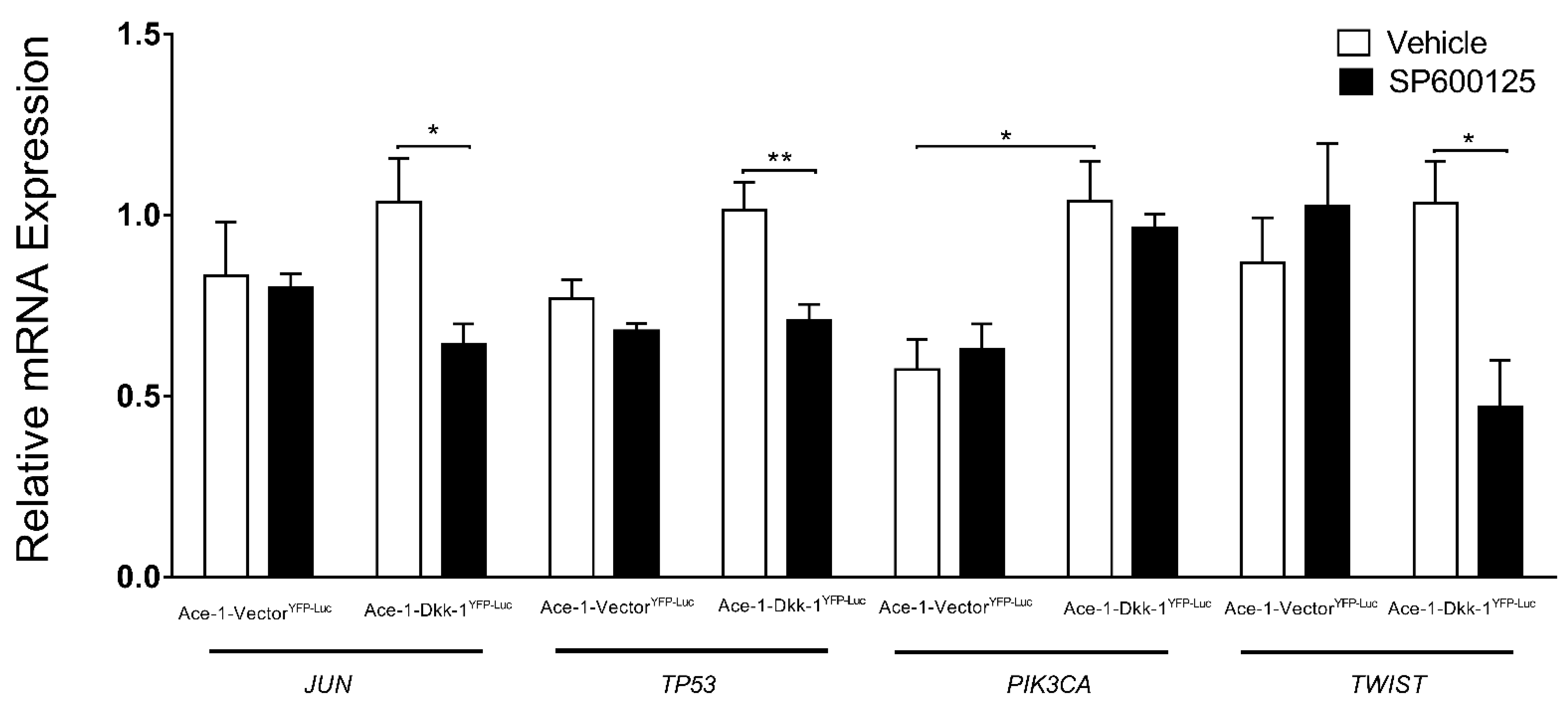
3.6. JNK Inhibitor (SP600125) Treatment In Vitro (RT-qPCR)
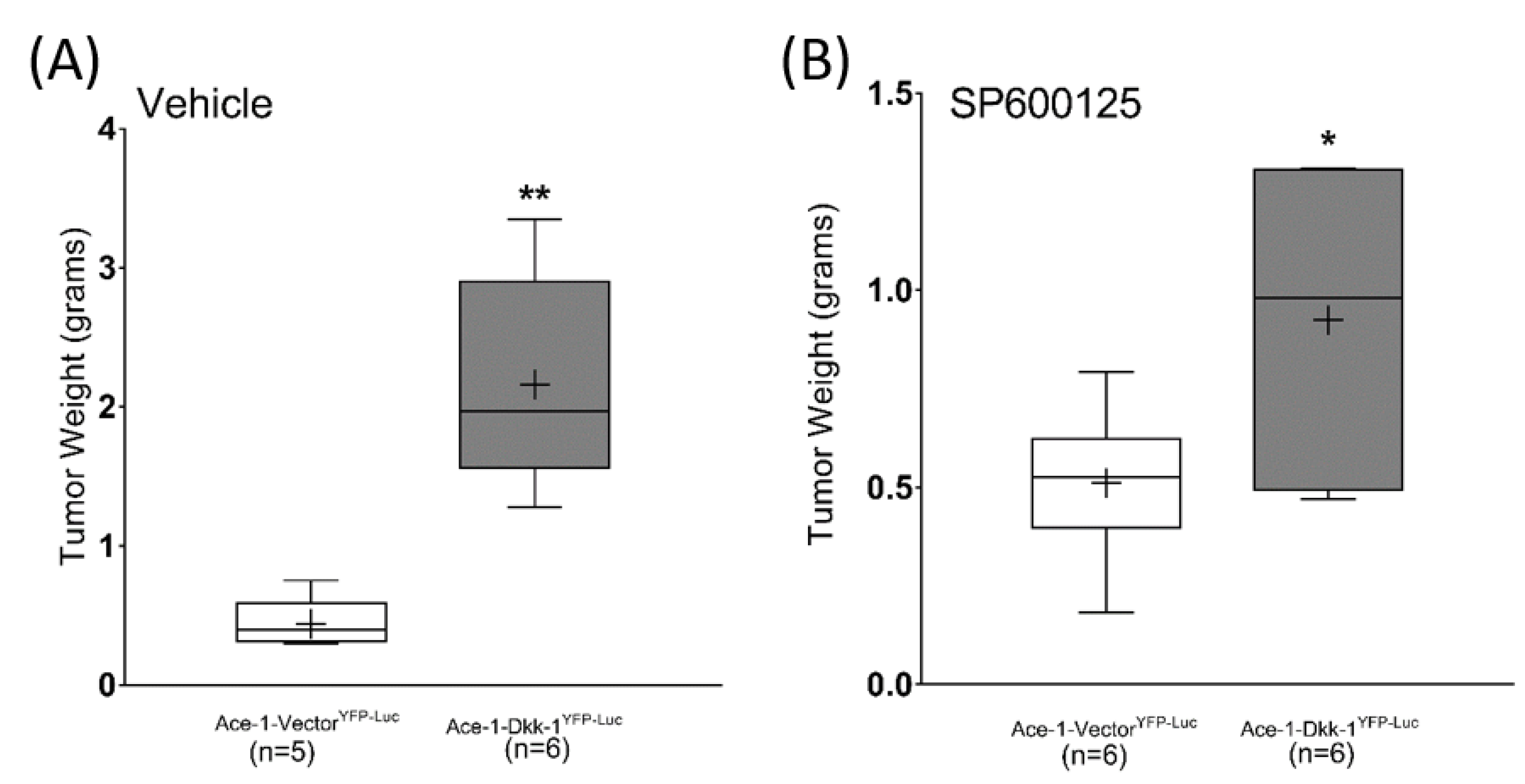
3.7. Subcutaneous Injection, JNK Inhibitor (SP600125) Treatment, and RT-qPCR
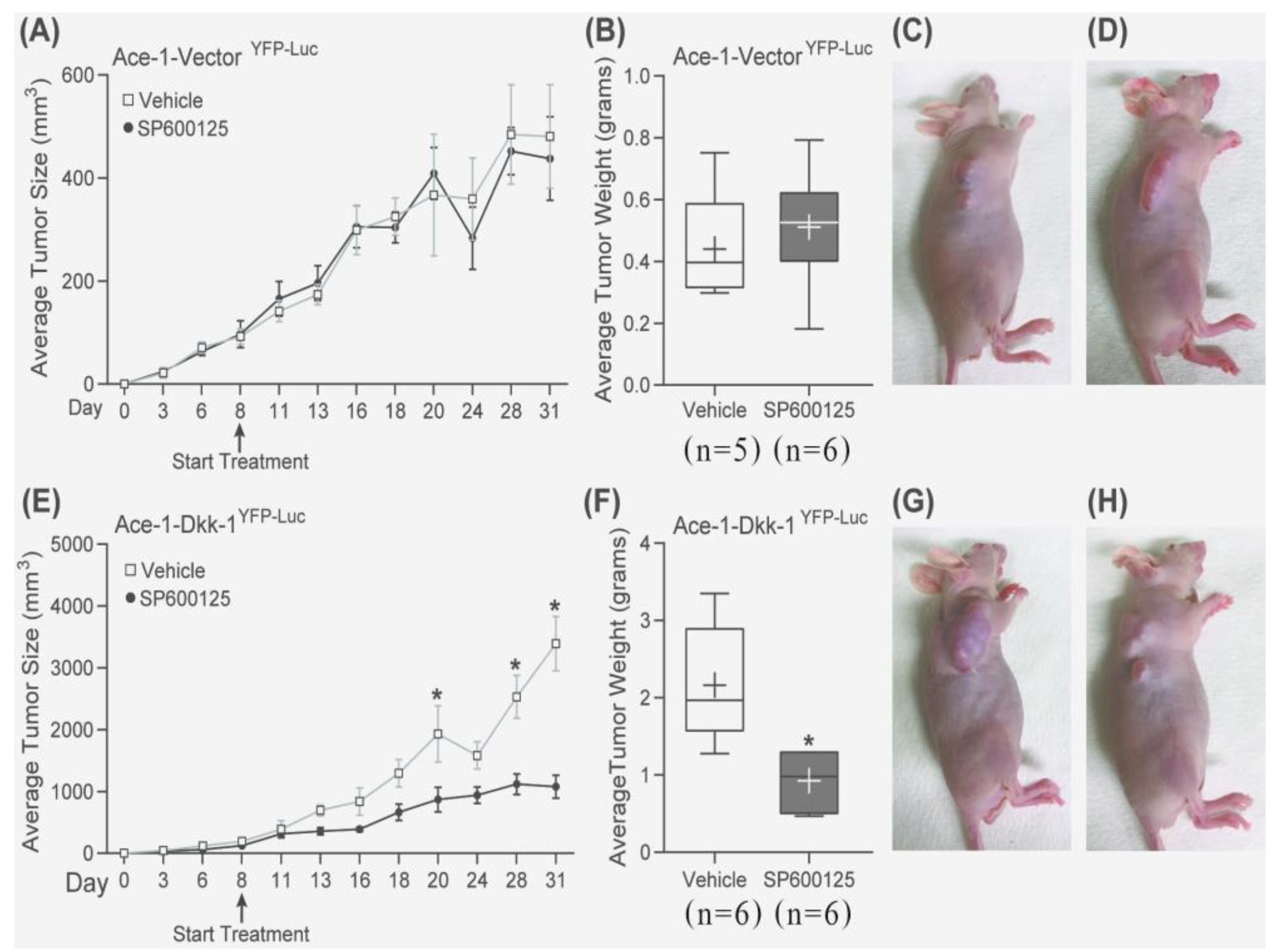
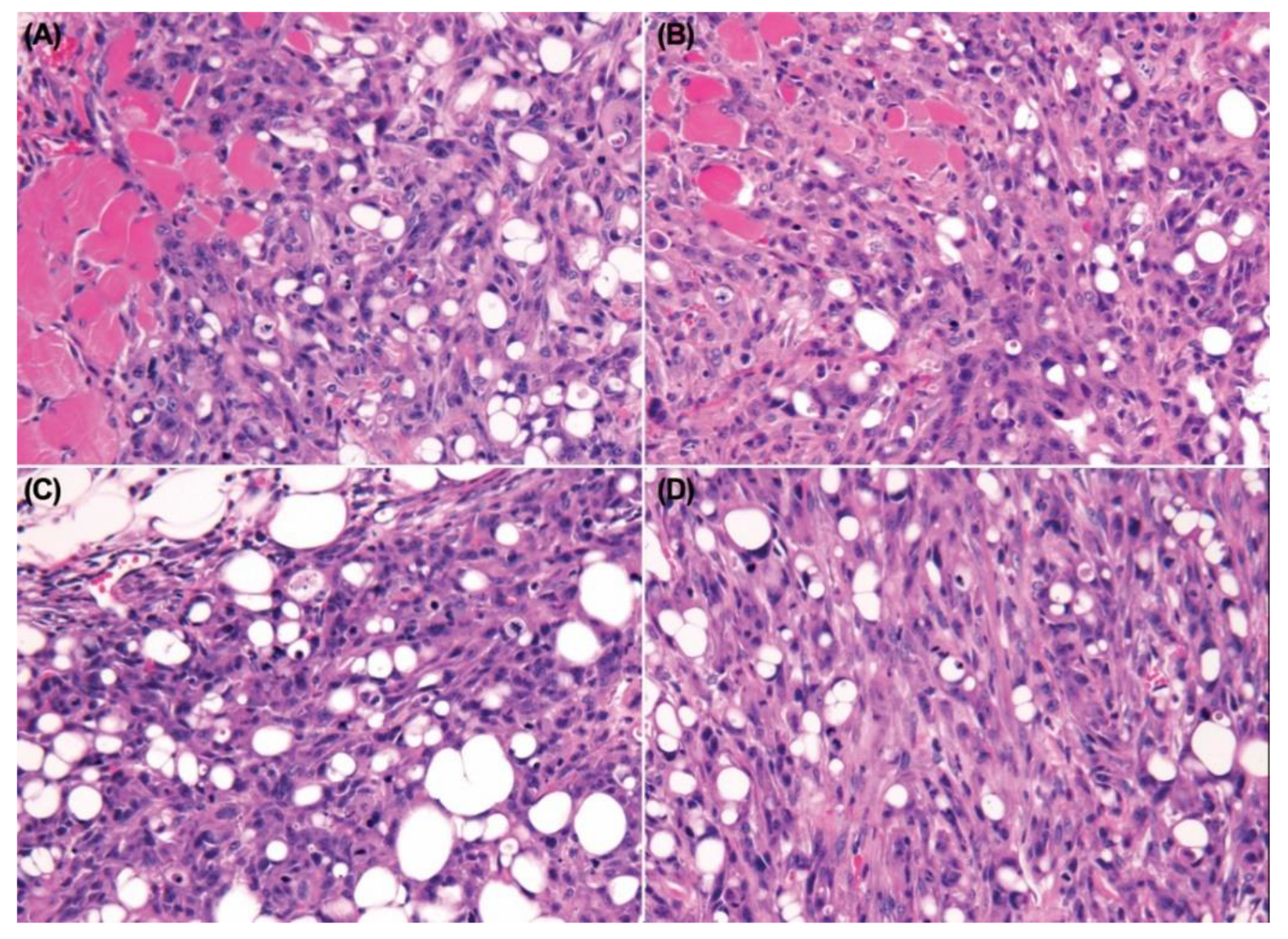
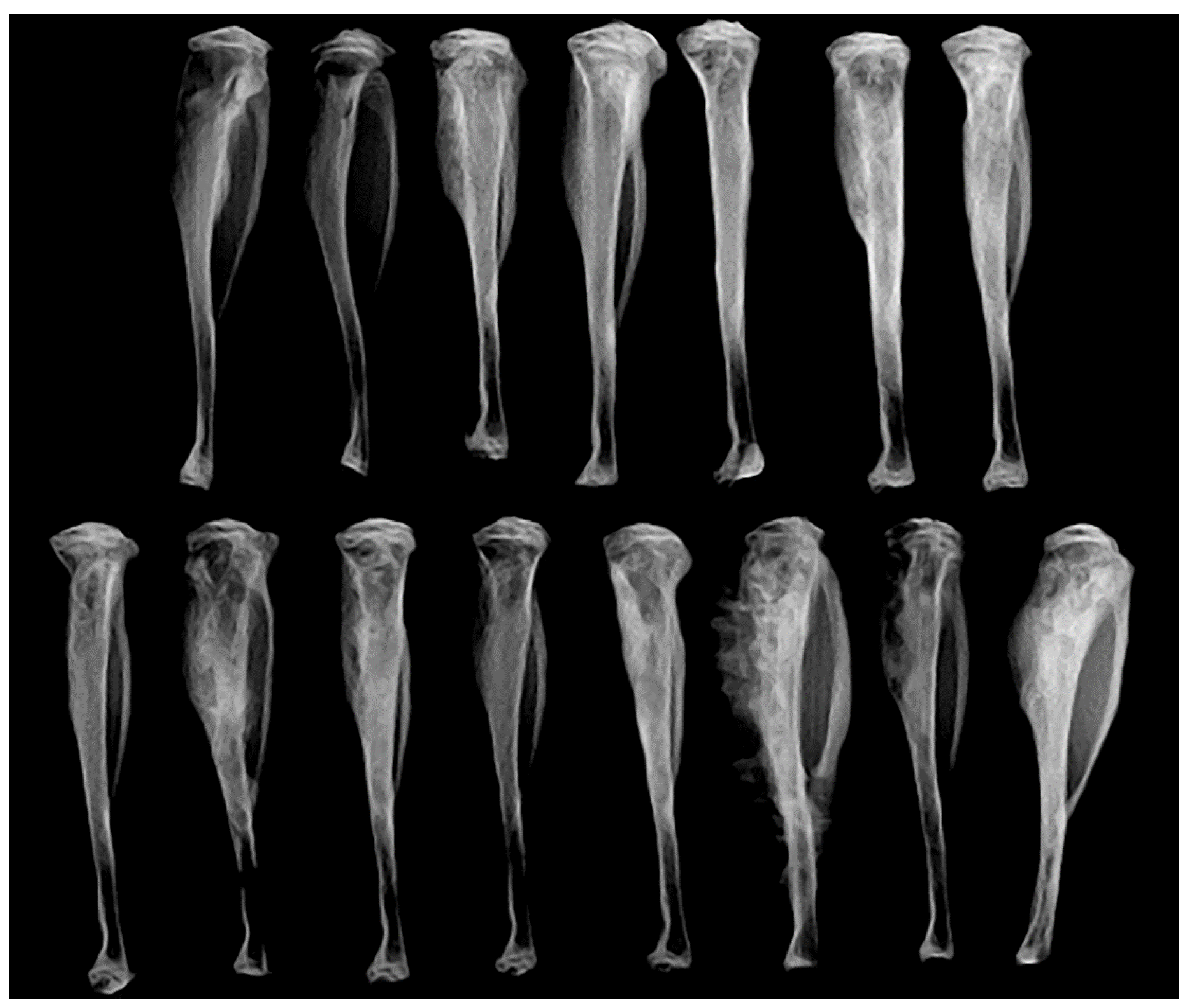
3.8. Intratibial Injection and JNK Inhibitor (SP600125) Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Hall, C.L.; Daignault, S.D.; Shah, R.B.; Pienta, K.J.; Keller, E.T. Dickkopf-1 expression increases early in prostate cancer development and decreases during progression from primary tumor to metastasis. Prostate 2008, 68, 1396–1404. [Google Scholar] [CrossRef] [PubMed]
- Suzman, D.L.; Boikos, S.A.; Carducci, M.A. Bone-targeting agents in prostate cancer. Cancer Metastasis Rev. 2014, 33, 619–628. [Google Scholar] [CrossRef]
- Thudi, N.K.; Martin, C.K.; Murahari, S.; Shu, S.T.; Lanigan, L.G.; Werbeck, J.L.; Keller, E.T.; McCauley, L.K.; Pinzone, J.J.; Rosol, T.J. Dickkopf-1 (DKK-1) stimulated prostate cancer growth and metastasis and inhibited bone formation in osteoblastic bone metastases. Prostate 2011, 71, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Albers, J.; Keller, J.; Baranowsky, A.; Beil, F.T.; Catala-Lehnen, P.; Schulze, J.; Amling, M.; Schinke, T. Canonical Wnt signaling inhibits osteoclastogenesis independent of osteoprotegerin. J. Cell Biol. 2013, 200, 537–549. [Google Scholar] [CrossRef]
- Takahashi, N.; Maeda, K.; Ishihara, A.; Uehara, S.; Kobayashi, Y. Regulatory mechanism of osteoclastogenesis by RANKL and Wnt signals. Front. Biosci. 2011, 16, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Glass, D.A., 2nd; Bialek, P.; Ahn, J.D.; Starbuck, M.; Patel, M.S.; Clevers, H.; Taketo, M.M.; Long, F.; McMahon, A.P.; Lang, R.A.; et al. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev. Cell 2005, 8, 751–764. [Google Scholar] [CrossRef]
