Experimental Animal Models of Arteriovenous Malformation: A Review
Abstract
:1. Introduction
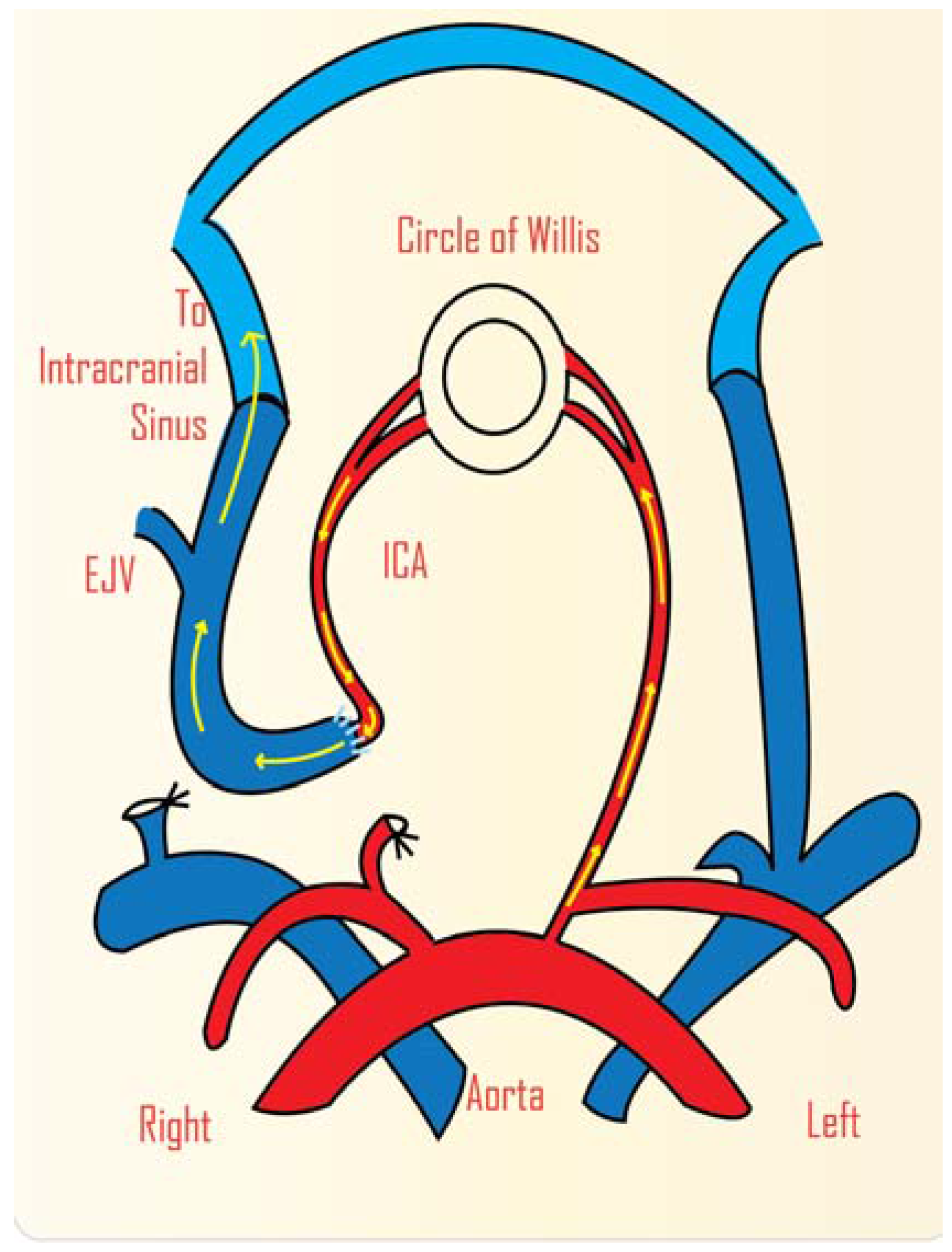
2. Study of AVM Haemodynamics

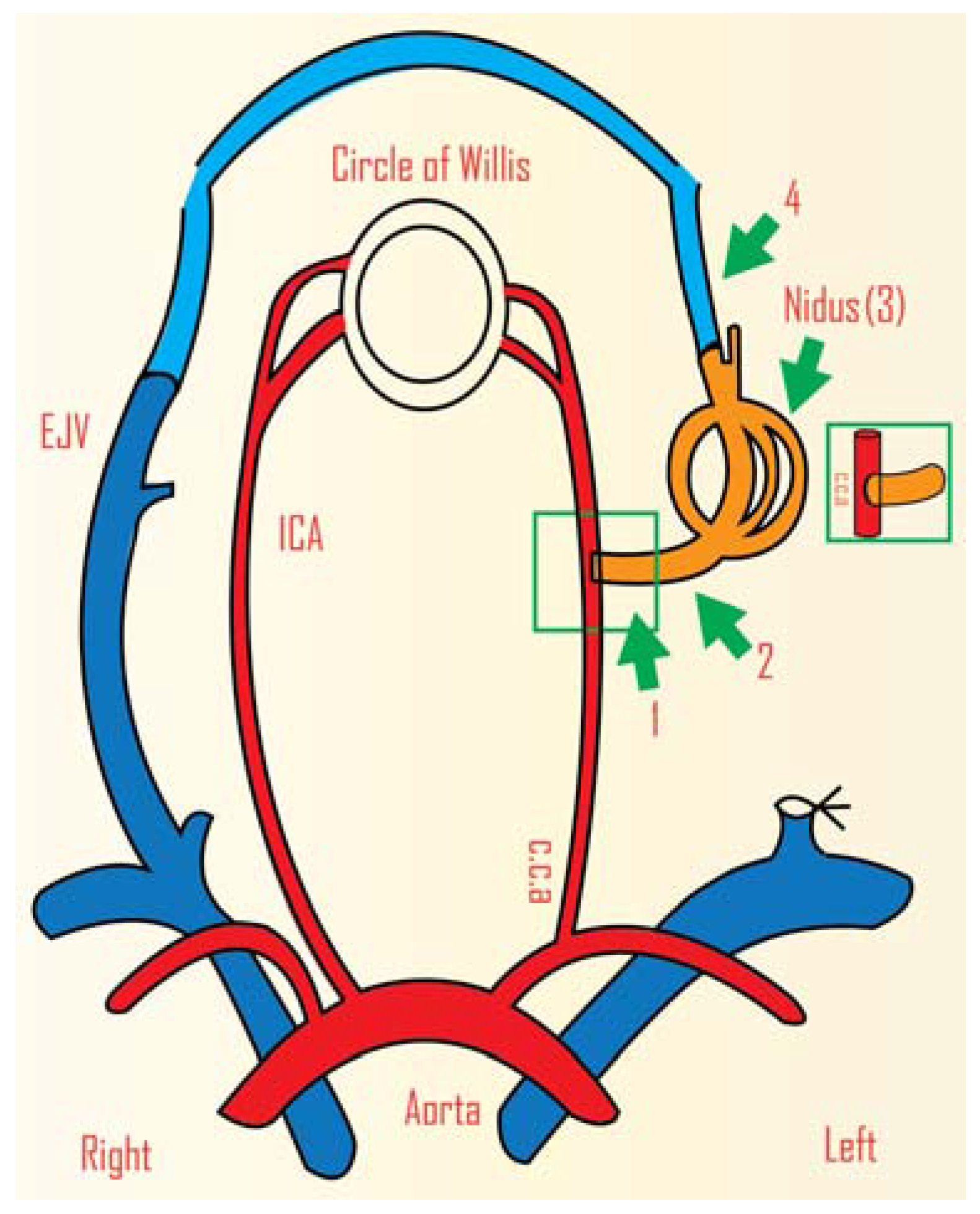

3. Radiosurgery
4. Genetic Studies
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Halim, A.X.; Johnston, S.C.; Singh, V.; McCulloch, C.E.; Bennett, J.P.; Achrol, A.S.; Sidney, S.; Young, W.L. Longitudinal risk of intracranial hemorrhage in patients with arteriovenous malformation of the brain within a defined population. Stroke 2004, 35, 1697–1702. [Google Scholar] [CrossRef] [PubMed]
- Leblanc, G.G.; Golanov, E.; Awad, I.A.; Young, W.L.; Biology of Vascular Malformations of the Brain NINDS Workshop Collaborators. Biology of vascular malformations of the brain. Stroke 2009, 40, e694–e702. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Choi, E.J.; McDougall, C.M.; Su, H. Brain arteriovenous malformation modeling, pathogenesis, and novel therapeutic targets. Transl. Stroke Res. 2014, 5, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Achrol, A.S.; Guzman, R.; Varga, M.; Adler, J.R.; Steinberg, G.K.; Chang, S.D. Pathogenesis and radiobiology of brain arteriovenous malformations: Implications for risk stratification in natural history and posttreatment course. Neurosurg. Focus 2009. [Google Scholar] [CrossRef] [PubMed]
- Jeffree, R.L.; Stoodley, M.A. Postnatal development of arteriovenous malformations. Pediatr. Neurosurg. 2009, 45, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Karunanayaka, A.; Windsor, A.; Stoodley, M.A. Comparison of an animal model of arteriovenous malformation with human arteriovenous malformation. J. Clin. Neurosci. 2010, 17, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz, M.; Regelsberger, J.; Zeumer, H.; Grzyska, U. Management of cerebral arteriovenous malformations associated with symptomatic congestive intracranial hypertension. Eur. Neurol. 2008, 59, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Hofmeister, C.; Stapf, C.; Hartmann, A.; Sciacca, R.R.; Mansmann, U.; TerBrugge, K.; Lasjaunias, P.; Mohr, J.P.; Mast, H.; Meisel, J. Demographic, morphological, and clinical characteristics of 1289 patients with brain arteriovenous malformation. Stroke 2000, 31, 1307–1310. [Google Scholar] [CrossRef] [PubMed]
- Grzyska, U.; Fiehler, J. Pathophysiology and treatment of brain AVMs. Clin. Neuroradiol. 2009, 1, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Friedlander, R.M. Clinical practice: Arteriovenous malformations of the brain. N. Engl. J. Med. 2007, 356, 2704–2712. [Google Scholar] [CrossRef] [PubMed]
