Overview of the Current State-of-the-Art for Bioaccumulation Models in Marine Mammals
Abstract
:1. Introduction
2. Available Modeling Software
3. Availability of Data or Parameters Required for Model Development and Evaluation
3.1. Data(sets) and Parameters
| Species | Chemical(s) | Model Type | Input | Absorption | Distribution | Metabolism | Elimination * | Statistical Model | Software | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Bottlenose dolphins (Tursiops truncatus) | ∑ 22 PCBs | One compartment PK or TK | Model fitted to specific dataset | R | [7] | |||||
| Killer whales (Orcinus orca) | ∑ 46 PCBs ∑ 9 PBDEs | One compartment PK or TK | Energy-based | 100% | Presumably diffusion limited | 1%–3% of total body burden goes to metabolic biotransformation, urinary and fecal excretion | Local SA, parameter values randomly drawn from ranges, method not specified | R | [15] | |
| Beluga whales (Delphinapterus leucas) | ∑ 64 PCBs | PBPK or PBTK | Energy-based | 80% for food 90% for milk | Flow limited | Half-life of 28 years | Fixed proportion of the intake rate | Not present | BASIC + upgrade to Visual Basic Applications (Excel) | [12] |
| Beluga whales (Delphinapterus leucas) | PCBs | PBPK or PBTK | Energy-based | 80% for food 90% for milk | Flow limited | Half-life of 12 years | Not present | BASIC | [13] | |
| Arctic ringed seals (Phoca hispida) | Several POPs (individual + sums) | PBPK or PBTK | Energy-based | 90% for food 90% for milk | Flow limited | First-order elimination rate constant for feces, biotransformation and respiration | Not present | BASIC + upgrade to Visual Basic Applications (Excel) | [14] | |
| Killer whales (Orcinus orca) | ∑ PCBs | PBPK or PBTK | Energy-based | 90% | Flow limited | First-order elimination rate constant for feces, biotransformation and urine | Not present | BASIC + upgrade to Visual Basic Applications (Excel) | [8] | |
| Bottlenose dolphins (Tursiops truncatus) | ∑ PCBs | PBPK or PBTK | Energy-based | 90% | Flow limited | Elimination rate constants for feces, biotransformation urine and respiration | SA, statistical model not present | BASIC + upgrade to Visual Basic Applications (Excel) | [9] | |
| North Atlantic right whales (Eubalaena glacialis) | PCBs | Multi-compartment PK or TK | Energy-based | Energy (lipid)-based | Flow limited | First-order kinetics for excretion and metabolism | None | MATLAB | [16] | |
| Harbour porpoises (Phocoena phocoena) | PCB 153 | PBPK or PBTK | Mass-based | 90% | Flow limited | Metabolic half-life of 27.5 years | Excretion through feces was fitted to data | Local SA, statistical model not present | Berkeley Madonna | [17] |
| Harbour porpoises (Phocoena phocoena) | PCB 180, 101, 149, 118, 99, 170 | PBPK or PBTK | Mass-based | Fitted; compound-specific | Flow limited | Fitted; Congener specific elimination half-lives for metabolic biotransformation and feces | Not present | Berkeley Madonna | [18] | |
| Harbour porpoises (Phocoena phocoena) | PBDE 47, 99, 100, 153 | PBPK or PBTK | Mass-based | Fitted; compound-specific | Flow limited | Fitted; Congener specific elimination half-lives for metabolic biotransformation and feces | Local SA, statistical model not present | Berkeley Madonna | [19] | |
| Harbour porpoises (Phocoena phocoena) | p,p’-DDT, p,p’-DDE and p,p’-DDD | PBPK or PBTK | Mass-based | Estimated through MCMC simulations | Flow limited | Elimination half-lives for metabolic biotransformation and feces were estimated through MCMC simulations | Global SA, Bayesian approach, MCMC simulations for parameter estimations | AcslX/Libero | [20] | |
| Long-finned pilot whales (Globicephala melas) | PCB 153 | PBPK or PBTK | Mass-based | 90% | Flow limited | Elimination half-life for metabolic biotransformation, urine and feces of 27.5 years | Global SA, Bayesian approach, MCMC simulations for parameter estimations | Berkeley Madonna AcslX/Libero MCSim | [21] | |
| Harbour seals (Phoca vitulina) | PCBs | PBPK or PBTK | Mass-based | Not specified | Diffusion limited | Congener specific metabolic transformation rate constants | Rate constants for exhalation, feces, urine | Local SA, Monte Carlo simulations | Microsoft Excel 2000 and add-in Crystal Ball | [22] |
3.2. Parameters for Absorption, Distribution, Metabolic Biotransformation and Excretion
3.2.1. Absorption
3.2.2. Distribution
3.2.3. Metabolic Biotransformation
3.2.4. Excretion
4. Bioaccumulation Models: Structure and Equations
4.1. Structure


4.2. Equations
5. Bioaccumulation Models: Statistical Supplementation
 ) and simple Monte Carlo sampling (
) and simple Monte Carlo sampling (  ). Y-axis represents the parameter values (θ), X-axis represents the number of iterations (parameter drawings or model runs). Figure adjusted from Bernillon and Bois [53].
