1. Introduction
1.1. The Importance of Individual Decontamination
Contamination of persons, outer clothing/underwear, animals, food, feed, protective garments, terrain and other objects/materials can be expected during acts of chemical terrorism, war and the misuse/release of chemical warfare agents (CWA) or other hazardous substances. In such situations, decontamination represents a significant measure for active protection against the consequences of uncontrolled or accidental release of CWA or other hazardous substances into the environment. In case of accidents, the discovery of hazardous substances or terrorist acts in the Czech Republic (CR), Fire and Rescue Service Units (FRS CR) are responsible for being equipped with adequate devices of decontamination.
This paper focused on the decontamination of CWA and other hazardous substances. Fire-fighters recognize the term “individual decontamination” to be the decontamination of contaminated areas of the body, protective garments and/or materials/equipment immediately after contamination. This is performed individually or by mutual assistance using prescribed or improvised devices. The measures include personal decontamination as well as the decontamination of protective clothing and equipment used by fire-fighters in a contaminated area. Individual decontamination is not evaluated from the military point of view, but with regard to the needs of fire rescue units.
Individual decontamination is extremely important for reducing the maximum effects of contamination, as consequences of contamination by a toxic substance depend on the time elapsed between contamination and decontamination [
1,
2]. The general rule in this case is: “Better to perform the decontamination by improvised, less-effective devices as soon as possible after contamination than wait for more effective devices” [
3]. Skin decontamination after five minutes is already ineffective for some CWAs [
4].
1.2. Principles and Devices of Individual Decontamination
Individual decontamination can be divided into two general groups,
i.e., mechanical/physical and chemical. Modern devices of individual decontamination include both groups. Mechanical and physical methods remove toxic substances from the contaminated surface by dissolving, washing out, wiping with organic solvents or aqueous surfactant solutions and adsorption. The advantage of these procedures is their universal utility against toxic substances, temperature-independence, a longer lifetime and a reduced risk of undesirable interactions with decontaminated surfaces [
1]. However, these procedures do not eliminate the toxic contaminant, which may disqualify them for use in enclosed spaces and in cases where the waste disposal cannot be ensured. Chemical procedures decompose or convert toxic substances to non-toxic or less toxic products. Generally, the reagents are suitable for a specific group of contaminants (a few are universal), where the reagent affects various contaminants with different compositions [
4].
Contemporary examples of individual decontamination products are issued to modern military units. During the 20th century, the Army of the CR was equipped with an Individual Chemical Package IPB-80. The kit uses a natural sorbent, Fuller’s earth (powder form of activated montmorillonite; commercial name Desprach), for sorption and mechanical decontamination, and is used by a number of European armies for individual decontamination [
3]. Desprach is supplied in a plastic bottle (with a nozzle in the neck) for application to contaminated surfaces (
Figure 1) or onto wipes.
Figure 1.
Application of Desprach sorbent to a contaminated surface.
Figure 1.
Application of Desprach sorbent to a contaminated surface.
The M291 Skin Decontamination Kit was in use by the American Army several years ago [
5]. The kit uses a mixed ion-exchanger (Ambergard XE-555), which sorbs the toxic liquid, and the reactive properties of the ion exchanger partially decompose nerve and blistering warfare agents [
1,
2]. The material is provided as small cushions of non-woven fabric that is saturated with the synthetic ion exchange resin.
The nano-sorbent FAST-ACT is used for the adsorption, neutralization and decontamination of a wide variety of toxic liquids and gaseous substances (including CWA) [
6]. The primary mechanisms are rapid sorption and decomposition of the substance by the matrix. It is available as a powder in polyethylene containers of various volumes or in pressure bottles, and sufficient material for individual decontamination needs (0.5 kg sorbent) is provided in the application bottle. FAST-ACT is also available as a glove for individual decontamination, and is designed for the decontamination of smaller surfaces, equipment and individual protection (
Figure 2) [
7]. The M291 Decontamination kit and the FAST-ACT decontamination nano-sorbent are examples of combined physical and chemical decontamination methods, sometimes termed destructive sorption.
Figure 2.
Decontamination of a protective mask with a FAST-ACT decontamination glove.
Figure 2.
Decontamination of a protective mask with a FAST-ACT decontamination glove.
Another example of a kit that contains a loose decontamination mixture is the Polish Army IPP-95 kit. The plastic case contains plastic bags with the loose decontamination mixture (chloramine B, zinc oxide, magnesium stearate and a zeolite), a tube of decontamination ointment (sodium persulfate, magnesium stearate, urea and silicone oil) and wipes [
4].
A modern direction taken in the development of individual decontamination methods also includes carbon fibres. The materials consist of three-layers, where the lower and upper layers of non-woven fabric enclose activated carbon fibres [
8,
9]. The FIBERTECT line of products is available as gloves, cloths or perforated rolls of cloths in various sizes.
Decontamination solutions and emulsions have been traditionally used by the Soviet Union and successor countries, and they lead the world in the development of the new variants and skin-friendly decontamination methods [
4]. Examples are the IPP-11 (fabric saturated with decontamination liquid—a mixture of polyethylene glycols and lanthanum nitrate solution) and IPP-8 decontamination sets (solution of ethoxyethanol, isopropanol, dimethylformamide and sulfolane, in which metallic sodium is dissolved before use).
The RSDL decontamination sponge is offered by a number of producers (based on a Canadian patent) and is in use by various armed forces [
5]. The applicator is a sponge (~40 × 100 × 5 mm) saturated with the RSDL decontamination mixture, which is a solution of potassium salt and 2,3-butanedione monoxime in polyethylene glycol monoethyl ethers [
2]. Thorough tests have shown high decontamination efficiency for nerve agents and sulfur mustard [
10]. The sponge can be used to decontaminate various smaller surfaces, though its primary use is for the decontamination of skin [
11] (
Figure 3).
Figure 3.
Skin decontamination by RSDL sponge.
Figure 3.
Skin decontamination by RSDL sponge.
The sponge as the application device with oxime was included in some other devices. Other devices are developed throughout the world, devices that, besides having the enzyme immobilized on their material, contain activators of enzymes and indicators of enzyme depletion (colour, luminescence
etc.). Some devices can be used for the decontamination of air as a filter and for the decontamination of water [
12,
13].