- Klopfenstein, J.D.; Spetzler, R.F. Cerebral arteriovenous malformations: When is surgery indicated? Acta Neurochir. 2005, 147, 693–695. [Google Scholar] [CrossRef] [PubMed]
- Pik, J.H.; Morgan, M.K. Microsurgery for small arteriovenous malformations of the brain: Results in 110 consecutive patients. Neurosurgery 2000, 47, 571–577. [Google Scholar] [PubMed]
- Morgan, M.K.; Rochford, A.M.; Tsahtsarlis, A.; Little, N.; Faulder, K.C. Surgical risks associated with the management of Grade I and II brain arteriovenous malformations. Neurosurgery 2004, 54, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Ferch, R.D.; Morgan, M.K. High-grade arteriovenous malformations and their management. J. Clin. Neurosci. 2002, 9, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.K.; Drummond, K.J.; Grinnell, V.; Sorby, W. Surgery for cerebral arteriovenous malformation: Risks related to lenticulostriate arterial supply. J. Neurosurg. 1997, 86, 801–805. [Google Scholar] [CrossRef] [PubMed]
- McInerney, J.; Gould, D.A.; Birkmeyer, J.D.; Harbaugh, R.E. Decision analysis for small, asymptomatic intracranial arteriovenous malformations. Neurosurg. Focus 2001. [Google Scholar] [CrossRef]
- Karlsson, B.; Kihlstrom, L.; Lindquist, C.; Steiner, L. Gamma knife surgery for previously irradiated arteriovenous malformations. Neurosurgery 1998, 42, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Steiner, W. Results of curative laser microsurgery of laryngeal carcinomas. Am. J. Otolaryngol. 1993, 14, 116–121. [Google Scholar] [CrossRef]
- Friedman, W.A.; Bova, F.J.; Bollampally, S.; Bradshaw, P. Analysis of factors predictive of success or complications in arteriovenous malformation radiosurgery. Neurosurgery 2003, 52, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Friedman, W.A. Stereotactic radiosurgery of intracranial arteriovenous malformations. Neurosurg. Clin. N. Am. 2013, 24, 561–574. [Google Scholar] [CrossRef] [PubMed]
- Han, P.P.; Ponce, F.A.; Spetzler, R.F. Intention-to-treat analysis of Spetzler-Martin grades IV and V arteriovenous malformations: Natural history and treatment paradigm. J. Neurosurg. 2003, 98, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Heros, R.C. Spetzler-Martin grades IV and V arteriovenous malformations. J. Neurosurg. 2003, 98, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Chaloupka, J.C.; Vinuela, F.; Robert, J.; Duckwiler, G.R. An in vivo arteriovenous malformation model in swine: Preliminary feasibility and natural history study. Am. J. Neuroradiol. 1994, 15, 945–950. [Google Scholar] [PubMed]