). Y-axis represents the parameter values (θ), X-axis represents the number of iterations (parameter drawings or model runs). Figure adjusted from Bernillon and Bois [53].
 ) and simple Monte Carlo sampling (
) and simple Monte Carlo sampling (  ). Y-axis represents the parameter values (θ), X-axis represents the number of iterations (parameter drawings or model runs). Figure adjusted from Bernillon and Bois [53].
). Y-axis represents the parameter values (θ), X-axis represents the number of iterations (parameter drawings or model runs). Figure adjusted from Bernillon and Bois [53].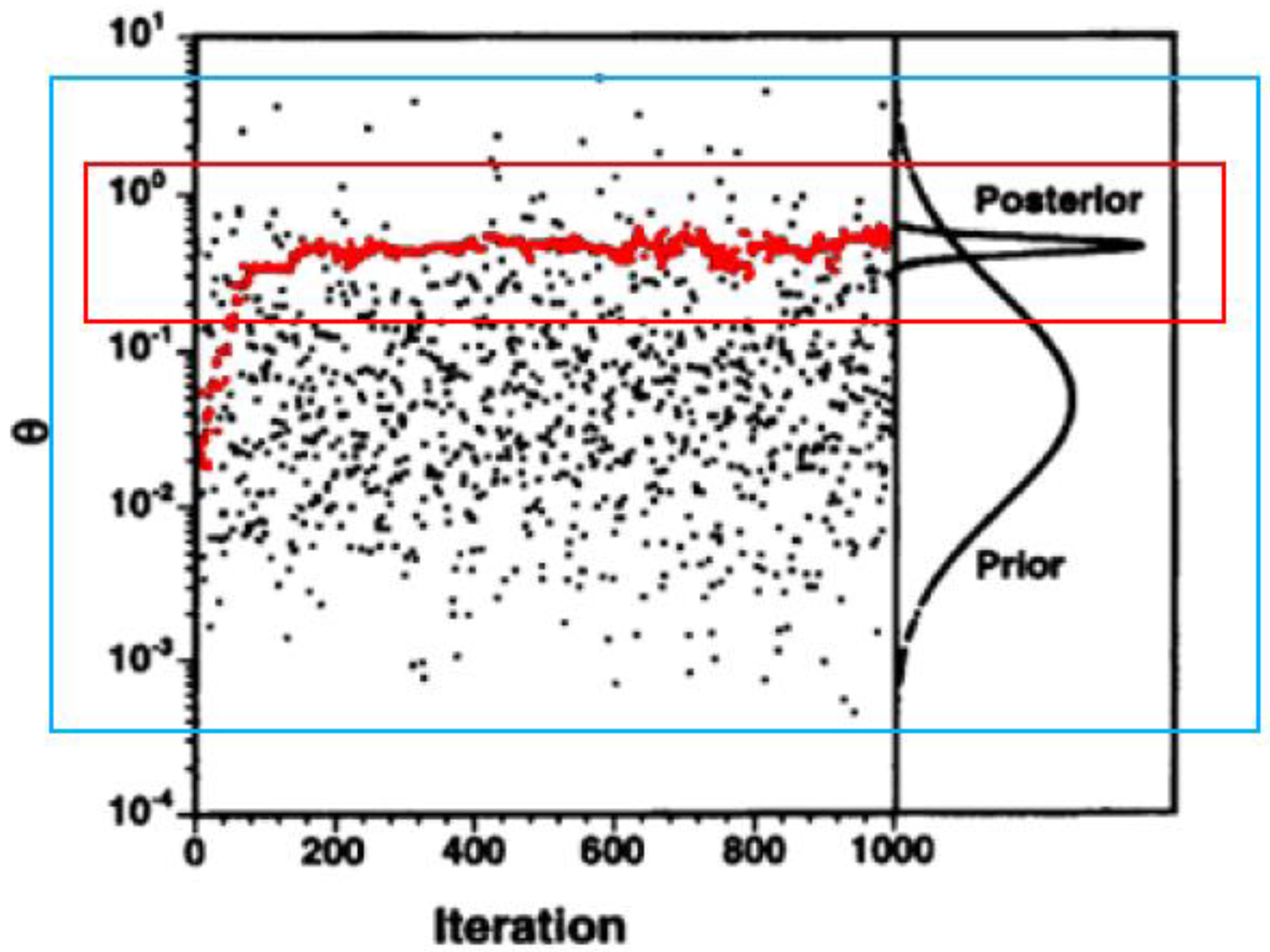
6. Concluding Remarks and Future Directions
6.1. Adding more Datasets
6.2. Adding more Compartments
6.3. Coupling to an Effects or Dynamic Model
6.4. Extrapolating to Other Species/Chemicals
Acknowledgments
Conflicts of Interest
References
- Timchalk, C.; Nolan, R.J.; Mendrala, A.L.; Dittenber, D.A.; Brzak, K.A.; Mattson, J.L. A physiologically based pharmacokinetic and pharmacodynamics (PBPK/PD) model for the organophosphate insecticide chlorpyrifos in rats and humans. Toxicol. Sci. 2002, 66, 34–53. [Google Scholar] [CrossRef]
- Ling, M.P.; Liao, C.M. A human PBPK/PD model to assess arsenic exposure risk through farmed tilapia consumption. Bull. Environ. Contam. Toxicol. 2009, 83, 108–114. [Google Scholar] [CrossRef]
- Liao, C.M.; Liang, H.M.; Chen, B.C.; Singh, S.; Tsai, J.W.; Chou, Y.H.; Lin, W.T. Dynamical coupling of PBPK/PD and AUC-based toxicity models for arsenic in tilapia Oreochromis mossambicus from blackfoot disease area in Taiwan. Environ. Pollut. 2005, 135, 221–233. [Google Scholar] [CrossRef]
- Abbas, R.; Hayton, W.L. A physiologically based pharmacokinetic and pharmacodynamics model for paraoxon in rainbow trout. Toxicol. Appl. Pharmacol. 1997, 145, 192–201. [Google Scholar] [CrossRef]
- Clewell, H.J., III; Andersen, M.E. Use of physiologically based pharmacokinetic modeling to investigate individual versus population risk. Toxicology 1996, 111, 315–329. [Google Scholar] [CrossRef]
- Lipscomb, J.C.; Haddad, S.; Poet, T.; Krishnan, K. Physiologically-Based Pharmacokinetic (PBPK) Models in Toxicity Testing and Risk Assessment. In New Technologies for Toxicity Testing; Balls, M., Combes, R.D., Bhogal, N., Eds.; Springer US: New York, NY, USA, 2012; pp. 76–95. [Google Scholar]
- Hall, A.J.; McConnell, B.J.; Rowles, T.K.; Aguilar, A.; Borrell, A.; Schwacke, L.; Reijnders, P.J.; Wells, R.S. Individual-based model framework to assess population consequences of polychlorinated biphenyl exposure in bottlenose dolphins. Environ. Health Perspect. 2006, 114, 60–64. [Google Scholar]