- Spencer, G.J.; Utting, J.C.; Etheridge, S.L.; Arnett, T.R.; Genever, P.G. Wnt signalling in osteoblasts regulates expression of the receptor activator of NFkappaB ligand and inhibits osteoclastogenesis in vitro. J. Cell Sci. 2006, 119, 1283–1296. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Kobayashi, Y.; Udagawa, N.; Uehara, S.; Ishihara, A.; Mizoguchi, T.; Kikuchi, Y.; Takada, I.; Kato, S.; Kani, S.; et al. Wnt5a-Ror2 signaling between osteoblast-lineage cells and osteoclast precursors enhances osteoclastogenesis. Nat. Med. 2012, 18, 405–412. [Google Scholar] [CrossRef]
- Le, P.N.; McDermott, J.D.; Jimeno, A. Targeting the Wnt pathway in human cancers: Therapeutic targeting with a focus on OMP-54F28. Pharmacol. Ther. 2015, 146, 1–11. [Google Scholar] [CrossRef]
- Schulte, G. International Union of Basic and Clinical Pharmacology. LXXX. The class Frizzled receptors. Pharmacol. Rev. 2010, 62, 632–667. [Google Scholar] [CrossRef]
- Papachristou, D.J.; Batistatou, A.; Sykiotis, G.P.; Varakis, I.; Papavassiliou, A.G. Activation of the JNK–AP-1 signal transduction pathway is associated with pathogenesis and progression of human osteosarcomas. Bone 2003, 32, 364–371. [Google Scholar] [CrossRef]
- Bennett, B.L.; Sasaki, D.T.; Murray, B.W.; O’Leary, E.C.; Sakata, S.T.; Xu, W.; Leisten, J.C.; Motiwala, A.; Pierce, S.; Satoh, Y.; et al. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc. Natl. Acad. Sci. USA 2001, 98, 13681–13686. [Google Scholar] [CrossRef] [PubMed]
- Curtin, J.F.; Cotter, T.G. JNK regulates HIPK3 expression and promotes resistance to Fas-mediated apoptosis in DU 145 prostate carcinoma cells. J. Biol. Chem. 2004, 279, 17090–17100. [Google Scholar] [CrossRef]
- Yang, Y.M.; Bost, F.; Charbono, W.; Dean, N.; McKay, R.; Rhim, J.S.; Depatie, C.; Mercola, D. C-Jun NH(2)-terminal kinase mediates proliferation and tumor growth of human prostate carcinoma. Clin. Cancer Res. 2003, 9, 391–401. [Google Scholar] [PubMed]
- Tan, H.; Zhang, G.; Yang, X.; Jing, T.; Shen, D.; Wang, X. Peimine inhibits the growth and motility of prostate cancer cells and induces apoptosis by disruption of intracellular calcium homeostasis through Ca(2+) /CaMKII/JNK pathway. J. Cell BioChem. 2020, 121, 81–92. [Google Scholar] [CrossRef]