- Massoud, T.F.; Ji, C.; Vinuela, F.; Guglielmi, G.; Robert, J.; Duckwiler, G.R.; Gobin, Y.P. An experimental arteriovenous malformation model in swine: Anatomic basis and construction technique. Am. J. Neuroradiol. 1994, 15, 1537–1545. [Google Scholar] [PubMed]
- Qian, Z.; Climent, S.; Maynar, M.; Uson-Garallo, J.; Lima-Rodrigues, M.A.; Calles, C.; Robertson, H.; Castaneda-Zuniga, W.R. A simplified arteriovenous malformation model in sheep: Feasibility study. Am. J. Neuroradiol. 1999, 20, 765–770. [Google Scholar] [PubMed]
- Lv, M.M.; Fan, X.D.; Su, L.X. Is a swine model of arteriovenous malformation suitable for human extracranial arteriovenous malformation? A preliminary study. Cardiovasc. Intervent. Radiol. 2013, 36, 1364–1370. [Google Scholar] [CrossRef] [PubMed]
- Klisch, J.; Requejo, F.; Yin, L.; Eissner, B.; Schumacher, M. The two-in-one model: A new variation of the arteriovenous malformation model in swine. Neuroradiology 2001, 43, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Lawton, M.T.; Jacobowitz, R.; Spetzler, R.F. Redefined role of angiogenesis in the pathogenesis of dural arteriovenous malformations. J. Neurosurg. 1997, 87, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.M.; Spetzler, R.F.; Bederson, J.B.; Kurbat, J.M.; Zabramski, J.M. Genesis of a dural arteriovenous malformation in a rat model. J. Neurosurg. 1995, 83, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Yassari, R.; Sayama, T.; Jahromi, B.S.; Aihara, Y.; Stoodley, M.; Macdonald, R.L. Angiographic, hemodynamic and histological characterization of an arteriovenous fistula in rats. Acta Neurochir. 2004, 146, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Sekhon, L.H.; Morgan, M.K.; Spence, I. Normal perfusion pressure breakthrough: The role of capillaries. J. Neurosurg. 1997, 86, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.K.; Johnston, I.; Besser, M.; Baines, D. Cerebral arteriovenous malformations, steal, and the hypertensive breakthrough threshold. J. Neurosurg. 1987, 66, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Storer, K.; Tu, J.; Karunanayaka, A.; Smee, R.; Short, R.; Thorpe, P.; Stoodley, M. Coadministration of low-dose lipopolysaccharide and soluble tissue factor induces thrombosis after radiosurgery in an animal arteriovenous malformation model. Neurosurgery 2007, 61, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, K.; Kawahara, N.; Shin, M.; Tago, M.; Kishimoto, J.; Kurita, H.; Kawamoto, S.; Morita, A.; Kirino, T. The risk of hemorrhage after radiosurgery for cerebral arteriovenous malformations. N. Engl. J. Med. 2005, 352, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Storer, K.P.; Tu, J.; Stoodley, M.A.; Smee, R.I. Expression of endothelial adhesion molecules after radiosurgery in an animal model of arteriovenous malformation. Neurosurgery 2010, 67, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Morgan, W.R.; Majeski, J.A. Idiopathic arteriovenous renal vascular malformation treated by ex vivo repair. J. S. C. Med. Assoc. 1989, 85, 469–471. [Google Scholar] [PubMed]