- Hickie, B.E.; Ross, P.S.; MacDonald, R.W.; Ford, J.K.B. Killer whales (Orcinus orca) face protracted health risks associated with lifetime exposure to PCBs. Environ. Sci. Technol. 2007, 41, 6613–6619. [Google Scholar] [CrossRef]
- Hickie, B.E.; Cadieux, M.A.; Riehl, K.N.; Bossart, G.D.; Alava, J.J.; Fair, P.A. Modeling PCB-bioaccumulation in the bottlenose dolphin (Tursiops truncatus): Estimating a dietary threshold concentration. Environ. Sci. Technol. 2013, 47, 12314–12324. [Google Scholar] [CrossRef]
- Bouzom, F.; Ball, K.; Perdaems, N.; Walther, B. Physiologically based pharmacokinetic (PBPK) modeling tools: How to fit with our needs? Biopharm. Drug Dispos. 2012, 33, 55–71. [Google Scholar] [CrossRef]
- Schmitt, W.; Willmann, S. Physiologically-based pharmacokinetic modeling: Ready to be used. Drug Discov. Today 2005, 2, 125–132. [Google Scholar] [CrossRef]
- Hickie, B.E.; Mackay, D.; de Koning, J. Lifetime pharmacokinetic model for hydrophobic contaminants in marine mammals. Environ. Toxicol. Chem. 1999, 18, 2622–2633. [Google Scholar] [CrossRef]
- Hickie, B.E.; Kingsley, M.C.S.; Hodson, P.V.; Muir, D.C.G.; Béland, P.; Mackay, D. A modeling-based perspective on the past, present, and future polychlorinated biphenyl contamination of the St. Lawrence beluga whale (Delphinapterus leucas ) population. Can. J. Fish. Aquat. Sci. 2000, 57, 101–112. [Google Scholar] [CrossRef]
- Hickie, B.E.; Muir, D.C.G.; Addison, R.F.; Hoekstra, P.F. Development and application of bioaccumulation models to assess persistent organic pollutant temporal trends in arctic ringed seal (Phoca vitulina) populations. Sci. Total Environ. 2005, 351–352, 413–426. [Google Scholar]
- Mongillo, T.M.; Holmes, E.E.; Noren, D.P.; VanBlaricom, G.R.; Punt, A.E.; O’Neill, S.M.; Ylitalo, G.; Hanson, M.B.; Ross, P.S. Predicted polybrominated diphenyl ether (PBDE) and polychlorinated biphenyl (PCB) accumulation in southern resident killer whales. Mar. Ecol. Prog. Ser. 2012, 453, 263–277. [Google Scholar] [CrossRef]
- Klanjscek, T.; Nisbet, R.M.; Caswell, H.; Neubert, M.G. A model for energetics and bioaccumulation in marine mammals with applications to the right whale. Ecol. Appl. 2007, 17, 2233–2250. [Google Scholar] [CrossRef]
- Weijs, L.; Yang, R.S.H.; Covaci, A.; Das, K.; Blust, R. Physiologically based pharmacokinetic models for lifetime exposure to PCB 153 in male and female harbour porpoises (Phocoena phocoena): Model development and validation. Environ. Sci. Technol. 2010, 44, 7023–7030. [Google Scholar] [CrossRef]
