CrS2 Supported Transition Metal Single Atoms as Efficient Bifunctional Electrocatalysts: A Density Functional Theory Study
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Structural and Electronic Properties of TM@CrS2
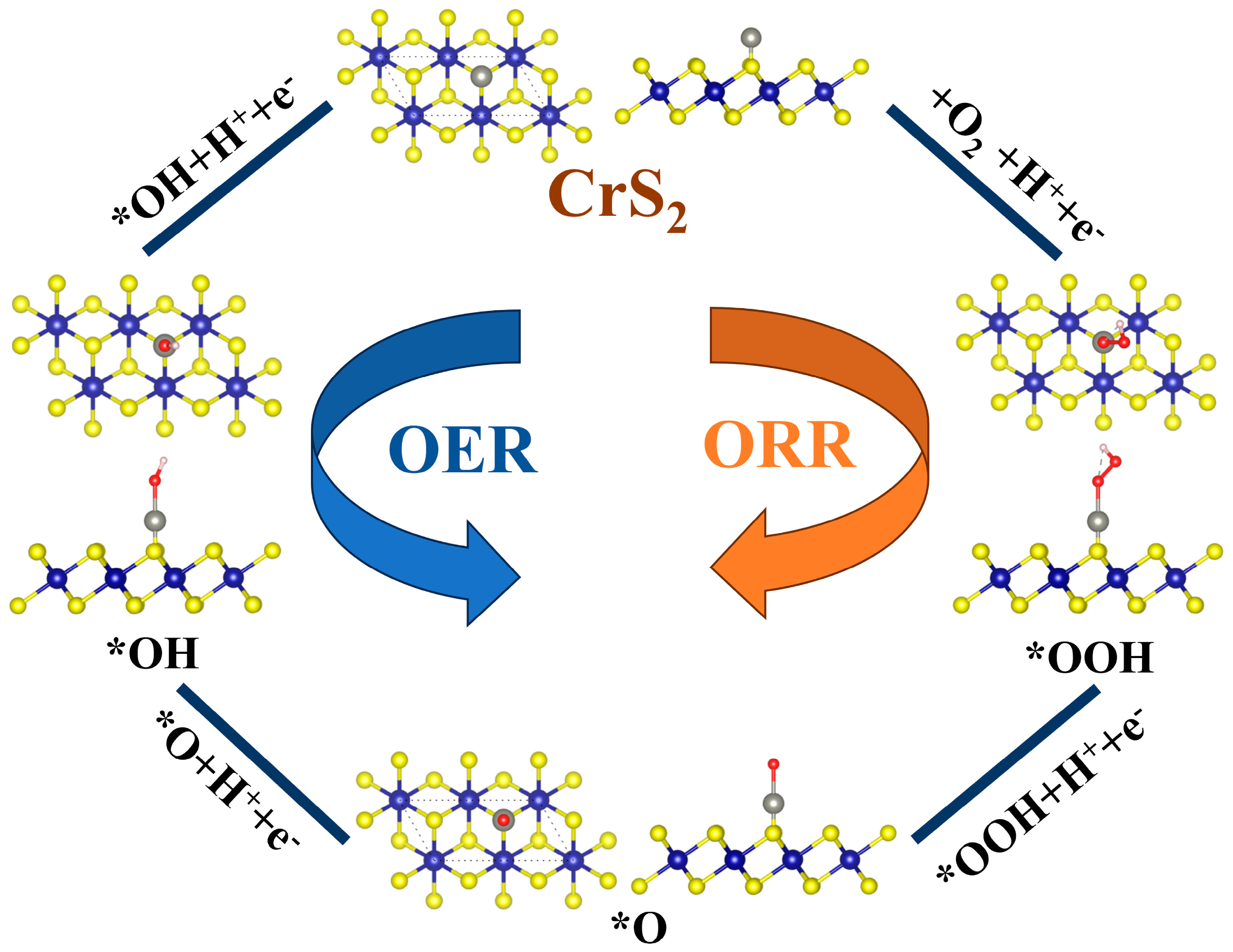
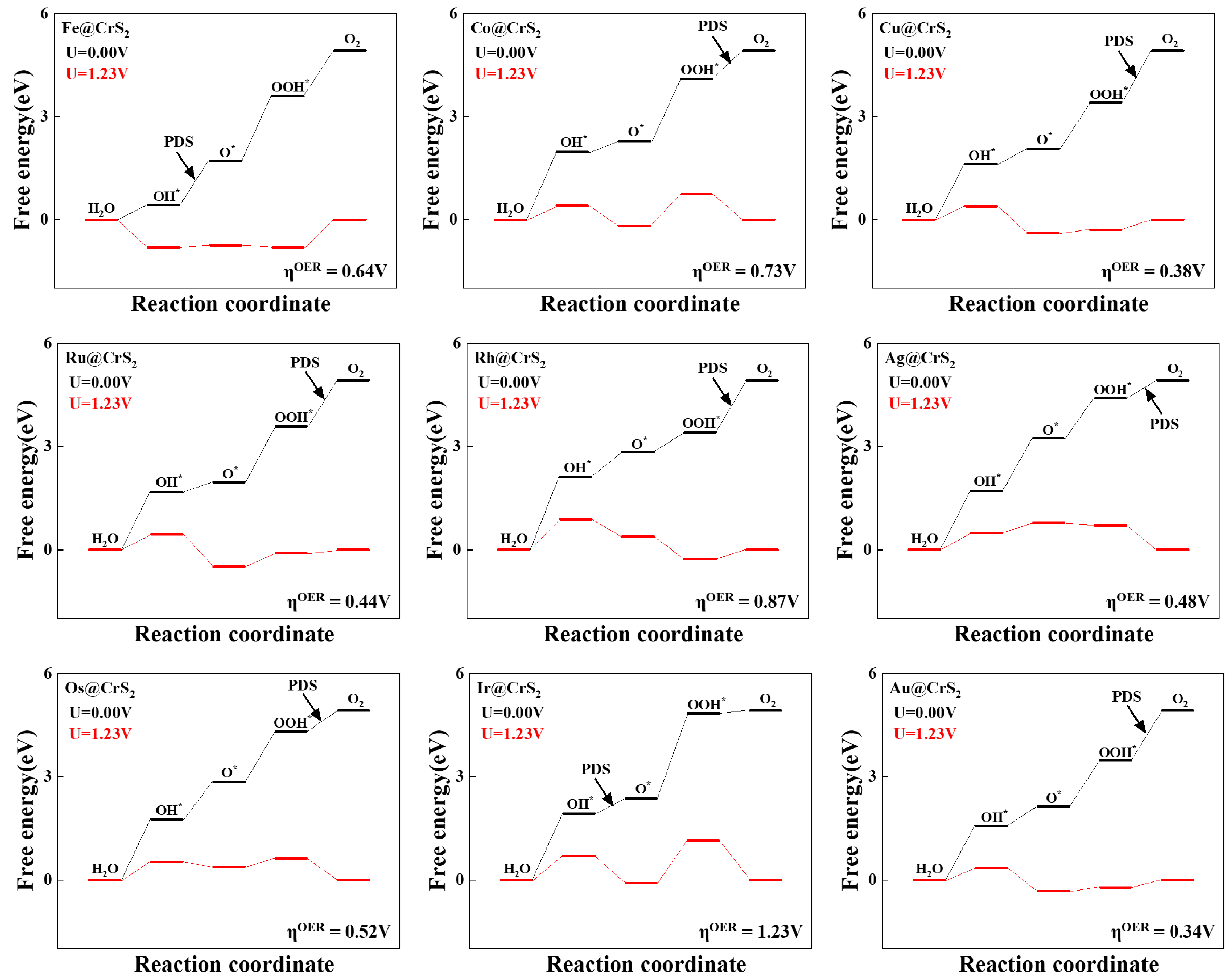
3.2. OER/ORR Activity and Catalytic Process of TM@CrS2
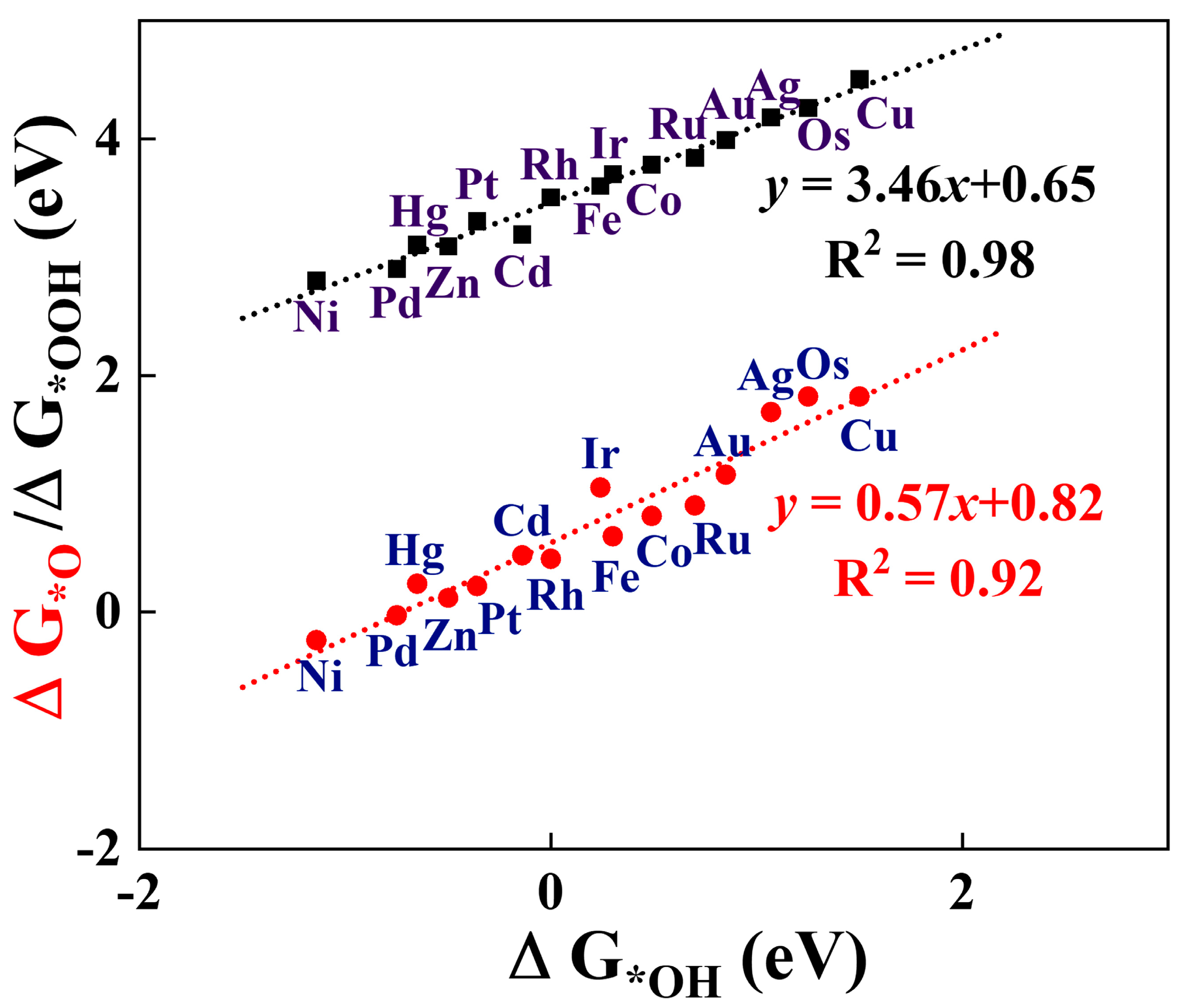
3.3. Reaction Mechanism Analysis of TM@CrS2
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sheng, T.; Tian, N.; Zhou, Z.-Y.; Lin, W.-F.; Sun, S.-G. Designing Pt-Based Electrocatalysts with High Surface Energy. ACS Energy Lett. 2017, 2, 1892–1900. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Fang, B.; Li, H.; Bi, X.T.; Wang, H. Progress in modified carbon support materials for Pt and Pt-alloy cathode catalysts in polymer electrolyte membrane fuel cells. Prog. Mater. Sci. 2016, 82, 445–498. [Google Scholar] [CrossRef]
- Sanchez Casalongue, H.G.; Ng, M.L.; Kaya, S.; Friebel, D.; Ogasawara, H.; Nilsson, A. In Situ observation of surface species on iridium oxide nanoparticles during the oxygen evolution reaction. Angew. Chem. Int. Ed. Engl. 2014, 53, 7169–7172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Y.; Cheng, C.; Yang, Z.; Hermansson, K. Tuning the ORR activity of Pt-based Ti(2)CO(2) MXenes by varying the atomic cluster size and doping with metals. Nanoscale 2020, 12, 12497–12507. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, H.; Zhao, S.; Xie, C.; Huang, Z.; Wang, S. Insights into the Dynamic Evolution of Defects in Electrocatalysts. Adv. Mater. 2023, 35, e2209680. [Google Scholar] [CrossRef] [PubMed]
- Yeh, K.Y.; Wasileski, S.A.; Janik, M.J. Electronic structure models of oxygen adsorption at the solvated, electrified Pt(111) interface. Phys. Chem. Chem. Phys. 2009, 11, 10108–10117. [Google Scholar] [CrossRef]
