p16, pRb, and p53 in Feline Oral Squamous Cell Carcinoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Immunostaining
2.3. p16 mRNA in 3 FOSCC Cell Lines
2.4. Papillomavirus L1 Major Capsid DNA Investigation
2.5. PCR Product Purification and Sequencing
2.6. Statistical Analysis
3. Results
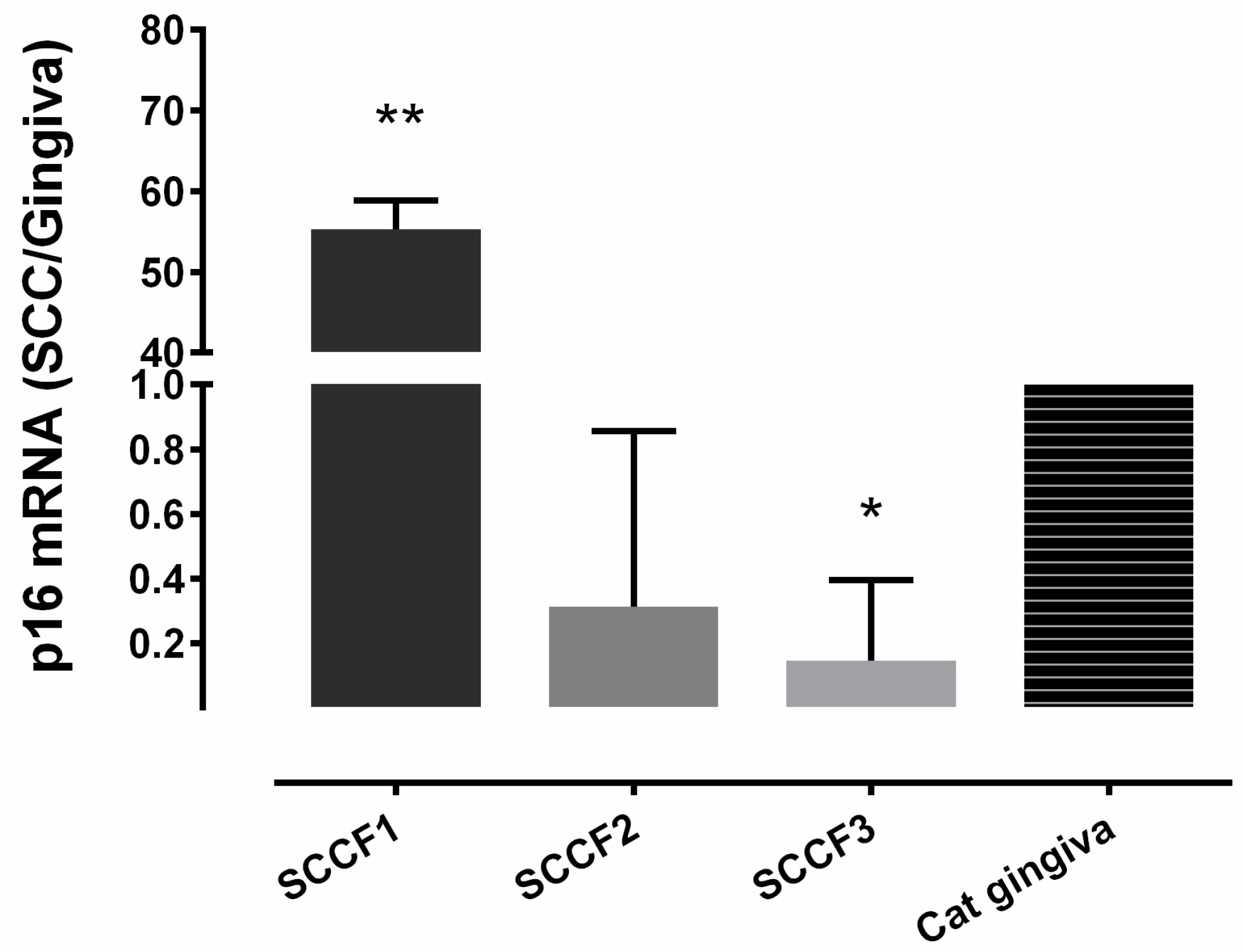
3.1. Relative p16 mRNA Quantification in 3 FOSCC Cell Lines
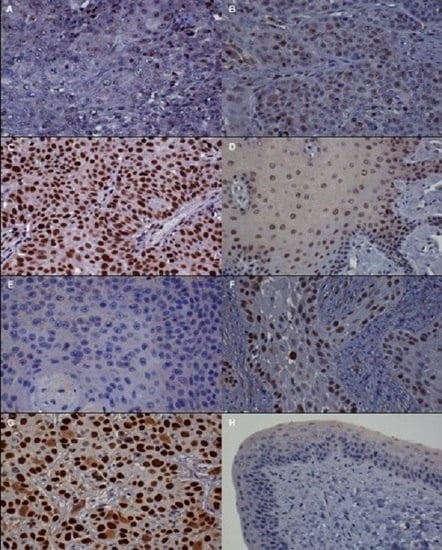
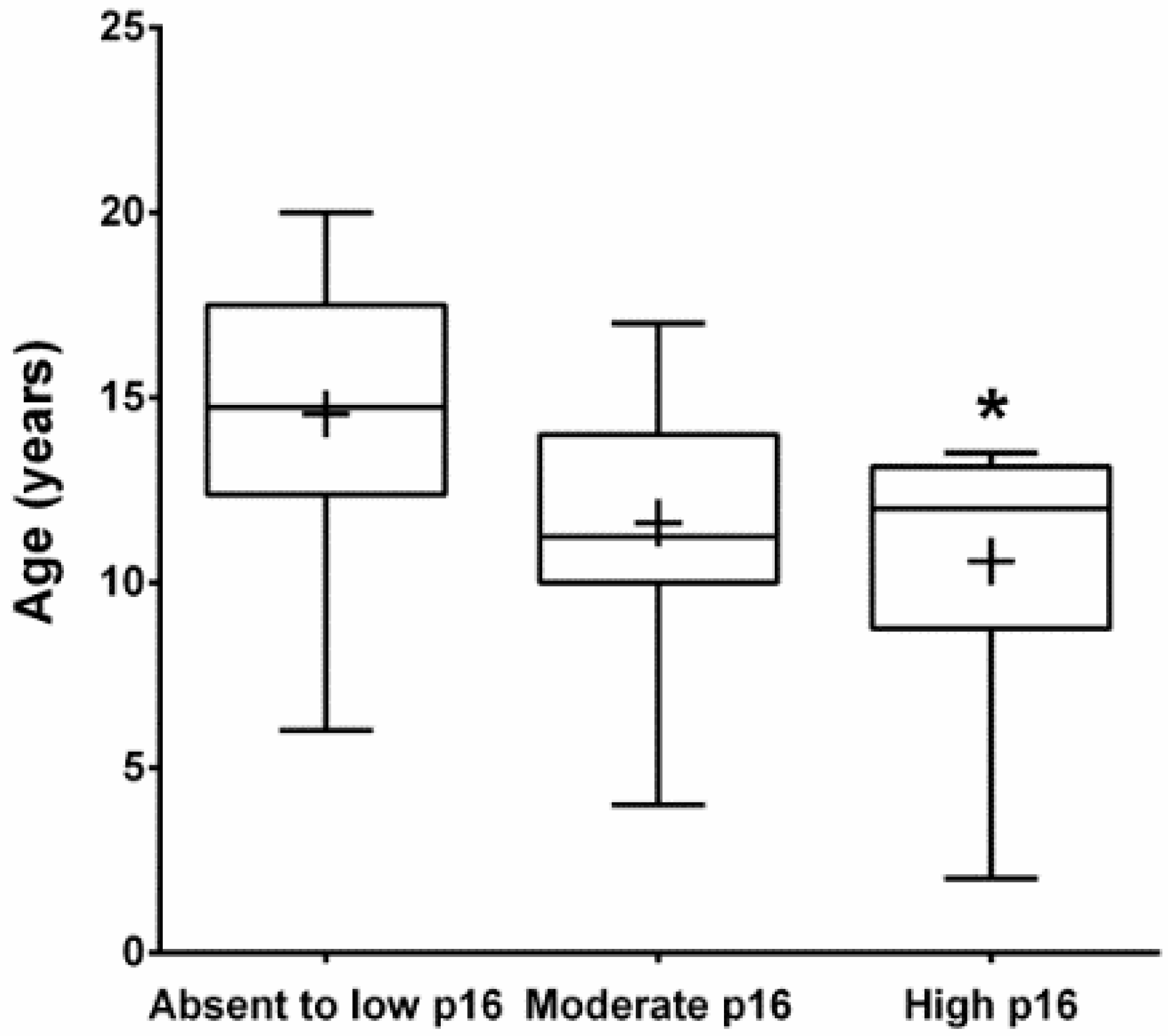
3.2. Immunohistochemistry (IHC)
3.3. Papillomavirus L1 capsid DNA amplification:
3.4. Sequencing
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| FOSCC | Feline oral squamous cell carcinoma |
| HPV | Human papillomavirus |
| PV | Papillomavirus |
| FcaPV | Feline catus papillomavirus |
| IHC | Immunohistochemistry |
| FFPE | Formalin-fixed, paraffin-embedded |
| PCR | Polymerase chain reaction |
| RT-PCR | Reverse transcriptase PCR |
| HNSCC | Head and neck squamous cell carcinoma |
References
- Hayes, A.M.; Adams, V.J.; Scase, T.J.; Murphy, S. Survival of 54 cats with oral squamous cell carcinoma in United Kingdom general practice. J. Small Anim. Pract. 2007, 48, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.K.; Tannehill-Gregg, S.H.; Wolfe, T.D.; Rosol, T.J. Bone-invasive oral squamous cell carcinoma in cats: Pathology and expression of parathyroid hormone-related protein. Vet. Pathol. 2011, 48, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Supsavhad, W.; Dirksen, W.P.; Martin, C.K.; Rosol, T.J. Animal models of head and neck squamous cell carcinoma. Vet. J. 2016, 210, 7–16. [Google Scholar] [CrossRef] [PubMed]
