Ultrastructural and Proteomic Analyses Revealed the Mechanism by Which Foliar Spraying of Se Nanoparticles Alleviated the Toxicity of Microplastics in Pistia stratiotes L.
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Plant Materials
2.2. Experimental Materials and Experimental Design
2.3. Dose Selection and Environmental Relevance
2.4. Physiological and Bioinformatic Analyses
2.4.1. Measurement of Physiological Traits
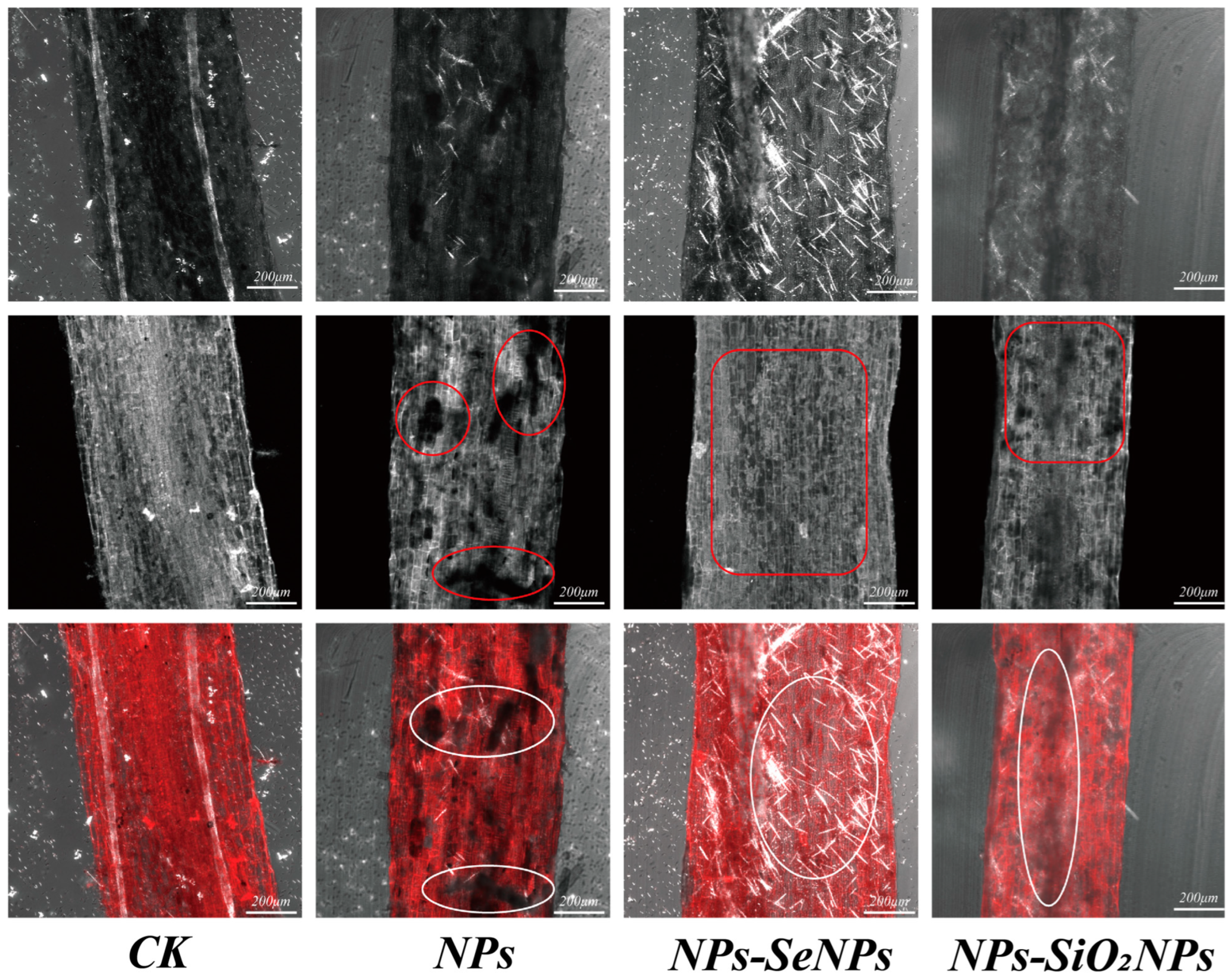
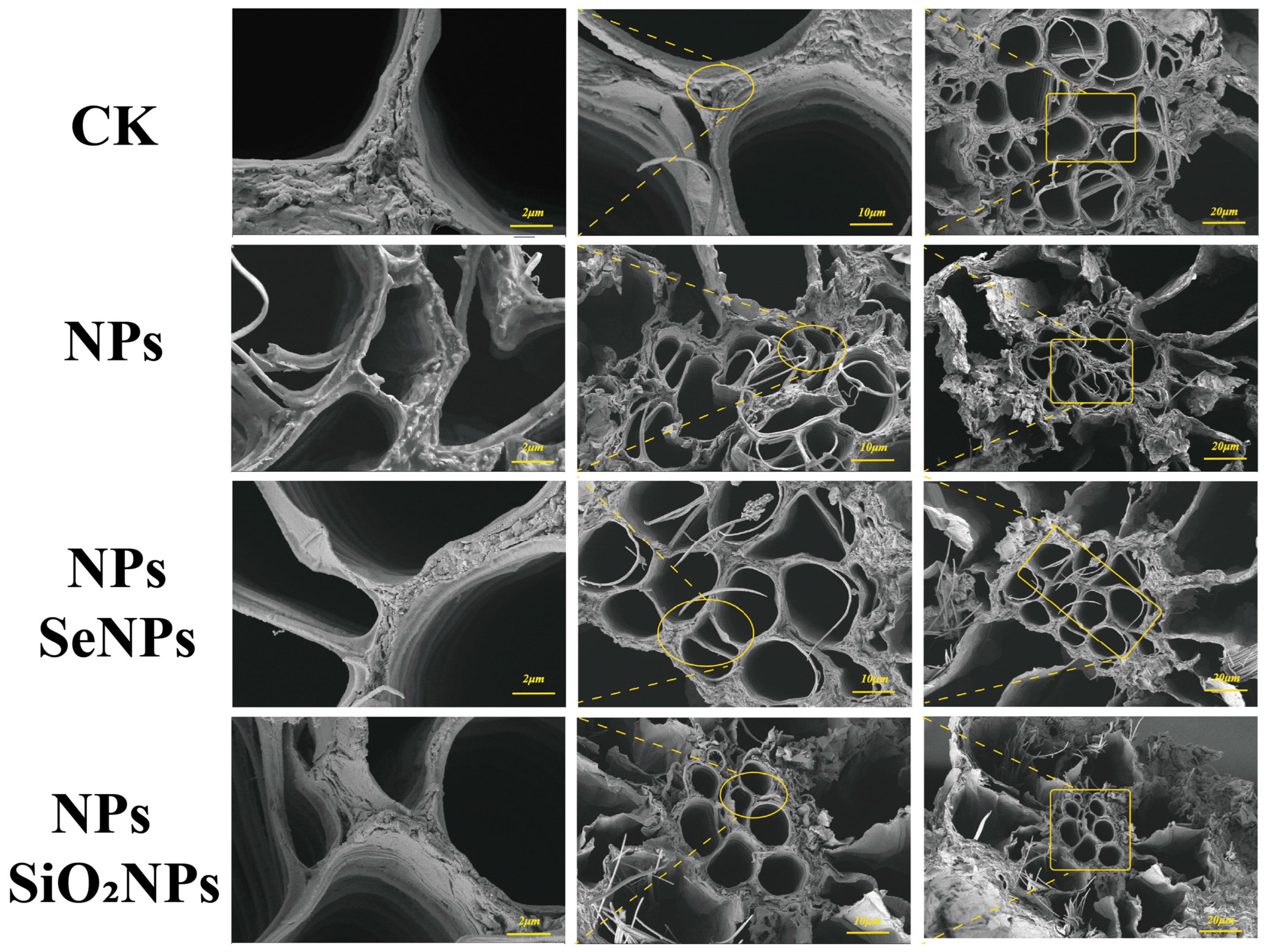
2.4.2. SEM Analysis
2.4.3. LSCM Analysis