Two-solution type kits are available where the solutions are mixed at the moment of application in the nozzle of a two-chamber applicator. These decontamination agents are (in this case) substances that decompose both organophosphates and yperites by oxidation,
i.e., hypochlorite [
14] and peroxides [
2]. These active components are most frequently mixed with a surfactant to facilitate the removal of contaminants from surfaces. A surfactant-sodium hypochlorite mixture is the basis of the German Alldecont decontamination kit [
4] and the MDF LSA-100 surfactant-hydrogen peroxide mixture, which produces a decontamination foam Sandia [
2,
5]. The Czech foam applicator (Hvezda,
Figure 4) dispenses a mixture of surfactant and hydrogen peroxide [
15].
Figure 4.
Decontamination of surfaces by the Hvezda two-chamber foam applicator.
Figure 4.
Decontamination of surfaces by the Hvezda two-chamber foam applicator.
1.3. Security of Individual Decontamination at Units of FRS CR
FRS CR units are equipped with devices for mass decontamination. Decontamination procedures employ tanker trucks, fire engines and hand sprayers, sources of pressurized water, hot water and first of all the station of the decontamination technique and station of the decontamination of persons with high capacity. The equipment is capable of using special decontamination mixtures, with a simple and rapid deployment, as well as ensuring the collection of contaminated waste-water. While mass decontamination could be performed, FRS CR units were not able to perform individual decontamination until 2013.
The rule that for the maximal reduction of contamination effects, it is more important to perform decontamination by improvised and less effective devices as soon as possible after the contamination was not followed. There is a low frequency of large-scale contamination of fire-fighters and equipment on one side, and on the other side a high frequency of partial contamination of protective suits or small objects, which could be instantly removed or decontaminated by other devices of individual decontamination.
The absence of individual decontamination methods for fire units is not limited to the Czech Republic. The International Technical Committee for Prevention and Extinction of Fires or CTIF (Comité Technique International de prevention et d’extincion du Feu) discussed at its 2013 meeting that the majority of European fire and rescue brigades are not equipped with devices of individual decontamination, which significantly reduces their level of preparedness to the release of CWA and other hazardous substances.
In order to temporarily address this deficit, the FRS CR decided to equip fire-fighters with improvised kits called Device for Individual Fire-fighter Decontamination (INDEHA) (
Figure 5) produced by chemical laboratories of the FRS CR. The kit allows for decontamination by wiping with cloth swabs saturated with ethanol. This is a classical physical principle whose application to individual decontamination has been used and recommended for some time [
16]. The kit also solves the waste problem, as used wipes are gathered in a hermetically sealed container, which serves as the cover for the kit. The waste is treated with solid sodium hydroxide, and during transport, the presence of ethanol creates a favourable environment for the decomposition of contaminants. The decontamination of wastes is carried out by laboratories of the FRS CR [
17].
Figure 5.
Components of the individual fire-fighter decontamination kit.
Figure 5.
Components of the individual fire-fighter decontamination kit.
Several currently existing methods available in the Czech Republic were compared in order to design equipment providing FRS CR units with adequate devices of individual decontamination. The representatives of various methods were chosen for the testing. Desprach sorbent uses simple sorption, whereas the FAST-ACT nano-sorbent and decontamination gloves use destructive sorption. Liquid chemical decontamination agents were represented by the RSDL decontamination sponge and the Czech Hvezda prototype applicator for hydrogen peroxide foam. The improvised Czech INDEHA approach, based on wiping surfaces with ethanol, was also evaluated.
Decontamination efficiency, properties of the devices and waste risks were evaluated. The selection of surfaces was representative of materials and situations the FRS CR would actually encounter (painted surfaces and materials used for chemical protection). When all principles are followed during a response to a dangerous substance, skin contamination (fire-fighter) should not occur. Therefore, individual decontamination procedures were tested on chemical protective suit material and steel plates coated with common exterior paint. The test surfaces were contaminated with VX and sulfur mustard. Since fire units also respond to the release of hazardous industrial substances, decontamination efficiency for other hazardous substances was needed and for this purpose o-cresol and acrylonitrile were used. Decontamination efficiencies were determined on the basis of residual contamination values, which were compared to permissible residual contamination values. Values for CWA and contact with unprotected skin are taken from the Czech Defense Standard [
18], and for hazardous industrial substances, the estimated values for the highest permissible surface contamination were derived from the highest permissible concentrations in air. Another indicator was the decontamination efficiency in percent as a ratio of the difference between initial and residual contamination and the initial contamination value multiplied by 100.
2. Results and Discussion
2.1. Decontamination Efficiency
The evaluation of residual surface contamination present on protective suit material and painted steel plate after treatment with VX shown is shown in
Table 1. The most effective treatment for both surfaces was Desprach. Permissible residual contamination values were not achieved using the FAST-ACT decontamination glove (both surfaces) or the Hvezda foam (painted steel plate). The foam also caused a relatively high degree of paint destruction. The FAST-ACT sorbent, RSDL decontamination sponge and wiping of the surface with ethanol (INDEHA kit) showed comparable efficiencies and permissible residual contamination values for VX on both test surfaces.
Table 1.
Average values of residual surface contamination (RC), relative standard deviation (SR) and decontamination efficiency (DE) for O-ethyl-S-(diisopropyl-aminoethyl)methylthiophosphonate (VX) at an initial contamination level of 2.1 g/m2 (permissible residual contamination 1 mg/m2).
Table 1.