- Shaulian, E.; Karin, M. AP-1 as a regulator of cell life and death. Nat. Cell Biol. 2002, 4, E131–E136. [Google Scholar] [CrossRef]
- Eferl, R.; Wagner, E.F. AP-1: A double-edged sword in tumorigenesis. Nat. Rev. Cancer 2003, 3, 859–868. [Google Scholar] [CrossRef]
- Rivat, C.; Le Floch, N.; Sabbah, M.; Teyrol, I.; Redeuilh, G.; Bruyneel, E.; Mareel, M.; Matrisian, L.M.; Crawford, H.C.; Gespach, C.; et al. Synergistic cooperation between the AP-1 and LEF-1 transcription factors in activation of the matrilysin promoter by the src oncogene: Implications in cellular invasion. FASEB J. 2003, 17, 1721–1723. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ouyang, X.; Jessen, W.J.; Al-Ahmadie, H.; Serio, A.M.; Lin, Y.; Shih, W.J.; Reuter, V.E.; Scardino, P.T.; Shen, M.M.; Aronow, B.J.; et al. Activator protein-1 transcription factors are associated with progression and recurrence of prostate cancer. Cancer Res. 2008, 68, 2132–2144. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y.; He, B.; You, L.; Xu, Z.; Mazieres, J.; Reguart, N.; Mikami, I.; Batra, S.; Jablons, D.M. Dickkopf-1 antagonizes Wnt signaling independent of beta-catenin in human mesothelioma. BioChem. Biophys. Res. Commun. 2004, 323, 1246–1250. [Google Scholar] [CrossRef]
- Niehrs, C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene 2006, 25, 7469–7481. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.L.; Keller, E.T. The role of Wnts in bone metastases. Cancer Metastasis Rev. 2006, 25, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Rachner, T.D.; Thiele, S.; Gobel, A.; Browne, A.; Fuessel, S.; Erdmann, K.; Wirth, M.P.; Frohner, M.; Todenhofer, T.; Muders, M.H.; et al. High serum levels of Dickkopf-1 are associated with a poor prognosis in prostate cancer patients. BMC Cancer 2014, 14, 649. [Google Scholar] [CrossRef] [PubMed]