- Altschuler, E.; Lunsford, L.D.; Kondziolka, D.; Wu, A.; Maitz, A.H.; Sclabassi, R.; Martinez, A.J.; Flickinger, J.C. Radiobiologic models for radiosurgery. Neurosurg. Clin. N. Am. 1992, 3, 61–77. [Google Scholar] [PubMed]
- Pietila, T.A.; Zabramski, J.M.; Thellier-Janko, A.; Duveneck, K.; Bichard, W.D.; Brock, M.; Spetzler, R.F. Animal model for cerebral arteriovenous malformation. Acta Neurochir. 2000, 142, 1231–1240. [Google Scholar] [PubMed]
- Numazawa, S.; Sasaki, T.; Sato, S.; Watanabe, Y.; Watanabe, Z.; Kodama, N. Experimental model of intracranial arteriovenous shunting in the acute stage. Neurol. Med. Chir. 2005, 45, 288–293. [Google Scholar] [CrossRef]
- Pollock, B.E. Stereotactic radiosurgery for arteriovenous malformations. Neurosurg. Clin. N. Am. 1999, 10, 281–290. [Google Scholar] [PubMed]
- Gobin, Y.P.; Laurent, A.; Merienne, L.; Schlienger, M.; Aymard, A.; Houdart, E.; Casasco, A.; Lefkopoulos, D.; George, B.; Merland, J.J. Treatment of brain arteriovenous malformations by embolization and radiosurgery. J. Neurosurg. 1996, 85, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Jahan, R.; Solberg, T.D.; Lee, D.; Medin, P.; Tateshima, S.; de Salles, A.; Sayre, J.; Vinters, H.V.; Vinuela, F. An arteriovenous malformation model for stereotactic radiosurgery research. Neurosurgery 2007, 61, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Mut, M.; Oge, K.; Zorlu, F.; Undeger, U.; Erdem, S.; Ozcan, O.E. Effects of ionizing radiation on brain tissue surrounding arteriovenous malformations: An experimental study in a rat caroticojugular fistula model. Neurosurg. Rev. 2004, 27, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Frankenberg-Schwager, M. Induction, repair and biological relevance of radiation-induced DNA lesions in eukaryotic cells. Radiat. Environ. Biophys. 1990, 29, 273–292. [Google Scholar] [CrossRef] [PubMed]
- Lunec, J. Free radicals: Their involvement in disease processes. Ann. Clin. Biochem. 1990, 27, 173–182. [Google Scholar] [CrossRef] [PubMed]
- De Salles, A.A.; Solberg, T.D.; Mischel, P.; Massoud, T.F.; Plasencia, A.; Goetsch, S.; de Souza, E.; Vinuela, F. Arteriovenous malformation animal model for radiosurgery: The rete mirabile. Am. J. Neuroradiol. 1996, 17, 1451–1458. [Google Scholar] [PubMed]