Average values of residual surface contamination (RC), relative standard deviation (SR) and decontamination efficiency (DE) for O-ethyl-S-(diisopropyl-aminoethyl)methylthiophosphonate (VX) at an initial contamination level of 2.1 g/m2 (permissible residual contamination 1 mg/m2).
| Surface | Decontamination device devices | RC (mg/m2) | SR (%) | DE (%) |
|---|
| Protective suit material | Desprach Sorbent | 0.23 | 6.8 | 99.99 |
| FAST-ACT Sorbent | 0.66 | 4.8 | 99.97 |
| FAST-ACT Glove | 4.90 | 8.2 | 99.77 |
| RSDL Sponge | 0.64 | 3.8 | 99.97 |
| Hvezda Foam applicator | 0.77 | 11.7 | 99.96 |
| INDEHA | 0.92 | 3.9 | 99.96 |
| Painted steel plate | Desprach Sorbent | 0.17 | 7.9 | 99.99 |
| FAST-ACT Sorbent | 0.93 | 7.3 | 99.96 |
| FAST-ACT Glove | 1.60 | 6.3 | 99.92 |
| RSDL Sponge | 0.22 | 16.0 | 99.99 |
| Hvezda Foam applicator | 1.31 | 7.0 | 99.94 |
| INDEHA | 0.76 | 3.8 | 99.96 |
Table 2 summarizes the results for sulfur mustard decontamination of test surfaces by various methods. Unlike VX, where the methods had roughly the same efficiency for protective suit material and painted steel plate, it was necessary to evaluate the surfaces separately for sulfur mustard. For protective suit material, the highest decontamination efficiency was achieved using the FAST-ACT sorbent, Hvezda foam and wiping with ethanol. Values below the permissible contamination level were achieved with the RSDL sponge and Desprach sorbent, while the decontamination glove was not effective. For the painted steel plate, wiping the surface with ethanol was most effective, but Hvezda foam and Desprach showed also a good degree of effectiveness. Residual surface contamination values after decontamination with FAST-ACT sorbent, decontamination glove and RSDL sponge were comparable and slightly exceeded the permissible value.
As a representative of stable polar contaminants, o-cresol was chosen, and the evaluation of residual surface contamination after decontamination is shown in
Table 3. The FAST-ACT decontamination glove was not effective, because the glove might not have been capable of absorbing the initial high density contamination (50 g/m
2). FAST-ACT sorbent did not efficiently decontaminate the protective suit material, while the Desprach sorbent and Hvezda foam were substantially more effective. The RSDL decontamination sponge was more efficient than the FAST-ACT sorbent. This is understandable, because the RSDL decontamination solution contains alcohols and their derivatives. Even though the sponge was a chemical decontamination method, physical methods will be used for the decontamination of cresols and similar contaminants. However, it must be noted that the RSDL sponge was designed for decontamination of CWA, not hazardous industrial agents, while the FAST-ACT sorbent informational literature specifically mentioned cresol.
Table 2.
Average surface residual contamination values (RC) for surfaces, relative standard deviations (SR) and decontamination efficiencies (DE) for 10 g/m2 sulfur mustard initial contamination (permissible residual contamination 10 mg/m2).
Table 2.
Average surface residual contamination values (RC) for surfaces, relative standard deviations (SR) and decontamination efficiencies (DE) for 10 g/m2 sulfur mustard initial contamination (permissible residual contamination 10 mg/m2).
| Surface | Decontamination device devices | RC (mg/m2) | SR (%) | DE (%) |
|---|
| Protective suit material | Desprach Sorbent | 5.7 | 4.2 | 99.94 |
| FAST-ACT Sorbent | <0.5 | - | 100 |
| FAST-ACT Glove | 15.0 | 8.2 | 99.85 |
| RSDL Sponge | 9.3 | 4.8 | 99.91 |
| Hvezda Foam applicator | <0.5 | - | 100 |
| INDEHA | 1.6 | 16.5 | 99.98 |
| Painted steel plate | Desprach Sorbent | 4.7 | 10.0 | 99.95 |
| FAST-ACT Sorbent | 12.1 | 8.1 | 99.88 |
| FAST-ACT Glove | 15.2 | 8.2 | 99.85 |
| RSDL Sponge | 14.3 | 7.0 | 99.86 |
| Hvezda Foam applicator | 3.0 | 8.9 | 99.97 |
| INDEHA | <0.5 | - | 100 |
Table 3.
Average values for residual surface contamination (RC), relative standard deviation (SR) and decontamination efficiency (DE) for o-cresol at an initial contamination level of 50 g/m2 (permissible residual contamination 100 mg/m2).
Table 3.
Average values for residual surface contamination (RC), relative standard deviation (SR) and decontamination efficiency (DE) for o-cresol at an initial contamination level of 50 g/m2 (permissible residual contamination 100 mg/m2).
| Surface | Decontamination device devices | RC (mg/m2) | SR (%) | DE (%) |
|---|
| Protective suit material | Desprach Sorbent | 250 | 6.5 | 99.50 |
| FAST-ACT Sorbent | 1500 | 3.8 | 97.00 |
| FAST-ACT Glove | 5400 | 15.2 | 89.20 |
| RSDL Sponge | 110 | 7.1 | 99.78 |
| Hvezda Foam applicator | 280 | 5.7 | 99.44 |
| INDEHA | 8.0 | 14.9 | 99.98 |
| Painted steel plate | Desprach Sorbent | 4.7 | 10.0 | 99.95 |
| FAST-ACT Sorbent | 12.1 | 8.1 | 99.88 |
| FAST-ACT Glove | 15.2 | 8.2 | 99.85 |
| RSDL Sponge | 14.3 | 7.0 | 99.86 |
| Hvezda Foam applicator | 3.0 | 8.9 | 99.97 |
| INDEHA | 8.0 | 17.3 | 99.98 |
Decontamination results for acrylonitrile are shown in
Table 4. A significant difference was observed between the decontamination of protective suit material (decontaminated by wiping with ethanol or a decontamination sponge) and the painted steel plate (decontaminated by all methods). The most effective procedure for the removal of acrylonitrile from surfaces was wiping with ethanol. Unlike previous contaminants, the worst results were seen with the Desprach sorbent. For protective suit material, comparable results were achieved with the FAST-ACT sorbent and Hvezda foam, and the worst results with the FAST-ACT glove. For decontamination of painted steel plate, the decontamination glove was very effective. Residual contamination values for the paint after decontamination by Desprach and FAST- ACT sorbents, Hvezda foam and RSDL sponge were comparable, and contamination levels were less than the permissible contamination levels.
Table 4.
Average values for residual surface contamination (RC), relative standard deviation (SR) and decontamination efficiency (DE) for acrylonitrile at an initial contamination level of 50 g/m2 (permissible residual contamination 10 mg/m2).
Table 4.