- MacEwen, E.G. Spontaneous tumors in dogs and cats: Models for the study of cancer biology and treatment. Cancer Metastasis Rev. 1990, 9, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Ballegeer, E.A.; Madrill, N.J.; Berger, K.L.; Agnew, D.W.; McNiel, E.A. Evaluation of hypoxia in a feline model of head and neck cancer using (6)(4)Cu-ATSM positron emission tomography/computed tomography. BMC Cancer 2013. [Google Scholar] [CrossRef] [PubMed]
- Leemans, C.R.; Braakhuis, B.J.; Brakenhoff, R.H. The molecular biology of head and neck cancer. Nat. Rev. Cancer 2011, 11, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Ganci, F.; Sacconi, A.; Manciocco, V.; Covello, R.; Spriano, G.; Fontemaggi, G.; Blandino, G. Molecular genetics and biology of head and neck squamous cell carcinoma: Implications for diagnosis, prognosis and treatment. In Head and Neck Cancer; Agulnik, M., Ed.; InTech Europe: Rijeka, Croatia, 2012; pp. 73–122. [Google Scholar]
- Snyder, L.A.; Bertone, E.R.; Jakowski, R.M.; Dooner, M.S.; Jennings-Ritchie, J.; Moore, A.S. P53 expression and environmental tobacco smoke exposure in feline oral squamous cell carcinoma. Vet. Pathol. 2004, 41, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Yuen, P.W.; Man, M.; Lam, K.Y.; Kwong, Y.L. Clinicopathological significance of p16 gene expression in the surgical treatment of head and neck squamous cell carcinomas. J. Clin. Pathol. 2002, 55, 58–60. [Google Scholar] [CrossRef] [PubMed]
- Langendijk, J.A.; Psyrri, A. The prognostic significance of p16 overexpression in oropharyngeal squamous cell carcinoma: Implications for treatment strategies and future clinical studies. Ann. Oncol. 2010, 21, 1931–1934. [Google Scholar] [CrossRef] [PubMed]
- Reed, A.L.; Califano, J.; Cairns, P.; Westra, W.H.; Jones, R.M.; Koch, W.; Ahrendt, S.; Eby, Y.; Sewell, D.; Nawroz, H.; et al. High frequency of p16 (CDKN2A/MTS-1/INK4a) inactivation in head and neck squamous cell carcinoma. Cancer Res. 1996, 56, 3630–3633. [Google Scholar] [PubMed]