- Eslamibidgoli, M.J.; Eikerling, M.H. Electrochemical Formation of Reactive Oxygen Species at Pt (111)—A Density Functional Theory Study. ACS Catal. 2015, 5, 6090–6098. [Google Scholar] [CrossRef]
- Wang, A.; Niu, H.; Wang, X.; Wan, X.; Xie, L.; Zhang, Z.; Wang, J.; Guo, Y. Two-dimensional metal–organic frameworks as efficient electrocatalysts for bifunctional oxygen evolution/reduction reactions. J. Mater. Chem. A 2022, 10, 13005–13012. [Google Scholar] [CrossRef]
- Wei, X.; Jiang, C.; Xu, H.; Ouyang, Y.; Wang, Z.; Lu, C.; Lu, X.; Pang, J.; Dai, F.; Bu, X. Synergistic Effect of Organic Ligands on Metal Site Spin States in 2D Metal–Organic Frameworks for Enhanced ORR Performance. ACS Catal. 2023, 13, 15663–15672. [Google Scholar] [CrossRef]
- Zhang, R.; Shehzad, N.; Zhang, L.; Ali, A.; Amin, B.; Shahid, I. Efficient electrocatalysts of single metal atom supported on defective graphene for oxygen reduction reaction (ORR): A first principles study. Chem. Phys. 2023, 570, 111888. [Google Scholar] [CrossRef]
- Zhang, W.; Lou, H.; Yang, G. 2D Metal-Free BSi(5) with an Intrinsic Metallicity and Remarkable HER Activity. J. Phys. Chem. Lett. 2023, 14, 11036–11042. [Google Scholar] [CrossRef] [PubMed]
- Karmodak, N.; Bursi, L.; Andreussi, O. Oxygen Evolution and Reduction on Two-Dimensional Transition Metal Dichalcogenides. J. Phys. Chem. Lett. 2022, 13, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Zhao, J. 1 T-MoSe(2) monolayer supported single Pd atom as a highly-efficient bifunctional catalyst for ORR/OER. J. Colloid Interface Sci. 2022, 605, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, F.; Camellone, M.F.; Tovt, A.; Tran, N.D.; Negreiros, F.R.; Vorokhta, M.; Skala, T.; Matolinova, I.; Myslivecek, J.; Matolin, V.; et al. Creating single-atom Pt-ceria catalysts by surface step decoration. Nat. Commun. 2016, 7, 10801. [Google Scholar] [CrossRef]