Average values for residual surface contamination (RC), relative standard deviation (SR) and decontamination efficiency (DE) for acrylonitrile at an initial contamination level of 50 g/m2 (permissible residual contamination 10 mg/m2).
| Surface | Decontamination device devices | RC (mg/m2) | SR (%) | DE (%) |
|---|
| Protective suit material | Desprach sorbent | 50 | 4.8 | 99.90 |
| FAST-ACT sorbent | 17 | 3.1 | 99.97 |
| FAST-ACT glove | 38 | 7.5 | 99.92 |
| RSDL sponge | 7.7 | 6.6 | 99.98 |
| Hvezda foam applicator | 21 | 5.9 | 99.96 |
| INDEHA | 1.6 | 13.7 | 100 |
| Painted steel plate | Desprach sorbent | 4.7 | 10.0 | 99.95 |
| FAST-ACT sorbent | 12.1 | 8.1 | 99.88 |
| FAST-ACT glove | 15.2 | 8.2 | 99.85 |
| RSDL sponge | 14.3 | 7.0 | 99.86 |
| Hvezda foam applicator | 3.0 | 8.9 | 99.97 |
| INDEHA | <0.2 | - | 100 |
After comparing the decontamination efficiencies of the various methods, the data indicate the following:
- (a)
-
Desprach sorbent was effective against CWA and had a reduced efficiency for hazardous industrial substances;
- (b)
-
The RSDL decontamination sponge and FAST-ACT sorbent were (with some exceptions) comparable in their decontamination efficiency;
- (c)
-
Wiping the surface with the FAST-ACT sorbent glove was least effective, with the exception of the acrylonitrile decontamination. Practical experience indicated that the sorbent properties were insufficient, the integrity of the glove material was difficult to ensure, as well as insufficient contact time between the active material and the contaminated surface;
- (d)
-
The FAST-ACT sorbent was not effective for the removal of cresol, even though this contaminant was listed in the informational literature. It is likely that the sorbent (and the Desprach sorbent) would function more efficiently if contaminated surfaces could be wiped with the material. However, the instructions do neither mention this option nor are devices provided to wipe the surfaces with [
7];
- (e)
-
Hvezda foam was highly efficient against sulfur mustard but less efficient against VX. When tested against industrial contaminants, its efficiency was comparable to the Desprach sorbent;
- (f)
-
The only procedure that reduced contamination levels of all substances on both surfaces to below permissible residual contamination levels was wiping with ethanol (INDEHA).
2.2. Decontamination of Places with Poor Accessibility and Vertical Surfaces
Small items also require decontamination, whereby difficult-to-access areas, such as internal corners and grooves should be taken into ccount, and the decontamination of these areas should be easily accomplished. A powdered sorbent could be applied practically everywhere (Desprach), and decontamination sponge, foam and wipes soaked in alcohol were not limited by size/space. The exception was the decontamination glove, where larger flat areas were more easily cleaned, while other areas were more difficult to clean. Vertical areas and places with poor accessibility should be reachable for decontamination, and could be decontaminated by chemical (decontamination sponge, foam applicator) and physical methods (wiping with ethanol). However, the application of solid sorbents posed problems, as decontamination was impossible without mechanical wiping.
2.3. Need of Water for Rinsing
The necessity of rinsing a decontaminated surface with water was significant, as water may not be available in a contaminated space. From this perspective, mechanical (decontamination glove, Desprach and FAST-ACT solid sorbents) and physical methods (wiping with ethanol) were superior, because a water rinse was unnecessary. The Hvezda foam required rinsing with water, since the material could have a significant negative impact on surfaces. It was unclear whether a water rinse was needed after decontamination with the RSDL sponge. The manual stated that the surface should be rinsed with water after 2 min “if available”, and also stated that the decontamination solution could have irritating effects. This suggested that skin should be rinsed, as should surfaces that might come into contact with exposed skin after decontamination. However the use of RSDL at temperatures below 0 °C is uncertain.
The necessity of a water rinse after decontamination might also be affected by freezing conditions, which would eliminate those methods requiring a water rinse when temperatures were below freezing. However, a temperature of −20 °C would not reduce the decontamination efficiency of sorbents and alcohols.
2.4. Economic Comparisons
A price comparison of methods was performed in the Czech Republic in 2013, as cost could be a significant factor in equipping fire and rescue brigades. A price was not available for the Desprach sorbent used by the CR armed forces, as it was no longer manufactured, and a price for the Hvezda foam prototype applicator had not been determined. That said, the price should not play a major role for the FRS CR, but other parameters that define the economic impact should be taken into account,
e.g., the lifetime (renewal frequency), the possibility of repeated applications for a method/material, as well as the area that could be decontaminated by the method/material. Economic parameters are summarized in
Table 5.
Table 5.
Overall evaluation of decontamination methods.
Table 5.
Overall evaluation of decontamination methods.
| Parameter | Desprach sorbent | FAST-ACT sorbent | FAST-ACT glove | RSDL sponge | Hvezda foam applicator | INDEHA kit |
|---|
| Kit contents | sorbent in plastic bottle | sorbent in plastic bottle | glove in cover | sponge in cover | 2 chambers with solutions | wipes, ethanol, NaOH |
| CWA Decontamination efficiency | very high | high | low | high | high | high |
| Decontamination efficiency for selected hazardous industrial substances | lowered | very low for cresol | very low for cresol | high | high | very high |
| Possibility of decontamination of places with poor accessibility | yes | yes | More difficult | yes | yes | yes |
| Possibility of decontaminating vertical areas | More difficult | no | yes | yes | yes | yes |
| Water need for rinsing | no | no | no | yes | yes | no |
| Possibility of decontamination under freezing conditions | yes | yes | yes | uncertain | no | yes |
| Risk of high waste toxicity after CWA decontamination | yes | no | yes | no | no for VX, yes for mustard | no |
| Risk of high waste toxicity after decontamination of hazardous industrial substances | yes | yes | yes | yes | yes | no |
| Waste disposal procedure available | no | no | no | too general | no | yes |
| Risks at actions of fire-fighters | unknown | unknown | unknown | dangerous reactions with high test hypochlorite, flammability | Oxidizing effects explosion and ignition | flammability |
| Expiration period (Years) | not known | 5 | 5 | 3 | 3 | not set |
| Decontamination area (dm2) | 10 | 50 | 4 | 4 | 4 | 9 |
| Multiple use | yes | yes | no | no | no | no |
| Price per (€) | no longer produced | 140 | 25 | 35 | unknown | 1 |
2.5. Waste Risks and Disposal
The risk from contaminated wastes cannot be overlooked for the various methods. It was obvious that procedures based on mechanical sorption and physical methods merely removed the contaminant from surfaces, yielding highly-contaminated waste material, while for the RSDL decontamination sponge and FAST-ACT sorbent, the manufacturers stated that the product decomposed CWA. The risks from wastes that remain 24 h after decontamination were evaluated by gas chromatography with mass detection by the head-space technique (GC/MS).