- Chen, Y.J.; Rau, K.M.; Chien, C.Y.; Fang, F.M.; Huang, T.L.; Chiu, T.J. High p16 expression predicts a positive response to chemoradiotherapy in stage IVa/b head and neck squamous cell carcinoma. Asian Pac. J. Cancer Prev. 2011, 12, 649–655. [Google Scholar]
- Singhi, A.D.; Westra, W.H. Comparison of human papillomavirus in situ hybridization and p16 immunohistochemistry in the detection of human papillomavirus-associated head and neck cancer based on a prospective clinical experience. Cancer 2010, 116, 2166–2173. [Google Scholar] [CrossRef] [PubMed]
- Lesnikova, I.; Lidang, M.; Hamilton-Dutoit, S.; Koch, J. Rapid, sensitive, type specific pcr detection of the E7 region of human papillomavirus type 16 and 18 from paraffin embedded sections of cervical carcinoma. Infect. Agent Cancer 2010. [Google Scholar] [CrossRef] [PubMed]
- Munday, J.S.; Aberdein, D. Loss of retinoblastoma protein, but not p53, is associated with the presence of papillomaviral DNA in feline viral plaques, bowenoid in situ carcinomas, and squamous cell carcinomas. Vet. Pathol. 2012, 49, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Tannehill-Gregg, S.; Kergosien, E.; Rosol, T.J. Feline head and neck squamous cell carcinoma cell line: Characterization, production of parathyroid hormone-related protein, and regulation by transforming growth factor-beta. In Vitro Cell Dev. Biol. Anim. 2001, 37, 676–683. [Google Scholar] [CrossRef]
- Martin, C.K.; Dirksen, W.P.; Shu, S.T.; Werbeck, J.L.; Thudi, N.K.; Yamaguchi, M.; Wolfe, T.D.; Heller, K.N.; Rosol, T.J. Characterization of bone resorption in novel in vitro and in vivo models of oral squamous cell carcinoma. Oral. Oncol. 2012, 48, 491–499. [Google Scholar] [CrossRef]