- Zhu, C.; Shi, Q.; Feng, S.; Du, D.; Lin, Y. Single-Atom Catalysts for Electrochemical Water Splitting. ACS Energy Lett. 2018, 3, 1713–1721. [Google Scholar] [CrossRef]
- Yang, S.; Tak, Y.J.; Kim, J.; Soon, A.; Lee, H. Support Effects in Single-Atom Platinum Catalysts for Electrochemical Oxygen Reduction. ACS Catal. 2017, 7, 1301–1307. [Google Scholar] [CrossRef]
- Li, T.; Ren, S.; Zhang, C.; Qiao, L.; Wu, J.; He, P.; Lin, J.; Liu, Y.; Fu, Z.; Zhu, Q.; et al. Cobalt single atom anchored on N-doped carbon nanoboxes as typical single-atom catalysts (SACs) for boosting the overall water splitting. Chem. Eng. J. 2023, 458, 141435. [Google Scholar] [CrossRef]
- Zhang, Z.; Qi, S.; Song, X.; Wang, J.; Zhang, W.; Zhao, M. Stable multifunctional single-atom catalysts adsorbed on pyrazine-modified graphyne. Appl. Surf. Sci. 2021, 553, 149464. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, Q.; Huang, Z.; Chen, H.; Zhang, J.; Chen, W.; Meng, G.; Wang, D. Multifunctional design of single-atom catalysts for multistep reactions. Sci. China Chem. 2023, 66, 1241–1260. [Google Scholar] [CrossRef]
- Zhao, L.; Yu, G.; Huang, X.; Chen, W. Realizing Efficient Catalytic Performance and High Selectivity for Oxygen Reduction Reaction on a 2D Ni(2)SbTe(2) Monolayer. Inorg. Chem. 2022, 61, 2284–2291. [Google Scholar] [CrossRef]
- Niu, W.; Pakhira, S.; Cheng, G.; Zhao, F.; Yao, N.; Mendoza-Cortes, J.L.; Koel, B.E. Reaction-driven restructuring of defective PtSe(2) into ultrastable catalyst for the oxygen reduction reaction. Nat. Mater. 2024, 23, 1704–1711. [Google Scholar] [CrossRef]
- Hussain, S.; Talib, S.H.; Mohamed, S.; Zhao, R.; Qurashi, A.; Li, J.; Lu, Z. Computational screening of oxygen and sulfur decorated MXene supported transitions metal single-atom catalysts for hydrogen evolution reaction. Int. J. Hydrogen Energy 2024, 53, 969–978. [Google Scholar] [CrossRef]