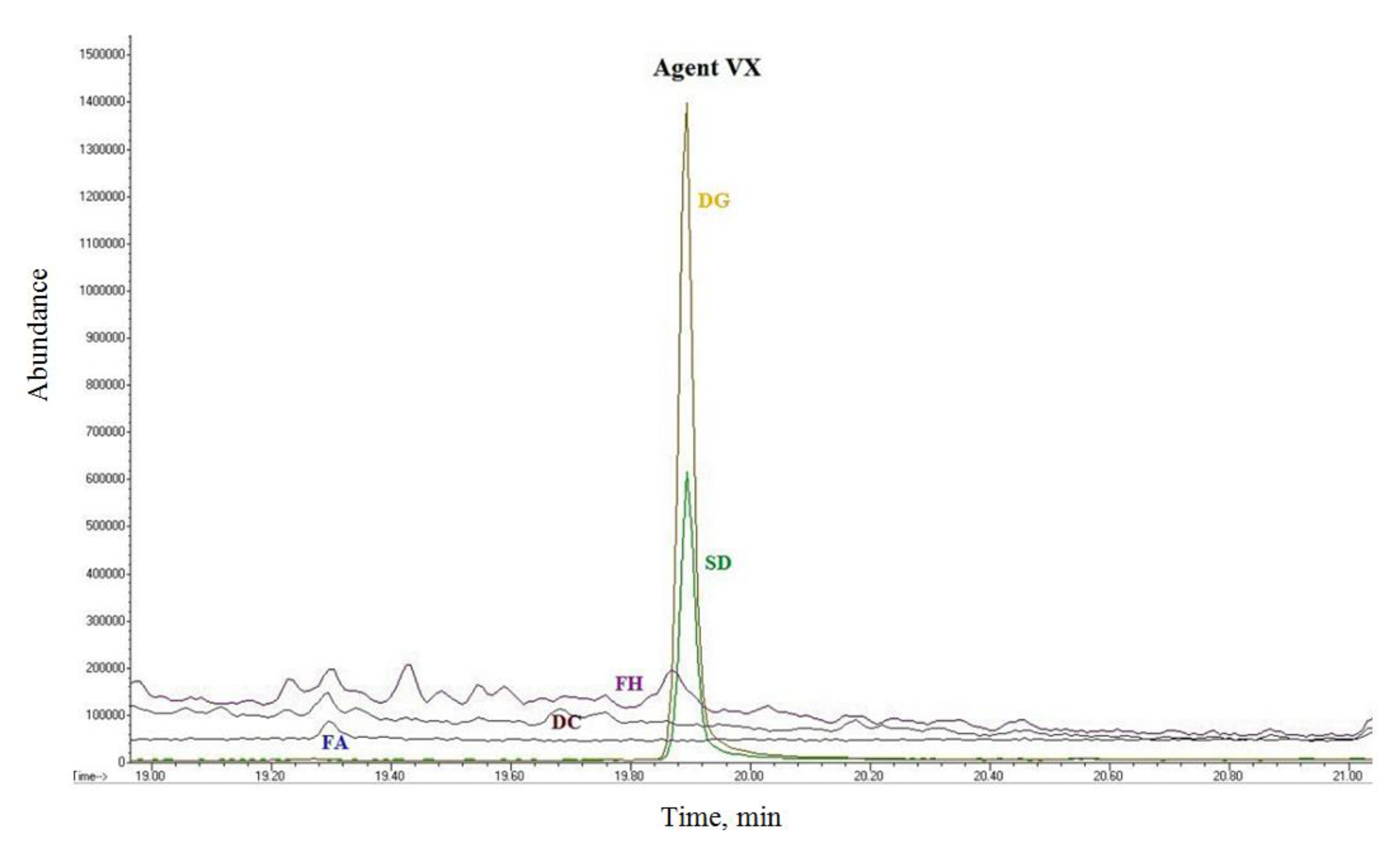
Figure 6 shows the retention time of VX for the analyses of waste materials after decontamination. Even after 24 h, significant amounts of VX were present in the Desprach sorbent and the FAST-ACT decontamination glove, which indicated that the waste material represented a significant risk of inhalation intoxication.
Figure 6 also shows the results for Hvezda foam, RSDL decontamination sponge and FAST-ACT sorbent, which did not show a VX peak, indicating that the toxin was decomposed.
Figure 6.
GC/MS chromatogram of waste after decontamination of O-ethyl-S-(diisopropylaminoethyl)-methylthiophosphonate (VX) with Desprach sorbent (SD), decontamination glove (DG), FAST-ACT sorbent (FA), RSDL decontaminationsponge (DC) and Hvezda foam (FH).
Figure 6.
GC/MS chromatogram of waste after decontamination of O-ethyl-S-(diisopropylaminoethyl)-methylthiophosphonate (VX) with Desprach sorbent (SD), decontamination glove (DG), FAST-ACT sorbent (FA), RSDL decontaminationsponge (DC) and Hvezda foam (FH).