- Munday, J.S.; Knight, C.G.; French, A.F. Evaluation of feline oral squamous cell carcinomas for p16CDKN2A protein immunoreactivity and the presence of papillomaviral DNA. Res. Vet. Sci. 2011, 90, 280–283. [Google Scholar] [CrossRef] [PubMed]
- Simmons, J.K.; Dirksen, W.P.; Hildreth, B.E., 3rd; Dorr, C.; Williams, C.; Thomas, R.; Breen, M.; Toribio, R.E.; Rosol, T.J. Canine prostate cancer cell line (Probasco) produces osteoblastic metastases in vivo. Prostate 2014, 74, 1251–1265. [Google Scholar] [CrossRef] [PubMed]
- Munday, J.S.; Kiupel, M.; French, A.F.; Howe, L. Amplification of papillomaviral DNA sequences from a high proportion of feline cutaneous in situ and invasive squamous cell carcinomas using a nested polymerase chain reaction. Vet. Dermatol. 2008, 19, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.Y.; Delius, H.; Halpern, A.L.; Bernard, H.U. Analysis of genomic sequences of 95 papillomavirus types: Uniting typing, phylogeny, and taxonomy. J. Virol. 1995, 69, 3074–3083. [Google Scholar] [PubMed]
- Munday, J.S.; Gibson, I.; French, A.F. Papillomaviral DNA and increased p16CDKN2A protein are frequently present within feline cutaneous squamous cell carcinomas in ultraviolet-protected skin. Vet. Dermatol. 2011, 22, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, L.L., Jr.; Pagano, G.M.; Li, M.; Tandon, R.; Holm, S.W.; White, D.K.; Lele, S.M. Overexpression of p16INK4 is a reliable marker of human papillomavirus-induced oral high-grade squamous dysplasia. Oral Surg Oral Med. Oral. Pathol. Oral. Radiol. Endod. 2006, 102, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Verschuur, H.P.; Irish, J.C.; O’Sullivan, B.; Goh, C.; Gullane, P.J.; Pintilie, M. A matched control study of treatment outcome in young patients with squamous cell carcinoma of the head and neck. Laryngoscope 1999, 109, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Shiboski, C.H.; Schmidt, B.L.; Jordan, R.C. Tongue and tonsil carcinoma: Increasing trends in the U.S. Population ages 20–44 years. Cancer 2005, 103, 1843–1849. [Google Scholar] [CrossRef] [PubMed]