- Weijs, L.; Covaci, A.; Yang, R.S.H.; Das, K.; Blust, R. A non-invasive approach to study lifetime exposure and bioaccumulation of PCBs in protected marine mammals: PBPK modeling in harbour porpoises. Toxicol. Appl. Pharmacol. 2011, 256, 136–145. [Google Scholar] [CrossRef]
- Weijs, L.; Covaci, A.; Yang, R.S.H.; Das, K.; Blust, R. Computational toxicology: Physiologically based pharmacokinetic models (PBPK) for lifetime exposure and bioaccumulation of polybrominated diphenyl ethers (PBDEs) in marine mammals. Environ. Pollut. 2012, 163, 134–141. [Google Scholar] [CrossRef]
- Weijs, L.; Yang, R.S.H.; Das, K.; Blust, R.; Covaci, A. Application of Bayesian population PBPK modeling and Markov chain Monte Carlo simulations to pesticide kinetics studies in protected marine mammals: DDT, DDE, DDD in harbour porpoises. Environ. Sci. Technol. 2013, 47, 4365–4374. [Google Scholar] [CrossRef]
- Weijs, L.; Roach, A.C.; Yang, R.S.H.; McDougall, R.; Lyons, M.; Housand, C.; Tibax, D.; Manning, T.M.; Chapman, J.C.; Edge, K.; et al. Lifetime PCB 153 bioaccumulation and pharmacokinetics in pilot whales: Bayesian population PBPK modeling and Markov chain Monte Carlo simulations. Chemosphere 2014, 94, 91–96. [Google Scholar] [CrossRef]
- Gobas, F.; Arnot, J. 2005 San Francisco Bay PCB Food Web Bioaccumulation Model. Final Technical Report. Available online: http://www.bacwa.org/Portals/0/Committees/Clean%20Estuary%20Partnership/Library/Task4.27-FoodWebModel.pdf (accessed on 5 June 2008).
- CRC. Handbook of Marine Mammal Medicine, 2nd ed.; Dierauf, L.A., Gulland, F.M.D., Eds.; CRC Press LCC: Boca Raton, FL, USA, 2001; p. 1120. [Google Scholar]
- Encyclopedia of Marine Mammals, 2nd ed.; William, P.; Bernd, W.; Thewissen, J. (Eds.) Academic Press: Sandiago, CA, USA, 2008; p. 1352.
- Oftedal, O.T. Lactation in whales and dolphins: Evidence of divergence between baleen- and toothed species. J. Mammary Gland Biol. Neoplasia 1997, 2, 205–230. [Google Scholar] [CrossRef]
- Brown, R.P.; Delp, M.D.; Lindstedt, S.L.; Rhomberg, L.R.; Beliles, R.P. Physiological parameter values for physiologically based pharmacokinetic models. Toxicol. Ind. Health 1997, 13, 407–484. [Google Scholar] [CrossRef]
- Noonburg, E.G.; Nisbet, R.M.; Klanjscek, T. Effects of life history variation on vertical transfer of toxicants in marine mammals. J. Theor. Biol. 2010, 264, 479–489. [Google Scholar] [CrossRef]