- De Salles, A.A.; Manchola, I. CO2 reactivity in arteriovenous malformations of the brain: A transcranial Doppler ultrasound study. J. Neurosurg. 1994, 80, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Spetzler, R.F.; Hargraves, R.W.; McCormick, P.W.; Zabramski, J.M.; Flom, R.A.; Zimmerman, R.S. Relationship of perfusion pressure and size to risk of hemorrhage from arteriovenous malformations. J. Neurosurg. 1992, 76, 918–923. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, H.; Murayama, Y.; Davis, C.R.; Howard, D.L.; Baumgardner, W.L.; Marks, M.P.; Do, H.M. Endovascular embolization of the swine rete mirabile with Eudragit-E 100 polymer. Am. J. Neuroradiol. 2007, 28, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Jahan, R.; Solberg, T.D.; Lee, D.; Medin, P.; Tateshima, S.; Sayre, J.; de Salles, A.; Vinters, H.V.; Vinuela, F. Stereotactic radiosurgery of the rete mirabile in swine: A longitudinal study of histopathological changes. Neurosurgery 2006, 58, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Massoud, T.F.; Hademenos, G.J. Transvenous retrograde nidus sclerotherapy under controlled hypotension (TRENSH): A newly proposed treatment for brain arteriovenous malformations—Concepts and rationale. Neurosurgery 1999, 45, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Kilic, K.; Konya, D.; Kurtkaya, O.; Sav, A.; Pamir, M.N.; Kilic, T. Inhibition of angiogenesis induced by cerebral arteriovenous malformations using gamma knife irradiation. J. Neurosurg. 2007, 106, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Stoodley, M.A.; Morgan, M.K.; Storer, K.P. Responses of arteriovenous malformations to radiosurgery: Ultrastructural changes. Neurosurgery 2006, 58, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Reddy, R.; Duong, T.T.; Fairhall, J.M.; Smee, R.I.; Stoodley, M.A. Durable thrombosis in a rat model of arteriovenous malformation treated with radiosurgery and vascular targeting. J. Neurosurg. 2014, 120, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Lawton, M.T.; Hamilton, M.G.; Spetzler, R.F. Multimodality treatment of deep arteriovenous malformations: Thalamus, basal ganglia, and brain stem. Neurosurgery 1995, 37, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, M.G.; Spetzler, R.F. The prospective application of a grading system for arteriovenous malformations. Neurosurgery 1994, 34, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Heros, R.C.; Korosue, K.; Diebold, P.M. Surgical excision of cerebral arteriovenous malformations: Late results. Neurosurgery 1990, 26, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Lawton, M.T.; Stewart, C.L.; Wulfstat, A.A.; Derugin, N.; Hashimoto, T.; Young, W.L. The transgenic arteriovenous fistula in the rat: An experimental model of gene therapy for brain arteriovenous malformations. Neurosurgery 2004, 54, 1463–1471. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Lam, T.; Boudreau, N.J.; Bollen, A.W.; Lawton, M.T.; Young, W.L. Abnormal balance in the angiopoietin-tie2 system in human brain arteriovenous malformations. Circ. Res. 2001, 89, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Letteboer, T.G.; Mager, J.J.; Snijder, R.J.; Koeleman, B.P.; Lindhout, D.; Ploos van Amstel, J.K.; Westermann, C.J. Genotype-phenotype relationship in hereditary haemorrhagic telangiectasia. J. Med. Genet. 2006, 43, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, S.; Mandzia, J.L.; ter Brugge, K.; Willinsky, R.A.; Faughnan, M.E. Angiographic and clinical characteristics of patients with cerebral arteriovenous malformations associated with hereditary hemorrhagic telangiectasia. Am. J. Neuroradiol. 2000, 21, 1016–1020. [Google Scholar] [PubMed]
- Willinsky, R.A.; Lasjaunias, P.; Terbrugge, K.; Burrows, P. Multiple cerebral arteriovenous malformations (AVMs): Review of our experience from 203 patients with cerebral vascular lesions. Neuroradiology 1990, 32, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Govani, F.S.; Shovlin, C.L. Hereditary haemorrhagic telangiectasia: A clinical and scientific review. Eur. J. Hum. Genet. 2009, 17, 860–871. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Hashimoto, T.; Tihan, T.; Young, W.L.; Perry, V.; Lawton, M.T. Growth and regression of arteriovenous malformations in a patient with hereditary hemorrhagic telangiectasia: Case report. J. Neurosurg. 2007, 106, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Tual-Chalot, S.; Oh, S.P.; Arthur, H.M. Mouse models of hereditary hemorrhagic telangiectasia: Recent advances and future challenges. Front. Genet. 2015. [Google Scholar] [CrossRef] [PubMed]
- Young, W.L.; Yang, G.Y. Are there genetic influences on sporadic brain arteriovenous malformations? Stroke 2004, 35, 2740–2745. [Google Scholar] [CrossRef] [PubMed]
- Arthur, H.M.; Ure, J.; Smith, A.J.; Renforth, G.; Wilson, D.I.; Torsney, E.; Charlton, R.; Parums, D.V.; Jowett, T.; Marchuk, D.A.; et al. Endoglin, an ancillary TGFbeta receptor, is required for extraembryonic angiogenesis and plays a key role in heart development. Dev. Biol. 2000, 217, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Marchuk, D.A.; Srinivasan, S.; Squire, T.L.; Zawistowski, J.S. Vascular morphogenesis: Tales of two syndromes. Hum. Mol. Genet. 2003, 12, R97–R112. [Google Scholar] [CrossRef] [PubMed]