- Munday, J.S.; Howe, L.; French, A.; Squires, R.A.; Sugiarto, H. Detection of papillomaviral DNA sequences in a feline oral squamous cell carcinoma. Res. Vet. Sci. 2009, 86, 359–361. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, S.H.; Newkirk, K.M.; Anis, E.A.; Brahmbhatt, R.; Frank, L.A.; Kania, S.A. Detection of human papillomavirus DNA in feline premalignant and invasive squamous cell carcinoma. Vet. Dermatol. 2011, 22, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Lie, A.K.; Kristensen, G. Human papillomavirus E6/E7 mRNA testing as a predictive marker for cervical carcinoma. Expert Rev. Mol. Diagn. 2008, 8, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Merlo, A.; Herman, J.G.; Mao, L.; Lee, D.J.; Gabrielson, E.; Burger, P.C.; Baylin, S.B.; Sidransky, D. 5′ CpG island methylation is associated with transcriptional silencing of the tumour suppressor p16/CDKN2A/MTS1 in human cancers. Nat. Med. 1995, 1, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Liggett, W.H., Jr.; Sidransky, D. Role of the p16 tumor suppressor gene in cancer. J. Clin. Oncol. 1998, 16, 1197–1206. [Google Scholar] [PubMed]
- Munday, J.S.; French, A.F.; Gibson, I.R.; Knight, C.G. The presence of p16 CDKN2A protein immunostaining within feline nasal planum squamous cell carcinomas is associated with an increased survival time and the presence of papillomaviral DNA. Vet. Pathol. 2013, 50, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Nguyen, D.; Zhang, W.W.; Kyritsis, A.P.; Roth, J.A. Cell cycle arrest and inhibition of tumor cell proliferation by the p16INK4a gene mediated by an adenovirus vector. Cancer Res. 1995, 55, 3250–3253. [Google Scholar] [PubMed]