- An, L.; Feng, J.; Zhang, Y.; Zhao, Y.-Q.; Si, R.; Wang, G.-C.; Cheng, F.; Xi, P.; Sun, S. Controllable tuning of Fe-N nanosheets by Co substitution for enhanced oxygen evolution reaction. Nano Energy 2019, 57, 644–652. [Google Scholar] [CrossRef]
- Abdelkareem, M.A.; Wilberforce, T.; Elsaid, K.; Sayed, E.T.; Abdelghani, E.A.M.; Olabi, A.G. Transition metal carbides and nitrides as oxygen reduction reaction catalyst or catalyst support in proton exchange membrane fuel cells (PEMFCs). Int. J. Hydrogen Energy 2021, 46, 23529–23547. [Google Scholar] [CrossRef]
- Jin, H.; Gu, Q.; Chen, B.; Tang, C.; Zheng, Y.; Zhang, H.; Jaroniec, M.; Qiao, S.-Z. Molten Salt-Directed Catalytic Synthesis of 2D Layered Transition-Metal Nitrides for Efficient Hydrogen Evolution. Chem 2020, 6, 2382–2394. [Google Scholar] [CrossRef]
- Ju, W.; Bagger, A.; Hao, G.P.; Varela, A.S.; Sinev, I.; Bon, V.; Cuenya, B.R.; Kaskel, S.; Rossmeisl, J.; Strasser, P. Understanding activity and selectivity of metal-nitrogen-doped carbon catalysts for electrochemical reduction of CO(2). Nat. Commun. 2017, 8, 944. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Luo, G.; Zhang, Q.; Hu, H.; Wang, D. Optimizing the ORR performance of PtMnM ternary intermetallics by tuning the surface strain. Sci. China Chem. 2024, 68, 536–542. [Google Scholar] [CrossRef]
- Hafner, J. Ab-initio simulations of materials using VASP: Density-functional theory and beyond. J. Comput. Chem. 2008, 29, 2044–2078. [Google Scholar] [CrossRef]
- Garcia-Rates, M.; Lopez, N. Multigrid-Based Methodology for Implicit Solvation Models in Periodic DFT. J. Chem. Theory Comput. 2016, 12, 1331–1341. [Google Scholar] [CrossRef]