- D’Amelio, P.; Roato, I.; Oderda, M.; Soria, F.; Zitella, A.; Ferracini, R.; Mengozzi, G.; Gontero, P.; Isaia, G.C. DKK-1 in prostate cancer diagnosis and follow up. BMC Clin. Pathol. 2014, 14, 11. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thudi, N.K.; Martin, C.K.; Nadella, M.V.; Fernandez, S.A.; Werbeck, J.L.; Pinzone, J.J.; Rosol, T.J. Zoledronic acid decreased osteolysis but not bone metastasis in a nude mouse model of canine prostate cancer with mixed bone lesions. Prostate 2008, 68, 1116–1125. [Google Scholar] [CrossRef] [PubMed]
- Jemaa, M.; Vitale, I.; Kepp, O.; Berardinelli, F.; Galluzzi, L.; Senovilla, L.; Marino, G.; Malik, S.A.; Rello-Varona, S.; Lissa, D.; et al. Selective killing of p53-deficient cancer cells by SP600125. EMBO Mol. Med. 2012, 4, 500–514. [Google Scholar] [CrossRef] [PubMed]
- Elshafae, S.M.; Hassan, B.B.; Supsavhad, W.; Dirksen, W.P.; Camiener, R.Y.; Ding, H.; Tweedle, M.F.; Rosol, T.J. Gastrin-releasing peptide receptor (GRPr) promotes EMT, growth, and invasion in canine prostate cancer. Prostate 2016, 76, 796–809. [Google Scholar] [CrossRef]
- Nusse, R.; Clevers, H. Wnt/beta-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell 2017, 169, 985–999. [Google Scholar] [CrossRef]
- Zeng, X.; Tamai, K.; Doble, B.; Li, S.; Huang, H.; Habas, R.; Okamura, H.; Woodgett, J.; He, X. A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature 2005, 438, 873–877. [Google Scholar] [CrossRef]
- Kikuchi, A.; Yamamoto, H.; Sato, A.; Matsumoto, S. New insights into the mechanism of Wnt signaling pathway activation. Int. Rev. Cell Mol. Biol. 2011, 291, 21–71. [Google Scholar] [CrossRef]
- Simmons, J.K.; Elshafae, S.M.; Keller, E.T.; McCauley, L.K.; Rosol, T.J. Review of Animal Models of Prostate Cancer Bone Metastasis. Vet. Sci. 2014, 1, 16–39. [Google Scholar] [CrossRef]
- Tweedle, M.F.; Ding, H.; Drost, W.T.; Dowell, J.; Spain, J.; Joseph, M.; Elshafae, S.M.; Menendez, M.I.; Gong, L.; Kothandaraman, S.; et al. Development of an orthotopic canine prostate cancer model expressing human GRPr. Prostate 2018. [Google Scholar] [CrossRef] [PubMed]