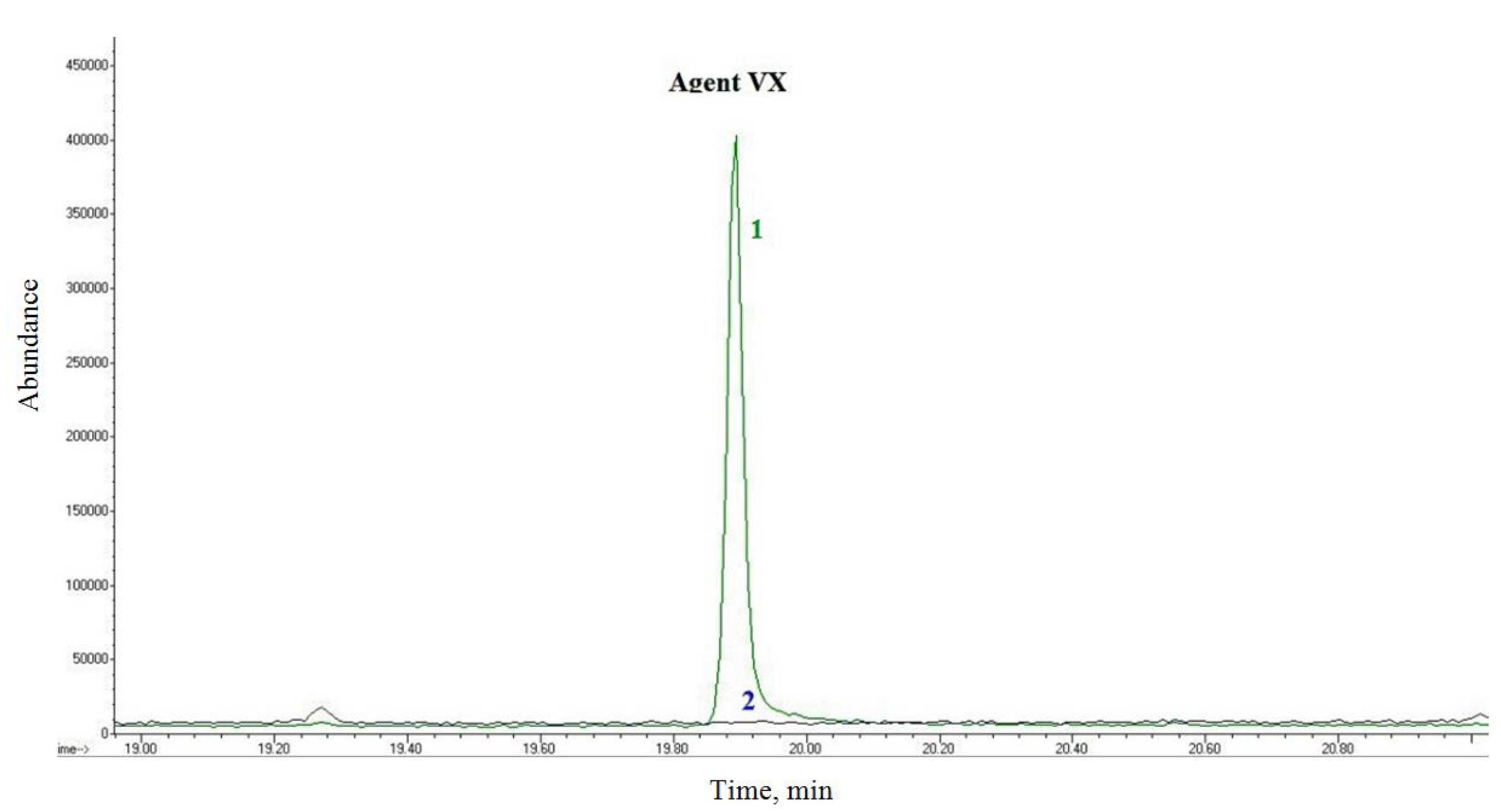
The FAST-ACT sorbent decomposed VX as described by the manufacturer’s literature, while the decontamination glove (which was filled with the same sorbent) still contained a significant amount of VX after 24 h. This indicated that the contaminant remained in the surface fabric layer and did not make contact with the sorbent, which may be the reason for the low decontamination efficiency. VX was also present in waste material after wiping with ethanol (
Figure 7), although when the INDEHA kit was used, wipes were collected in the container and treated with solid sodium hydroxide. In the highly-alkaline ethanol environment, VX was decomposed after 24 h and was no longer an inhalation risk.
Figure 7.
GC/MS chromatogram of O-ethyl-S-(diisopropylaminoethyl) methylthio-phosphonate (VX)-contaminated alcohol wipes after 24 h; 1—wipe without treatment, 2—wipe after treatment with solid sodium hydroxide.
Figure 7.
GC/MS chromatogram of O-ethyl-S-(diisopropylaminoethyl) methylthio-phosphonate (VX)-contaminated alcohol wipes after 24 h; 1—wipe without treatment, 2—wipe after treatment with solid sodium hydroxide.
Analyses of solid and liquid wastes were also performed for other contaminants [
17]. For sulfur mustard decontamination, results were similar to those observed for VX. The liquid waste that remained after decontamination with Hvezda foam contained non-decomposed sulfur mustard, used Desprach sorbent and the decontamination glove were toxic, while the FAST-ACT sorbent and the RSDL decontamination sponge efficiently decomposed sulfur mustard to 2-chlorethylvinylsulfide, 2-hydroxy-ethylvinylsulfide, divinylsulfide and p-dithiane (primarily). No sulfur mustard was found in ethanol wipes treated with solid sodium hydroxide after 24 h, although the above-mentioned decomposition products were found.
Analyses of the waste after the decontamination of o-cresol and acrylonitrile (hazardous industrial substances) indicated that the decontamination sponge and FAST-ACT sorbent did not decompose these contaminants to non-toxic products, because these were physical rather than chemical methods. However, one must remember that the RSDL sponge was designed for the decontamination of CWA. Both substances were also found in the liquid waste after application of Hvezda foam. When INDEHA kit waste was analysed after 24 h, the acrylonitrile was decomposed, and sodium propionate was present as the primary decomposition product [
17]. In the case of o-cresol, no decomposition occurred, but the conversion product (sodium cresolate) was not an inhalation hazard.
Overall, analyses indicated that dangerous wastes were produced, and that their toxicity depended on the toxicity of the original contaminant, giving rise to the question of what should be done with the waste materials. The available literature did not answer the question, although the RSDL decontamination sponge manual stated that the waste should be treated according to state requirements.