- Blochl, P.E. Projector augmented-wave method. Phys. Rev. B Condens. Matter 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Hardcastle, T.P.; Seabourne, C.R.; Zan, R.; Brydson, R.M.D.; Bangert, U.; Ramasse, Q.M.; Novoselov, K.S.; Scott, A.J. Mobile metal adatoms on single layer, bilayer, and trilayer graphene: Anab initioDFT study with van der Waals corrections correlated with electron microscopy data. Phys. Rev. B 2013, 87, 195430. [Google Scholar] [CrossRef]
- Moellmann, J.; Grimme, S. DFT-D3 Study of Some Molecular Crystals. J. Phys. Chem. C 2014, 118, 7615–7621. [Google Scholar] [CrossRef]
- Tang, W.; Sanville, E.; Henkelman, G. A grid-based Bader analysis algorithm without lattice bias. J. Phys. Condens. Matter 2009, 21, 084204. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, B.S.; Krishnamurty, S.; Pal, S. Influence of plane wave cut-off on structural and electronic properties in Sn-BEA and Ti-BEA zeolite water molecule interaction. Chem. Phys. Lett. 2010, 484, 374–379. [Google Scholar] [CrossRef]
- Nolan, M.; Grigoleit, S.; Sayle, D.C.; Parker, S.C.; Watson, G.W. Density functional theory studies of the structure and electronic structure of pure and defective low index surfaces of ceria. Surf. Sci. 2005, 576, 217–229. [Google Scholar] [CrossRef]
- Rossmeisl, J.; Qu, Z.W.; Zhu, H.; Kroes, G.J.; Nørskov, J.K. Electrolysis of water on oxide surfaces. J. Electroanal. Chem. 2007, 607, 83–89. [Google Scholar] [CrossRef]
- Rossmeisl, J.; Logadottir, A.; Nørskov, J.K. Electrolysis of water on (oxidized) metal surfaces. Chem. Phys. 2005, 319, 178–184. [Google Scholar] [CrossRef]
- Zhao, L.; Yu, G.; Lv, M.; Huang, X.; Zhu, H.; Chen, W. 2D RhTe Monolayer: A highly efficient electrocatalyst for oxygen reduction reaction. J. Colloid Interface Sci. 2023, 629 Pt A, 971–980. [Google Scholar] [CrossRef]
- Lian, Y.; Xu, J.; Zhou, W.; Lin, Y.; Bai, J. Research Progress on Atomically Dispersed Fe-N-C Catalysts for the Oxygen Reduction Reaction. Molecules 2024, 29, 771. [Google Scholar] [CrossRef]