- Clewell, R.A.; Clewell, H.J., III. Development and specification of physiologically based pharmacokinetic models for use in risk assessment. Regul. Toxicol. Pharmacol. 2008, 50, 129–143. [Google Scholar] [CrossRef]
- Duarte-Davidson, R.; Jones, K.C. Polychlorinated biphenyls (PCBs) in the UK population: Estimated intake, exposure and body burden. Sci. Total Environ. 1994, 151, 131–152. [Google Scholar] [CrossRef]
- Hui, C. Seawater consumption and water flux in the common dolphin Delphinus delphis. Physiol. Zool. 1981, 54, 430–440. [Google Scholar]
- Ortiz, R. Osmoregulation in marine mammals. J. Exp. Biol. 2001, 204, 1831–1844. [Google Scholar]
- Thomas, G.O.; Moss, S.E.W.; Asplund, L.; Hall, A.J. Absorption of decabromodiphenyl ether and other organohalogen chemicals by grey seals (Halichoerus grypus). Environ. Pollut. 2005, 133, 581–586. [Google Scholar] [CrossRef] [Green Version]
- Parham, F.M.; Kohn, M.C.; Matthews, H.B.; DeRosa, C.; Portier, C.J. Using structural information to create physiologically based pharmacokinetic models for all polychlorinated biphenyls. I. Tissue:blood partition coefficients. Toxicol. Appl. Pharmacol. 1997, 144, 340–347. [Google Scholar] [CrossRef]
- Thornton, S.J.; Hochachka, P.W.; Crocker, D.E.; Costa, D.P.; LeBoeuf, B.J.; Spielman, D.M.; Pelc, N.J. Stroke volume and cardiac output in juvenile elephant seals during forced dives. J. Exp. Biol. 2005, 208, 3637–3643. [Google Scholar] [CrossRef]
- Svendsgaard, D.J.; Ward, T.R.; Tilson, H.A.; Kodavanti, P.R.S. Emperical modeling of an in vitro activity of polychlorinated biphenyl congeners and mixtures. Environ. Health Perspect. 1997, 105, 1106–1115. [Google Scholar] [CrossRef]
- Braekevelt, E.; Tittlemier, S.A.; Tomy, G.T. Direct measurement of octanol-water partition coefficients of some environmentally relevant brominated diphenyl ether congeners. Chemosphere 2003, 51, 563–567. [Google Scholar] [CrossRef]
- Hayward, S.J.; Lei, Y.D.; Wania, F. Comparative evaluation of three high-performance liquid Chromatography-based Kow estimation methods for highly hydrophobic organic compounds: Polybrominated diphenyl ethers and hexabromocyclododecane. Environ. Toxicol. Chem. 2006, 25, 2018–2027. [Google Scholar] [CrossRef]
- Letcher, R.J.; Klasson-Wehler, E.; Bergman, A. Methyl Sulfone and Hydroxylated Metabolites of Polychlorinated Biphenyls. In The Handbook of Environmental Chemistry; Paasivirta, J., Ed.; Part K, New Types of Persistent Halogenated Compounds; Springer-Verlag: Heidelberg/Berlin, Germany, 2000; Volume 3. [Google Scholar]