- Pinzone, J.J.; Hall, B.M.; Thudi, N.K.; Vonau, M.; Qiang, Y.W.; Rosol, T.J.; Shaughnessy, J.D., Jr. The role of Dickkopf-1 in bone development, homeostasis, and disease. Blood 2009, 113, 517–525. [Google Scholar] [CrossRef]
- Li, F.; Chong, Z.Z.; Maiese, K. Vital elements of the Wnt-Frizzled signaling pathway in the nervous system. Curr. Neurovasc. Res. 2005, 2, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Hall, C.L.; Escara-Wilke, J.; Mizokami, A.; Keller, J.M.; Keller, E.T. Prostate cancer induces bone metastasis through Wnt-induced bone morphogenetic protein-dependent and independent mechanisms. Cancer Res. 2008, 68, 5785–5794. [Google Scholar] [CrossRef] [PubMed]
- Voorzanger-Rousselot, N.; Goehrig, D.; Journe, F.; Doriath, V.; Body, J.J.; Clezardin, P.; Garnero, P. Increased Dickkopf-1 expression in breast cancer bone metastases. Br. J. Cancer 2007, 97, 964–970. [Google Scholar] [CrossRef]
- Hall, C.L.; Bafico, A.; Dai, J.; Aaronson, S.A.; Keller, E.T. Prostate cancer cells promote osteoblastic bone metastases through Wnts. Cancer Res. 2005, 65, 7554–7560. [Google Scholar] [CrossRef]
- Lee, Y.C.; Cheng, C.J.; Bilen, M.A.; Lu, J.F.; Satcher, R.L.; Yu-Lee, L.Y.; Gallick, G.E.; Maity, S.N.; Lin, S.H. BMP4 promotes prostate tumor growth in bone through osteogenesis. Cancer Res. 2011, 71, 5194–5203. [Google Scholar] [CrossRef]
- Masuda, H.; Fukabori, Y.; Nakano, K.; Takezawa, Y.; CSuzuki, T.; Yamanaka, H. Increased expression of bone morphogenetic protein-7 in bone metastatic prostate cancer. Prostate 2003, 54, 268–274. [Google Scholar] [CrossRef]
- Carroll, S.H.; Ravid, K. Differentiation of mesenchymal stem cells to osteoblasts and chondrocytes: A focus on adenosine receptors. Expert Rev. Mol. Med. 2013, 15, e1. [Google Scholar] [CrossRef]
- Singleton, D.C.; Harris, A.L. Targeting the ATF4 pathway in cancer therapy. Expert Opin. Ther. Targets 2012, 16, 1189–1202. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, Z.; Wang, Y.; Chang, D.; Su, L.; Guo, Y.; Liu, C. TR1 promotes cell proliferation and inhibits apoptosis through cyclin A and CTGF regulation in non-small cell lung cancer. Tumour Biol. 2014, 35, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Paramio, J.M.; Navarro, M.; Segrelles, C.; Gomez-Casero, E.; Jorcano, J.L. PTEN tumour suppressor is linked to the cell cycle control through the retinoblastoma protein. Oncogene 1999, 18, 7462–7468. [Google Scholar] [CrossRef]