- Saunders, M.E.; MacKenzie, R.; Shipman, R.; Fransen, E.; Gilbert, R.; Jordan, R.C. Patterns of p53 gene mutations in head and neck cancer: Full-length gene sequencing and results of primary radiotherapy. Clin Cancer Res. 1999, 5, 2455–2463. [Google Scholar] [PubMed]
- Peltonen, J.K.; Helppi, H.M.; Paakko, P.; Turpeenniemi-Hujanen, T.; Vahakangas, K.H. P53 in head and neck cancer: Functional consequences and environmental implications of TP53 mutations. Head Neck. Oncol. 2010. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.J.; Lain, S.; Verma, C.S.; Fersht, A.R.; Lane, D.P. Awakening guardian angels: Drugging the p53 pathway. Nat. Rev. Cancer 2009, 9, 862–873. [Google Scholar] [CrossRef] [PubMed]
- Geradts, J.; Wilentz, R.E.; Roberts, H. Immunohistochemical [corrected] detection of the alternate INK4a-encoded tumor suppressor protein p14(ARF) in archival human cancers and cell lines using commercial antibodies: Correlation with p16(INK4a) expression. Mod. Pathol. 2001, 14, 1162–1168. [Google Scholar] [CrossRef] [PubMed]

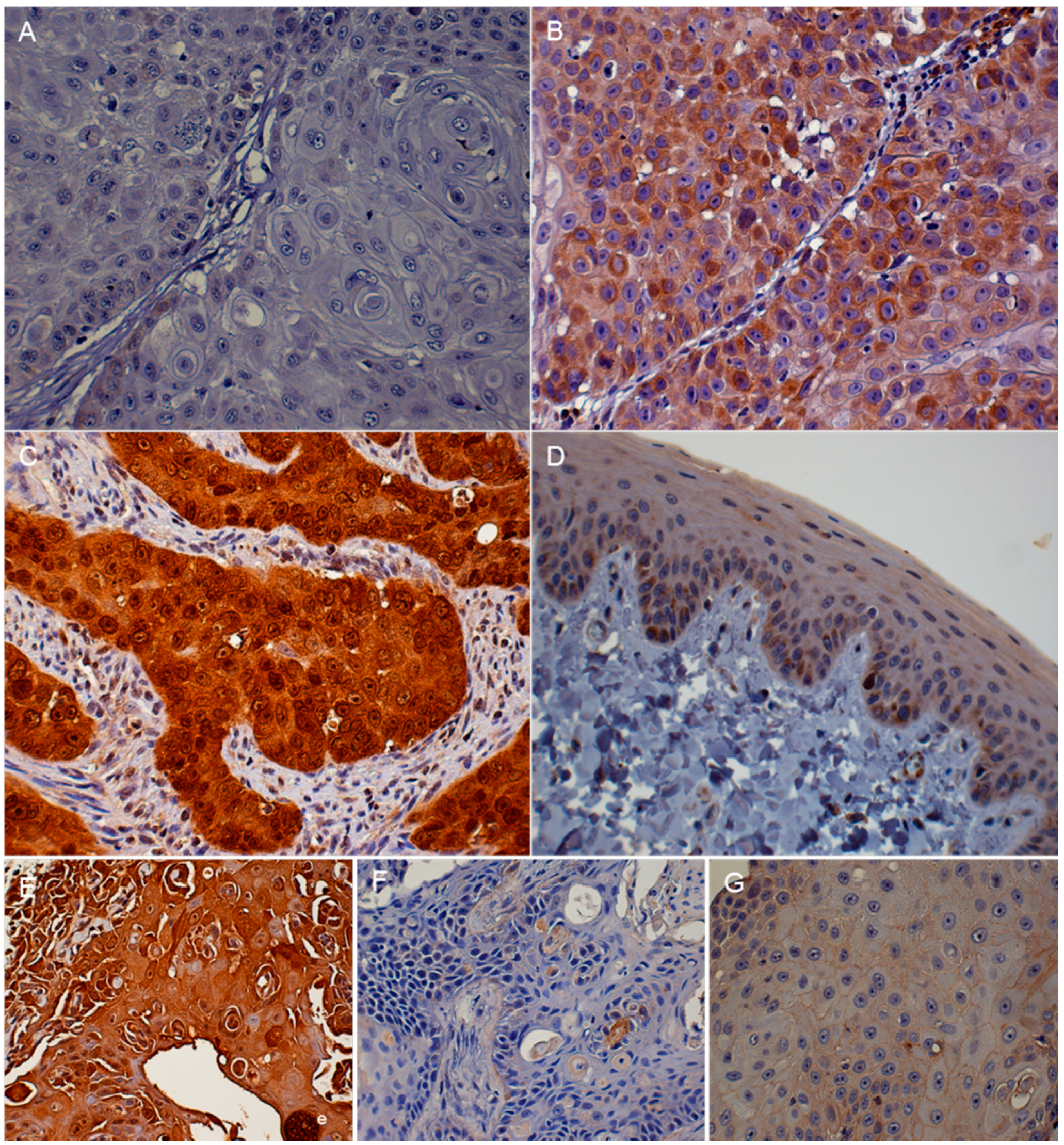
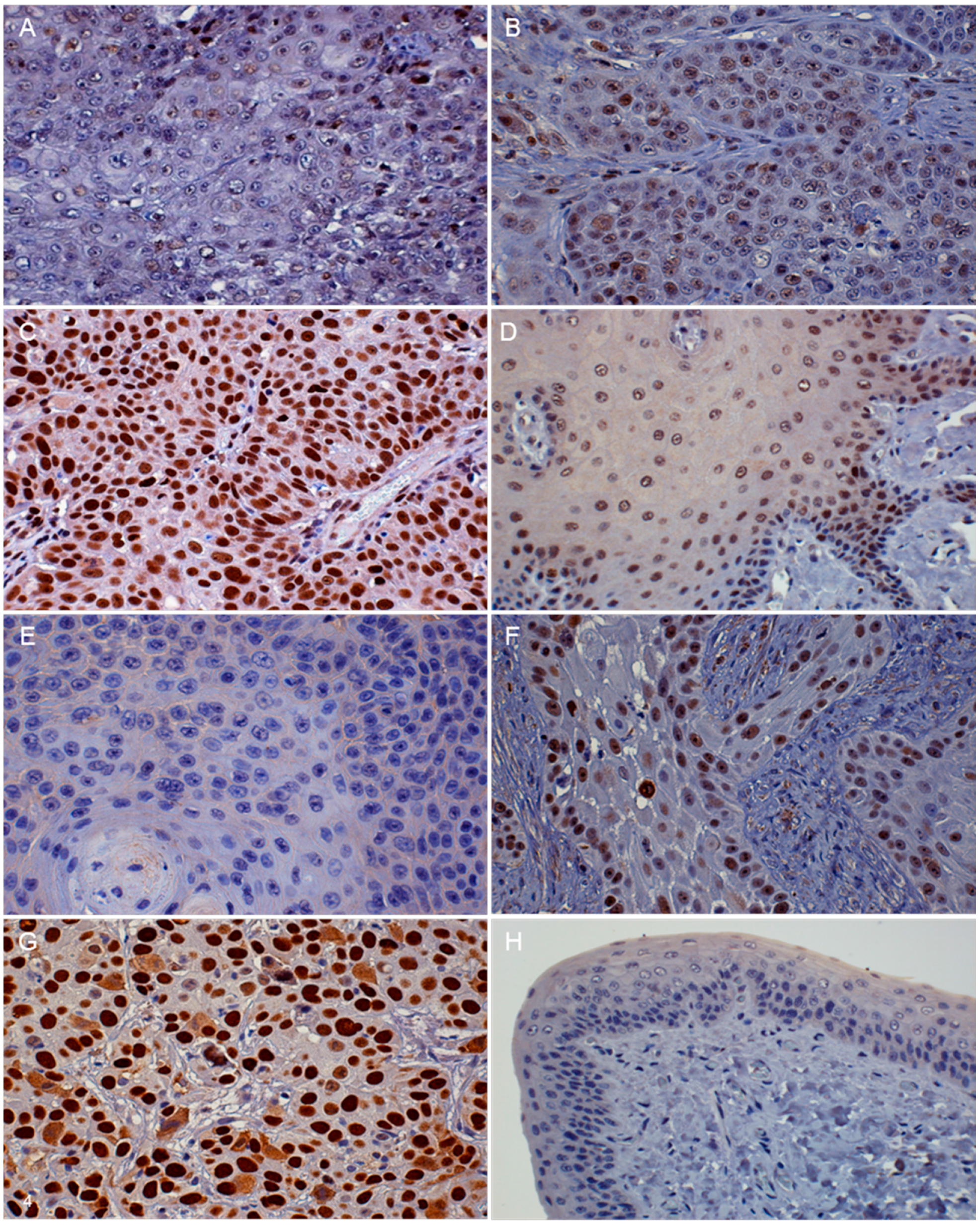
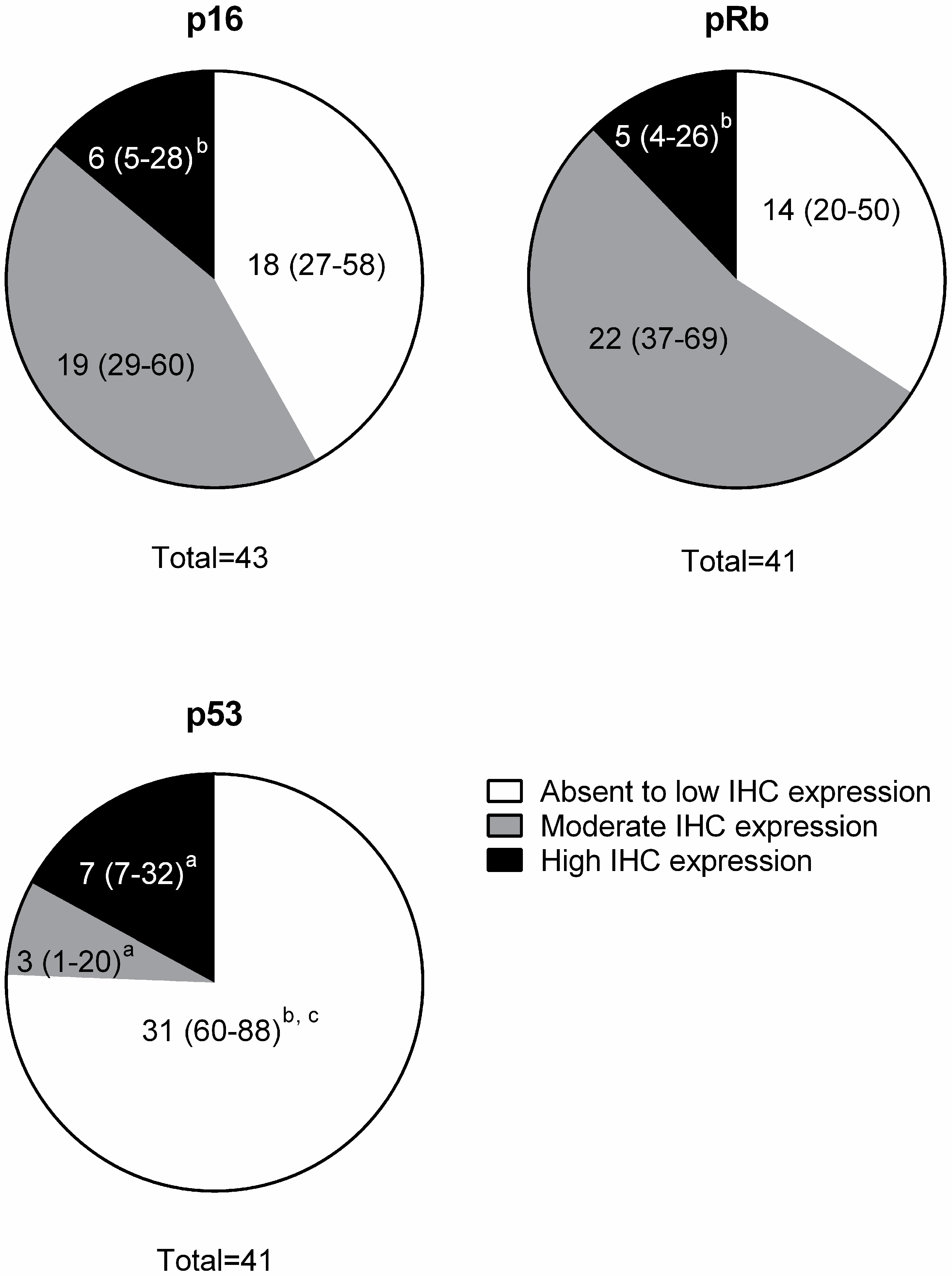

| Protein | Absent to Low | Moderate | High |
|---|---|---|---|
| p16 | <20% of cancer cells have low cytoplasmic intensity | 20%–50% of cancer cells have low to intermediate cytoplasmic and nuclear staining intensity | >50% of cancer cells have high cytoplasmic and nuclear staining |
| pRb and p53 | <20% of cancer cells have low to intermediate nuclear staining intensity | 20%–50% of cancer cells have high nuclear staining intensity, or >50% of cancer cells have low to intermediate nuclear staining intensity | >50% of cancer cells have high nuclear staining intensity |