It is necessary to note that decontamination of CWA by the armed forces would involve larger areas of contamination that would not equate to any small local incident, and a discarded sponge or contaminated sorbent would present a relatively insignificant hazard. On the other hand, fire units responding during peace-time would not encounter large contaminated areas, and a highly-contaminated glove, sponge or sorbent could pose a risk, while contaminated sorbent could be present on the ground and pose an inhalation risk. In order to avoid transferring contamination from place to place, fire units should be equipped with materials/methods for the decontamination of hazardous material and address disposal of contaminated wastes. Among the material/methods tested, only the improvised Czech fire-fighter INDEHA kit ensured safe waste disposal, as contaminated wipes were collected and treated with solid sodium hydroxide.
2.6. Overall Evaluation
A comparison of individual parameters and evaluations are given in
Table 5. The various material/methods are briefly evaluated as follows:
Desprach Sorbent was highly efficient against CWA and of medium efficiency against other dangerous substances. The material was easy to use, though difficulties arose in the decontamination of vertical surfaces, as fine sorbent particles containing the contaminant fell to the ground and could be carried into the air, posing an inhalation hazard. Waste toxicity was a crucial disadvantage. The sorbent could also be used for the decontamination of exposed skin.
FAST-ACT Sorbent had good decontamination efficiency. Its main advantage (compared to Desprach) was that it decomposed the absorbed CWA, so toxic waste was not produced. It was provided in a plastic bottle containing 0.5 kg sorbent, which allowed for multiple applications. A disadvantage was that it could only be used on horizontal surfaces.
The FAST-ACT decontamination glove contained the above-mentioned sorbent. However, compared to the loose sorbent, the glove had a low decontamination efficiency and did not decompose CWA. The glove could be used for the decontamination of vertical areas and ceilings, avoiding the disadvantage of the loose sorbent. Sufficient surface contact between the glove and contaminated surface was difficult to ensure. Waste analysis indicated that the soaked fabric did not transfer the contaminant to the sorbent, and the surface of the glove remained contaminated.
The RSDL decontamination sponge was an efficient device for decontamination of exposed skin and had favourable characteristics (low weight, small size, easy to apply, high efficiency). After wiping the surface contaminated with CWA, the contaminant was decomposed. With other dangerous substances, it provided a physical device of decontamination due to the use of an organic solvent. Despite the advantages, dangerous waste was still produced. The primary disadvantage was price, especially when the 3-year expiration period was considered.
The prototype Hvezda foam applicator was effective for the decontamination of CWA. A negative feature was that the foam damaged the paint applied to the steel plate. The applicator is not commercially available at this time.
The improvised Czech fire-fighter INDEHA kit, based on wiping with ethanol, had the least disadvantages and was also the most efficient device for decontamination of all contaminants tested. The procedure was the least expensive, could be used at temperatures well below 0 °C and was the only method to address the disposal of dangerous wastes.
3. Experimental
3.1. Surface Contamination
Comparison of decontamination efficiencies was carried for the following CWA and toxic industrial substances:
O-ethyl-S-(diisopropylaminoethyl)methylthiophosphonate (VX), VOZ 072 Zemianske Kostolany, Slovakia, 83% purity (potentiometric thiomercurymetric method using a sulfide ion-selective electrode); bis(2-chlorethyl)sulfide (sulfur mustard), VOZ 072 Zemianske Kostolany, Slovakia, 96% purity by potentiometric argentometric method using a sulfide ion-selective electrode; o-cresol, Lachema, Brno, CR, declared purity of >96%; and acrylonitrile, for analysis, PCK, Germany, declared purity of >98%.
The test surfaces were steel plates (100 mm × 100 mm × 1 mm) painted on one side with Universal gloss (Balakom, Jablunkov, Slovakia) exterior alkyd paint (paint age of at least 1 year) and chemical protective suit material, type SOO CO (Makyta, Puchov, Slovakia), 100 mm × 100 mm and seamless (the protective layer was a butyl rubber mixture and the carrier fabric was polyester).
The test surfaces were contaminated with drops of pure contaminant in a horizontal position and contamination was performed with an electronic eVol syringe (SGE Analytical Science, Ringwood, Australia).
VX contamination (100 mm × 100 mm), 25 drops (1.0 μL each), which represented (after conversion based on the weight of pure VX) a contamination of 2.1 g/m2. Sulfur mustard, 41 drops (2.0 μL each), corresponding (after conversion) to an initial contamination density of 10 g/m2. Contamination of surfaces by o-cresol was performed using 48 drops (10 μL each) corresponding to a contamination density of 50 g/m2. Acrylonitrile contamination used 62 drops (10 μL each), which corresponded to an initial contamination of 50 g/m2.
The samples were exposed to contaminants for 5 min in a horizontal position. For each surface, 10 replicates of the contaminant and decontamination procedure were performed.
3.2. Performance of Decontamination
All commercial decontamination materials/methods were applied/performed as described in the literature/manual provided, and test surfaces were decontaminated in a horizontal position. The specified decontamination procedures were also performed on uncontaminated surfaces (blank tests).
Desprach (Leciva, Prague, CR, batch numbers 030388 and 111190) squeeze-bottles were used, the entire contaminated surface (100 mm × 100 mm) was covered with sorbent and wiped for 30 s with cellulose wipes (50 mm × 50 mm). The Desprach was removed by shaking and any adhering material removed with a wipe. For surfaces contaminated with o-cresol and acrylonitrile at a greater contamination density, large drops were initially removed with dry cellulose wipes prior to treatment.
FAST-ACT decontamination nano-sorbent (NanoScale Corp., Manhattan, NY, USA, batch number 15-0192, exp. 10/2017) was applied from the bottle containing 0.5 kg of sorbent. The contaminated surface was sprayed with sorbent to a depth of 3 mm and after 3 min of sorption (when the decontamination effect was maximal [
7]), sorbent was removed from the surface by shaking. The FAST-ACT decontamination glove (RSDecon, Princeton, NJ, USA, batch number 15-0178, exp. 07/2017) was removed from the cover, placed on the hand and fixed around the wrist. The contaminated 100 mm × 100 mm area was wiped until the surface appeared clean, which required ~30 s.