- Redding, L.E.; Sohn, M.D.; McKone, T.E.; Chen, J.W.; Wang, S.L.; Hsieh, D.P.H.; Yang, R.S.H. Population physiologically based pharmacokinetic modeling for the human lactational transfer of PCB 153 with consideration of worldwide human biomonitoring results. Environ. Health Perspect. 2008, 116, 1629–1634. [Google Scholar] [CrossRef]
- Verner, M.A.; Charbonneau, M.; Lopez-Carrillo, L.; Haddad, S. Physiologically based pharmacokinetic modeling of persistent organic pollutants for lifetime exposure assessment: A new tool in breast cancer epidemiologic studies. Environ. Health Perspect. 2008, 116, 886–892. [Google Scholar] [CrossRef]
- Miniero, R.; de Felip, E.; Ferri, F.; di Domenico, A. An overview of TCDD half life in mammals and its correlation to body weight. Chemosphere 2001, 43, 839–844. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry (ATSDR). 2001; Polychlorinated biphenyls. US Department of Health and Human Services. Available online: http://www.atsdr.cdc.gov/toxfaqs/index.asp (accessed 5 June 2008). [Google Scholar]
- Staskal, D.F.; Diliberto, J.J.; DeVito, M.J.; Birnbaum, L.S. Toxicokinetics of BDE 47 in female mice: Effect of dose, rout of exposure, and time. Toxicol. Sci. 2005, 83, 215–223. [Google Scholar]
- Li, H.X.; Boon, J.P.; Lewis, W.E.; van den Berg, M.; Nyman, M.; Letcher, R.J. Hepatic microsomal cytochrome P450 enzyme activity in relation to in vitro metabolism/inhibition of polychlorinated biphenyls and testosterone in Baltic grey seal (Halichoerus grypus). Environ. Toxicol. Chem. 2003, 22, 636–644. [Google Scholar] [CrossRef]
- McKinney, M.A.; de Guise, S.; Martineau, D.; Béland, P.; Arukwe, A.; Letcher, R.J. Biotransformation of polybrominated diphenyl ethers and polychlorinated biphenyls in beluga whale (Delphinapterus leucas) and rat mammalian model using an in vitro hepatic microsomal assay. Aquat. Toxicol. 2006, 77, 87–97. [Google Scholar] [CrossRef]
- Innes, S.; Lavigne, D.M.; Earle, W.M.; Kovacs, K.M. Feeding rates of seals and whales. J. Anim. Ecol. 1987, 56, 115–130. [Google Scholar] [CrossRef]
- Kastelein, R.A. Food Consumption and Growth of Marine Mammals. Ph.D. Dissertation, Landbouwuniversiteit, Wageningen, The Netherlands, 1998; p. 647. [Google Scholar]
- Kastelein, R.A.; Hardeman, J.; Boer, H. Food Consumption and Body Weight of Harbour Porpoises (Phocoena phocoena). In The Biology of the Harbour Porpoise; Read, A.J., Wiepkema, P.R., Nachtigall, P.E., Eds.; De Spil Publishers: Woerden, The Netherlands, 1997; pp. 217–233. [Google Scholar]