- Bourdeau, A.; Dumont, D.J.; Letarte, M. A murine model of hereditary hemorrhagic telangiectasia. J. Clin. Investig. 1999, 104, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Satomi, J.; Mount, R.J.; Toporsian, M.; Paterson, A.D.; Wallace, M.C.; Harrison, R.V.; Letarte, M. Cerebral vascular abnormalities in a murine model of hereditary hemorrhagic telangiectasia. Stroke 2003, 34, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Wu, Y.Q.; Huey, M.; Arthur, H.M.; Marchuk, D.A.; Hashimoto, T.; Young, W.L.; Yang, G.Y. Vascular endothelial growth factor induces abnormal microvasculature in the endoglin heterozygous mouse brain. J. Cereb. Blood Flow Metab. 2004, 24, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Torsney, E.; Charlton, R.; Diamond, A.G.; Burn, J.; Soames, J.V.; Arthur, H.M. Mouse model for hereditary hemorrhagic telangiectasia has a generalized vascular abnormality. Circulation 2003, 107, 1653–1657. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Hanes, M.A.; Dickens, T.; Porteous, M.E.; Oh, S.P.; Hale, L.P.; Marchuk, D.A. A mouse model for hereditary hemorrhagic telangiectasia (HHT) type 2. Hum. Mol. Genet. 2003, 12, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.J.; Chen, W.; Jun, K.; Arthur, H.M.; Young, W.L.; Su, H. Novel brain arteriovenous malformation mouse models for type 1 hereditary hemorrhagic telangiectasia. PLoS ONE 2014, 9, e88511. [Google Scholar] [CrossRef] [PubMed]
- Park, S.O.; Wankhede, M.; Lee, Y.J.; Choi, E.J.; Fliess, N.; Choe, S.W.; Oh, S.H.; Walter, G.; Raizada, M.K.; Sorg, B.S.; et al. Real-time imaging of de novo arteriovenous malformation in a mouse model of hereditary hemorrhagic telangiectasia. J. Clin. Investig. 2009, 119, 3487–3496. [Google Scholar] [CrossRef] [PubMed]
- Milton, I.; Ouyang, D.; Allen, C.J.; Yanasak, N.E.; Gossage, J.R.; Alleyne, C.H., Jr.; Seki, T. Age-dependent lethality in novel transgenic mouse models of central nervous system arteriovenous malformations. Stroke 2012, 43, 1432–1435. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raj, J.A.; Stoodley, M. Experimental Animal Models of Arteriovenous Malformation: A Review. Vet. Sci. 2015, 2, 97-110. https://doi.org/10.3390/vetsci2020097
Raj JA, Stoodley M. Experimental Animal Models of Arteriovenous Malformation: A Review. Veterinary Sciences. 2015; 2(2):97-110. https://doi.org/10.3390/vetsci2020097
Chicago/Turabian StyleRaj, Jude Amal, and Marcus Stoodley. 2015. "Experimental Animal Models of Arteriovenous Malformation: A Review" Veterinary Sciences 2, no. 2: 97-110. https://doi.org/10.3390/vetsci2020097