- Wang, Y.; Mao, J.; Meng, X.; Yu, L.; Deng, D.; Bao, X. Catalysis with Two-Dimensional Materials Confining Single Atoms: Concept, Design, and Applications. Chem. Rev. 2019, 119, 1806–1854. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wei, W.; Wang, S.; Wang, H.; Huang, B.; Dai, Y. H4,4,4-graphyne with double Dirac points as high-efficiency bifunctional electrocatalysts for water splitting. J. Mater. Chem. A 2021, 9, 4082–4090. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhang, T.; Rappe, A.M. Topological Bismuth (1) Facet for Efficient Oxygen Reduction Cathode in Fuel Cells. J. Phys. Chem. C 2023, 127, 19879–19885. [Google Scholar] [CrossRef]
- Lu, R.; Quan, C.; Zhang, C.; He, Q.; Liao, X.; Wang, Z.; Zhao, Y. Establishing a theoretical insight for penta-coordinated iron-nitrogen-carbon catalysts toward oxygen reaction. Nano Res. 2022, 15, 6067–6075. [Google Scholar] [CrossRef]
- Liu, J.; Xu, H.; Zhu, J.; Cheng, D. Understanding the Pathway Switch of the Oxygen Reduction Reaction from Single- to Double-/Triple-Atom Catalysts: A Dual Channel for Electron Acceptance-Backdonation. JACS Au 2023, 3, 3031–3044. [Google Scholar] [CrossRef]
- Zhang, K.; Pan, M.; Liu, Z.; Wang, J.; Wang, Y.; Deng, H. Theoretically evaluating transition metal activated two-dimensional bilayer tetragonal AlN nanosheet for high-performance HER/OER/ORR electrocatalysts. Comput. Mater. Sci. 2024, 232, 112634. [Google Scholar] [CrossRef]
- Li, F.; Ai, H.; Liu, D.; Lo, K.H.; Pan, H. An enhanced oxygen evolution reaction on 2D CoOOH via strain engineering: An insightful view from spin state transition. J. Mater. Chem. A 2021, 9, 17749–17759. [Google Scholar] [CrossRef]
- Han, B.; Viswanathan, V.; Pitsch, H. First-Principles Based Analysis of the Electrocatalytic Activity of the Unreconstructed Pt(100) Surface for Oxygen Reduction Reaction. J. Phys. Chem. C 2012, 116, 6174–6183. [Google Scholar] [CrossRef]