- Backman, S.A.; Ghazarian, D.; So, K.; Sanchez, O.; Wagner, K.U.; Hennighausen, L.; Suzuki, A.; Tsao, M.S.; Chapman, W.B.; Stambolic, V.; et al. Early onset of neoplasia in the prostate and skin of mice with tissue-specific deletion of Pten. Proc. Natl. Acad. Sci. USA 2004, 101, 1725–1730. [Google Scholar] [CrossRef] [PubMed]
- Hofman, M.S.; Hicks, R.J.; Maurer, T.; Eiber, M. Prostate-specific Membrane Antigen PET: Clinical Utility in Prostate Cancer, Normal Patterns, Pearls, and Pitfalls. Radiographics 2018, 38, 200–217. [Google Scholar] [CrossRef]
- Gasinska, A.; Luczynska, E.; Wilk, W.; Cichocka, A. Differences in the expression of telomerase and prostate-specific membrane antigen in non-advanced prostatic cancer. Folia Histochem. Cytobiol. 2013, 51, 66–72. [Google Scholar] [CrossRef][Green Version]
- Kasperzyk, J.L.; Finn, S.P.; Flavin, R.; Fiorentino, M.; Lis, R.; Hendrickson, W.K.; Clinton, S.K.; Sesso, H.D.; Giovannucci, E.L.; Stampfer, M.J.; et al. Prostate-specific membrane antigen protein expression in tumor tissue and risk of lethal prostate cancer. Cancer Epidemiol. Biomark. Prev. 2013, 22, 2354–2363. [Google Scholar] [CrossRef] [PubMed]
- Obradovich, J.; Walshaw, R.; Goullaud, E. The Influence of Castration on the Development of Prostatic Carcinoma in the Dog 43 Cases (1978–1985). J. Vet. Intern. Med. 1987, 1, 183–187. [Google Scholar] [CrossRef]
- Sorenmo, K.U.; Goldschmidt, M.; Shofer, F.; Goldkamp, C.; Ferracone, J. Immunohistochemical characterization of canine prostatic carcinoma and correlation with castration status and castration time. Vet. Comp. Oncol. 2003, 1, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Shorning, B.Y.; Dass, M.S.; Smalley, M.J.; Pearson, H.B. The PI3K-AKT-mTOR Pathway and Prostate Cancer: At the Crossroads of AR, MAPK, and WNT Signaling. Int. J. Mol. Sci. 2020, 21, 4507. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.S.; Schultz, N.; Hieronymus, H.; Gopalan, A.; Xiao, Y.; Carver, B.S.; Arora, V.K.; Kaushik, P.; Cerami, E.; Reva, B.; et al. Integrative genomic profiling of human prostate cancer. Cancer Cell 2010, 18, 11–22. [Google Scholar] [CrossRef]
- Kagey, M.H.; He, X. Rationale for targeting the Wnt signalling modulator Dickkopf-1 for oncology. Br. J. Pharmacol. 2017, 174, 4637–4650. [Google Scholar] [CrossRef]
- Rinehart-Kim, J.; Johnston, M.; Birrer, M.; Bos, T. Alterations in the gene expression profile of MCF-7 breast tumor cells in response to c-Jun. Int. J. Cancer 2000, 88, 180–190. [Google Scholar] [CrossRef]
- Smith, L.M.; Wise, S.C.; Hendricks, D.T.; Sabichi, A.L.; Bos, T.; Reddy, P.; Brown, P.H.; Birrer, M.J. cJun overexpression in MCF-7 breast cancer cells produces a tumorigenic, invasive and hormone resistant phenotype. Oncogene 1999, 18, 6063–6070. [Google Scholar] [CrossRef] [PubMed]
- Ecke, T.H.; Schlechte, H.H.; Hubsch, A.; Lenk, S.V.; Schiemenz, K.; Rudolph, B.D.; Miller, K. TP53 mutation in prostate needle biopsies--comparison with patients follow-up. Anticancer Res. 2007, 27, 4143–4148. [Google Scholar] [PubMed]
- Ecke, T.H.; Schlechte, H.H.; Schiemenz, K.; Sachs, M.D.; Lenk, S.V.; Rudolph, B.D.; Loening, S.A. TP53 gene mutations in prostate cancer progression. Anticancer Res. 2010, 30, 1579–1586. [Google Scholar]
- Sirohi, D.; Devine, P.; Grenert, J.P.; van Ziffle, J.; Simko, J.P.; Stohr, B.A. TP53 structural variants in metastatic prostatic carcinoma. PLoS ONE 2019, 14, e0218618. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, K.; Jung, H.H.; Lee, E.; Cho, E.Y.; Lee, K.H.; Bae, S.Y.; Lee, S.K.; Kim, S.W.; Lee, J.E.; et al. Association between Mutation and Expression of TP53 as a Potential Prognostic Marker of Triple-Negative Breast Cancer. Cancer Res. Treat. 2016, 48, 1338–1350. [Google Scholar] [CrossRef]