The RSDL decontamination sponge (E-Z-EM Canada Inc., Quebec, QC, Canada, batch number 60827, exp. 02/2015) was used to wipe the surface for 30 s, and the surface (100 mm × 100 mm) was rinsed after 2 min with 50 mL water.
The Hvezda foam two-chamber applicator prototype was provided by Decomkov (Prague, Czech Republic). Just prior to application, both components were mixed in the applicator, shaken and the foam applied to a depth of 2 mm. Decontamination time was 5 min, and the foam was rinsed with 50 mL water.
For decontamination with the improvised INDEHA kit, ethanol-saturated gauze wipes (75 mm × 75 mm) were used. Initially, contaminant drops were removed with gauze wipes and another gauze wipe impregnated with ~3 mL of alcohol was used to wipe the surface. The procedure was repeated with 2 additional wipes. The test surface (100 mm × 100 mm) was cleaned with 4 wipes for 7 s each.
3.3. Sampling and Preparation of Surface Samples
Sampling of the painted steel surface was performed with a wiping technique, using three cotton wool wipes, two dipped in solvent and the third dry. The cotton wool wipes were placed into Erlenmeyer flasks (250 mL), 25 mL of the relevant solvent was added, the opening sealed with a ground-glass stopper and the flasks were shaken for 30 min. The protective suit material (100 mm × 100 mm) was place in the flask (250 mL), 25 mL of the appropriate solvent was added, the neck sealed with a ground-glass stopper and the sample was shaken for 30 min. Methanol was used as the solvent for VX extraction, ethanol for the extraction of sulfur mustard, 0.3 mol/L sodium hydroxide for the extraction of o-cresol and 10% sodium hydroxide for the extraction of acrylonitrile. Solvents and chemicals used for extractions were at least of analysis purity level (Merck, Darmstadt, Germany). Samples were extracted on a laboratory Orbi-Shaker (Benchmark Scientific, South Plainfield, NJ, USA). Volumes of the extraction solution were diluted with the appropriate solvent and contaminant content was analysed, while similar extractions and analyses were performed with uncontaminated samples (blank tests).
3.5. Determination of Sulfur Mustard
Sulfur mustard was reacted with alkaline thymolphthalein, where the esterified carboxylic acid was converted to a colourless lactone in an acid environment. After acidification of the reaction mixture, the presence of sulfur mustard was indicated by a yellow or orange colour (in the absence of sulfur mustard, the solution remained colourless) and the colour intensity was proportional to the concentration of sulfur mustard in the solution. Ethanol extract (2 mL) was added to 2 mL reagent (2.0 g thymolphtalein in 75 mL ethanol, 12.5 mL of 1 mol/L sodium hydroxide added and ethanol to a final volume of 100 mL), mixed and heated for 20 min in a 77 °C water bath. The sample was cooled, 1 drop of acetic acid (99.8%) was added, mixed and the absorbance was measured at 448 nm against the blank. All chemicals were of high purity (Merck, Darmstadt, Germany). The concentration of sulfur mustard in the extract was determined based on standards of known concentration (linear at a 1–30 mg/L concentration range). The residual surface contamination by sulfur mustard was reported in mg/m2.
3.6. Determination of o-cresol
Extracts (in 0.3 mol/L sodium hydroxide) were measured at 286 nm, where the maximal absorbance of o-cresolate was observed. Extracts (or dilutions in 0.3 mol/L sodium hydroxide) were measured in a 1.00 cm quartz cuvette at 250–500 nm and a reference wavelength of 500 nm. The concentrations of o-cresol in the extracts (mg/m2) were determined by comparison to standards of known o-cresol concentrations (1–50 mg/L at 286 nm).
3.7. Determination of Acrylonitrile
Acrylonitrile was subjected to alkaline hydrolysis (boiling) and the equivalent amount of ammonia present was determined by reaction with Nessler’s reagent (aqueous solution of 30 mml/L mercuric iodide, 42 mmol/L potassium iodide and 4 mol/L sodium hydroxide), yielding a yellow colour. All chemicals were of for analysis purity (Merck, Darmstadt, Germany). Extracts (5 mL, in 10% sodium hydroxide) were sealed in screw-capped tubes, heated for 10 min in boiling water, cooled and 0.1 mL Nessler’s reagent added. Absorption was measured at 380–500 nm in a 1.00 cm glass cuvette against a reference wavelength of 800 nm. Acrylonitrile in the extract was determined by comparison to solutions of known concentrations (0.1–5 mg/L) at 400 nm and residual surface contamination reported as mg/m2.
3.8. Surface Contamination
Residual surface contamination levels were statistically evaluated using the EffiValidation 3.0 (CR) software [
19]. The Grubbs test, Grubbs pair test and Dixon test were used for the determination of statistically remote data. Based on the results of these tests, no results could be excluded, and further data analyses were based on 10 replicates. Average surface residual contamination (mg/m
2) and relative repeatability (relative standard deviation in %) values were calculated, which indicated the reproducibility and reliability of the decontamination procedures. The device efficiencies for individual decontamination methods were further expressed as percent decontamination efficiencies, which were calculated as the difference between initial and residual contamination divided by the initial contamination multiplied by 100.
3.9. Analysis of Contaminants in Waste after Decontamination
After decontamination, materials (sorbents, sponges, wipes, gloves) were hermetically sealed in vials for 24 h and analysed by gas chromatography with mass detection (GC/MS) using the head-space technique after solid phase micro-extraction (SPME, Carboxen/Polydimethysiloxane fibre, Supelco, Bellefonte, PA, USA). Vials containing the samples were heated in an 80 °C water-bath for 30 min, the septum perforated by the SPME holder and the fibre (previously conditioned at 300 °C for one hour) injected into the vial. The period of sorption took 5 min without ejecting the vial from the thermostat. The fibre was loaded into the injector port of the GC/MSD 7890/5975C (Agilent Technologies, Inc., Wilmington, DE, USA) equipped with a HP-5MS column (Agilent 19091SA-433, 30 m length, ø of 250 μm, phase 0.25 μm) with the following parameters: 1.2 mL/min He carrier gas, 290 °C T inlet, 290 °C T interface GC/MSD, scan range 35–800 amu, 10:1 split; GC program: 40 °C for 2 min, from 40 °C to 280 °C at 10 °C/min and 280 °C for 10 min.