| Signalment | IHC Results | ||||||
|---|---|---|---|---|---|---|---|
| No. | Breed | Sex | Age (Year) | Location | p16 | pRb | p53 |
| 1 | DSH | FS | 14 | Gingiva | Low | Moderate | Low |
| 2 | DSL | MC | 20 | Gingiva | Low | Low | N/A |
| 3 | DSH | F | 11 | Gingiva | High | High | Low |
| 4 | Himalayan | FS | 16 | Gingiva | Moderate | Moderate | Low |
| 5 | DSH | MC | 15 | Gingiva | Moderate | Moderate | Moderate |
| 6 | DSH | FS | N/A | Gingiva | Moderate | Moderate | Low |
| 7 | Himalayan | MC | 12 | Gingiva | Low | High | Low |
| 8 | DSH | MC | 11 | Gingiva | Moderate | Moderate | High |
| 9 | DSH | MC | 17 | Gingiva | Moderate | Moderate | Low |
| 10 | DSH | MC | 13 | Tongue | Low | High | High |
| 11 | Siamese | FS | 13 | Tongue | Low | Low | Moderate |
| 12 | DSH | MC | 16 | Tongue | Low | Moderate | Low |
| 13 | DLH | MC | 11 | Tongue | Moderate | Low | Low |
| 14 | DSH | FS | 13 | Tongue | Low | Low | Low |
| 15 | Exotic SH | F | 10 | Tongue | Moderate | N/A | Low |
| 16 | DSH | MC | 11.5 | Tongue | Moderate | Moderate | Low |
| 17 | DLH | F | 10 | Tongue | Moderate | Moderate | Low |
| 18 | DSH | MC | 6 | Tongue | Low | Low | High |
| 19 | DSH | FS | 15.5 | Tongue | Low | Low | High |
| 20 | DSH | F | 10 | Tongue | Moderate | Moderate | Low |
| 21 | DSH | F | 12 | Sublingual | Low | Low | Moderate |
| 22 | Angora | FS | 7 | Sublingual | Low | Moderate | Low |
| 23 | DSH | MC | 4 | Sublingual | Moderate | Low | Low |
| 24 | DSH | M | 14+ | Sublingual | Moderate | Low | High |
| 25 | Persian | FS | 13.5 | Sublingual | High | Moderate | Low |
| 26 | DSH | MC | 12 | Sublingual | Moderate | Moderate | Low |
| 27 | DSH | M | 12.5 | Sublingual | Low | Low | Low |