- Kulkarni, A.; Siahrostami, S.; Patel, A.; Norskov, J.K. Understanding Catalytic Activity Trends in the Oxygen Reduction Reaction. Chem. Rev. 2018, 118, 2302–2312. [Google Scholar] [CrossRef]




| Pristine | E | Average Bond Length (Å) | ΔQ (e) | Binding Site | |
|---|---|---|---|---|---|
| Eb (eV) | Ecoh (eV) | ||||
| Fe | −6.42 | −2.93 | 2.27 | 0.04 | H |
| Co | −5.65 | −3.29 | 2.18 | 0.21 | H |
| Ni | −5.40 | −4.23 | 2.12 | 0.34 | TS |
| Cu | −4.14 | −4.47 | 2.18 | 0.45 | H |
| Zn | −2.94 | −4.92 | 2.24 | 0.23 | TS |
| Ru | −7.00 | −3.11 | 2.23 | 0.37 | TS |
| Rh | −6.05 | −3.57 | 2.32 | 0.46 | H |
| Pd | −4.17 | −3.48 | 2.29 | −0.06 | H |
| Ag | −0.22 | −2.02 | 2.50 | 0.56 | TCr |
| Cd | −1.87 | −4.76 | 2.46 | 0.63 | H |
| Os | −7.79 | −2.75 | 2.30 | 0.31 | TS |
| Ir | −6.97 | −3.62 | 2.30 | 0.57 | H |
| Pt | −5.41 | −4.06 | 2.27 | 0.54 | TS |
| Au | −2.72 | −4.08 | 2.37 | 0.36 | H |
| Hg | −0.64 | −4.63 | 2.87 | 0.28 | TS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y. CrS2 Supported Transition Metal Single Atoms as Efficient Bifunctional Electrocatalysts: A Density Functional Theory Study. ChemEngineering 2025, 9, 43. https://doi.org/10.3390/chemengineering9030043
Wang Y. CrS2 Supported Transition Metal Single Atoms as Efficient Bifunctional Electrocatalysts: A Density Functional Theory Study. ChemEngineering. 2025; 9(3):43. https://doi.org/10.3390/chemengineering9030043
Chicago/Turabian StyleWang, Ying. 2025. "CrS2 Supported Transition Metal Single Atoms as Efficient Bifunctional Electrocatalysts: A Density Functional Theory Study" ChemEngineering 9, no. 3: 43. https://doi.org/10.3390/chemengineering9030043
APA StyleWang, Y. (2025). CrS2 Supported Transition Metal Single Atoms as Efficient Bifunctional Electrocatalysts: A Density Functional Theory Study. ChemEngineering, 9(3), 43. https://doi.org/10.3390/chemengineering9030043