- Kastelein, R.A.; Staal, C.; Wiepkema, P.R. Food consumption and body mass of captive harbour seals Phoca vitulina. Aquat. Mamm. 2005, 31, 34–42. [Google Scholar] [CrossRef]
- Lockyer, C. All creatures great and smaller: A study in cetacean life history energetics. J. Mar. Biol. Assoc. UK 2007, 87, 1035–1045. [Google Scholar] [CrossRef]
- Cullon, D.L.; Jeffries, S.J.; Ross, P.S. Persistent organic pollutants in the diet of harbour seals (Phoca vitulina) inhabiting Puget Sound, Washington (USA), and the Strait of Georgia, British Columbia (Canada): A food basket approach. Environ. Toxicol. Chem. 2005, 24, 2562–2572. [Google Scholar] [CrossRef]
- Emond, C.; Raymer, J.H.; Studabaker, W.B.; Garner, C.E.; Birnbaum, L.S. A physiologically based pharmacokinetic model for developmental exposure to BDE-47 in rats. Toxicol. Appl. Pharmacol. 2010, 242, 290–298. [Google Scholar] [CrossRef]
- McNally, K.; Cotton, R.; Loizou, G.D. A workflow for global sensitivity analysis of PBPK models. Front. Pharmacol. 2011, 2, 1–21. [Google Scholar]
- Bernillon, P.; Bois, F.Y. Statistical issues in toxicokinetic modeling: A Bayesian perspective. Environ. Health Perspect. 2000, 108, 883–893. [Google Scholar] [CrossRef]
- Hack, C.E. Bayesian analysis of physiologically based toxicokinetic and toxicodynamic models. Toxicology 2006, 221, 241–248. [Google Scholar] [CrossRef]
- Weijs, L. Development of Physiologically Based Pharmacokinetic Models for the Bioaccumulation of Persistent Organic Pollutants in Marine Mammals. Ph.D. Dissertation, University of Antwerp, Antwerpen, Belgium, May 2013. [Google Scholar]
- Das, K.; Vossen, A.; Tolley, K.; Vikingsson, G.; Thron, K.; Baumgärtner, W.; Siebert, U. Interfollicular fibrosis in the thyroid of the harbour porpoise: An endocrine disruption? Arch. Environ. Contam. Toxicol. 2006, 52, 720–729. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Weijs, L.; Hickie, B.E.; Blust, R.; Covaci, A. Overview of the Current State-of-the-Art for Bioaccumulation Models in Marine Mammals. Toxics 2014, 2, 226-246. https://doi.org/10.3390/toxics2020226
Weijs L, Hickie BE, Blust R, Covaci A. Overview of the Current State-of-the-Art for Bioaccumulation Models in Marine Mammals. Toxics. 2014; 2(2):226-246. https://doi.org/10.3390/toxics2020226
Chicago/Turabian StyleWeijs, Liesbeth, Brendan E. Hickie, Ronny Blust, and Adrian Covaci. 2014. "Overview of the Current State-of-the-Art for Bioaccumulation Models in Marine Mammals" Toxics 2, no. 2: 226-246. https://doi.org/10.3390/toxics2020226