| 28 | DLS | FS | 17 | Sublingual | Low | High | High |
| 29 | DSH | FS | 19 | Sublingual | Low | Moderate | Low |
| 30 | DSH | FS | 14 | Sublingual | Moderate | Moderate | Low |
| 31 | DLH | MC | 17 | Sublingual | Low | Moderate | Low |
| 32 | DSH | FS | 19 | Sublingual | Low | N/A | Low |
| 33 | DSH | MC | 13 | Mandi or Maxi | High | Moderate | High |
| 34 | DSH | MC | 12 | Mandi or Maxi | High | Low | Low |
| 35 | DLH | F | 6.5 | Mandi or Maxi | Moderate | Moderate | Low |
| 36 | DSH | MC | 12 | Mandi or Maxi | High | Moderate | Low |
| 37 | DSH | F | 16 | Mandi or Maxi | Low | Low | Low |
| 38 | DSH | F | 20 | Mandi or Maxi | Low | Low | Low |
| 39 | DSH | FS | 2 | Mandi or Maxi | High | Moderate | Low |
| 40 | DLH | MC | 14 | Mandi or Maxi | Moderate | Low | Low |
| 41 | Himalayan | F | 11 | Mandi or Maxi | Moderate | Moderate | Low |
| 42 | DSH | MC | 13 | Mandi or Maxi | Moderate | Moderate | Low |
| 43 | DSH | FS | 9 | Mandi or Maxi | Moderate | High | N/A |
| Sample | p16 IHC | p53 IHC | pRB IHC |
|---|---|---|---|
| SCCF1 | High | Low | Low |
| SCCF2 | Low | Low | Low |
| SCCF3 | Low | Moderate | Low |
| FBISC | High | Low | Low |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Supsavhad, W.; Dirksen, W.P.; Hildreth, B.E.; Rosol, T.J. p16, pRb, and p53 in Feline Oral Squamous Cell Carcinoma. Vet. Sci. 2016, 3, 18. https://doi.org/10.3390/vetsci3030018
Supsavhad W, Dirksen WP, Hildreth BE, Rosol TJ. p16, pRb, and p53 in Feline Oral Squamous Cell Carcinoma. Veterinary Sciences. 2016; 3(3):18. https://doi.org/10.3390/vetsci3030018
Chicago/Turabian StyleSupsavhad, Wachiraphan, Wessel P. Dirksen, Blake E. Hildreth, and Thomas J. Rosol. 2016. "p16, pRb, and p53 in Feline Oral Squamous Cell Carcinoma" Veterinary Sciences 3, no. 3: 18. https://doi.org/10.3390/vetsci3030018