- Khan, M.A.; Chen, H.C.; Zhang, D.; Fu, J. Twist: A molecular target in cancer therapeutics. Tumour Biol. 2013, 34, 2497–2506. [Google Scholar] [CrossRef] [PubMed]
- Mironchik, Y.; Winnard, P.T., Jr.; Vesuna, F.; Kato, Y.; Wildes, F.; Pathak, A.P.; Kominsky, S.; Artemov, D.; Bhujwalla, Z.; Van Diest, P.; et al. Twist overexpression induces in vivo angiogenesis and correlates with chromosomal instability in breast cancer. Cancer Res. 2005, 65, 10801–10809. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Mani, S.A.; Donaher, J.L.; Ramaswamy, S.; Itzykson, R.A.; Come, C.; Savagner, P.; Gitelman, I.; Richardson, A.; Weinberg, R.A. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 2004, 117, 927–939. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Supsavhad, W.; Hassan, B.B.; Simmons, J.K.; Dirksen, W.P.; Elshafae, S.M.; Kohart, N.A.; Demirer, A.A.; Rosol, T.J. Effect of Dickkopf-1 (Dkk-1) and SP600125, a JNK Inhibitor, on Wnt Signaling in Canine Prostate Cancer Growth and Bone Metastases. Vet. Sci. 2021, 8, 153. https://doi.org/10.3390/vetsci8080153
Supsavhad W, Hassan BB, Simmons JK, Dirksen WP, Elshafae SM, Kohart NA, Demirer AA, Rosol TJ. Effect of Dickkopf-1 (Dkk-1) and SP600125, a JNK Inhibitor, on Wnt Signaling in Canine Prostate Cancer Growth and Bone Metastases. Veterinary Sciences. 2021; 8(8):153. https://doi.org/10.3390/vetsci8080153
Chicago/Turabian StyleSupsavhad, Wachiraphan, Bardes B. Hassan, Jessica K. Simmons, Wessel P. Dirksen, Said M. Elshafae, Nicole A. Kohart, Aylin A. Demirer, and Thomas J. Rosol. 2021. "Effect of Dickkopf-1 (Dkk-1) and SP600125, a JNK Inhibitor, on Wnt Signaling in Canine Prostate Cancer Growth and Bone Metastases" Veterinary Sciences 8, no. 8: 153. https://doi.org/10.3390/vetsci8080153
APA StyleSupsavhad, W., Hassan, B. B., Simmons, J. K., Dirksen, W. P., Elshafae, S. M., Kohart, N. A., Demirer, A. A., & Rosol, T. J. (2021). Effect of Dickkopf-1 (Dkk-1) and SP600125, a JNK Inhibitor, on Wnt Signaling in Canine Prostate Cancer Growth and Bone Metastases. Veterinary Sciences, 8(8), 153. https://doi.org/10.3390/vetsci8080153