2.5. Proteome Profiling
2.6. Statistical Analysis
2.7. Research Consent Statement
3. Results and Discussion
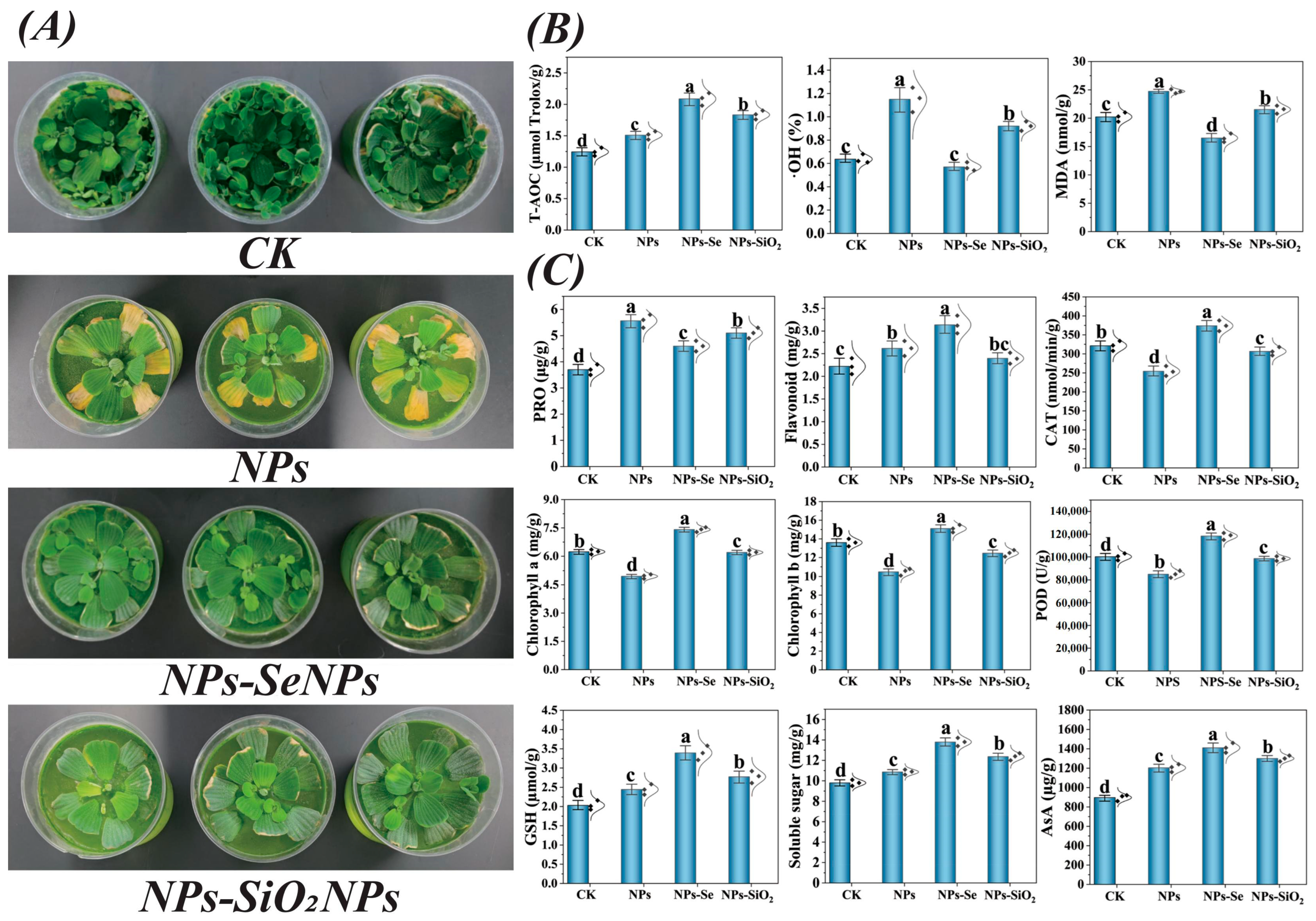
3.1. Plant Morphology Under Different Treatments
3.2. SeNPs Enhance the Antioxidant Enzyme Activities of P. stratiotes
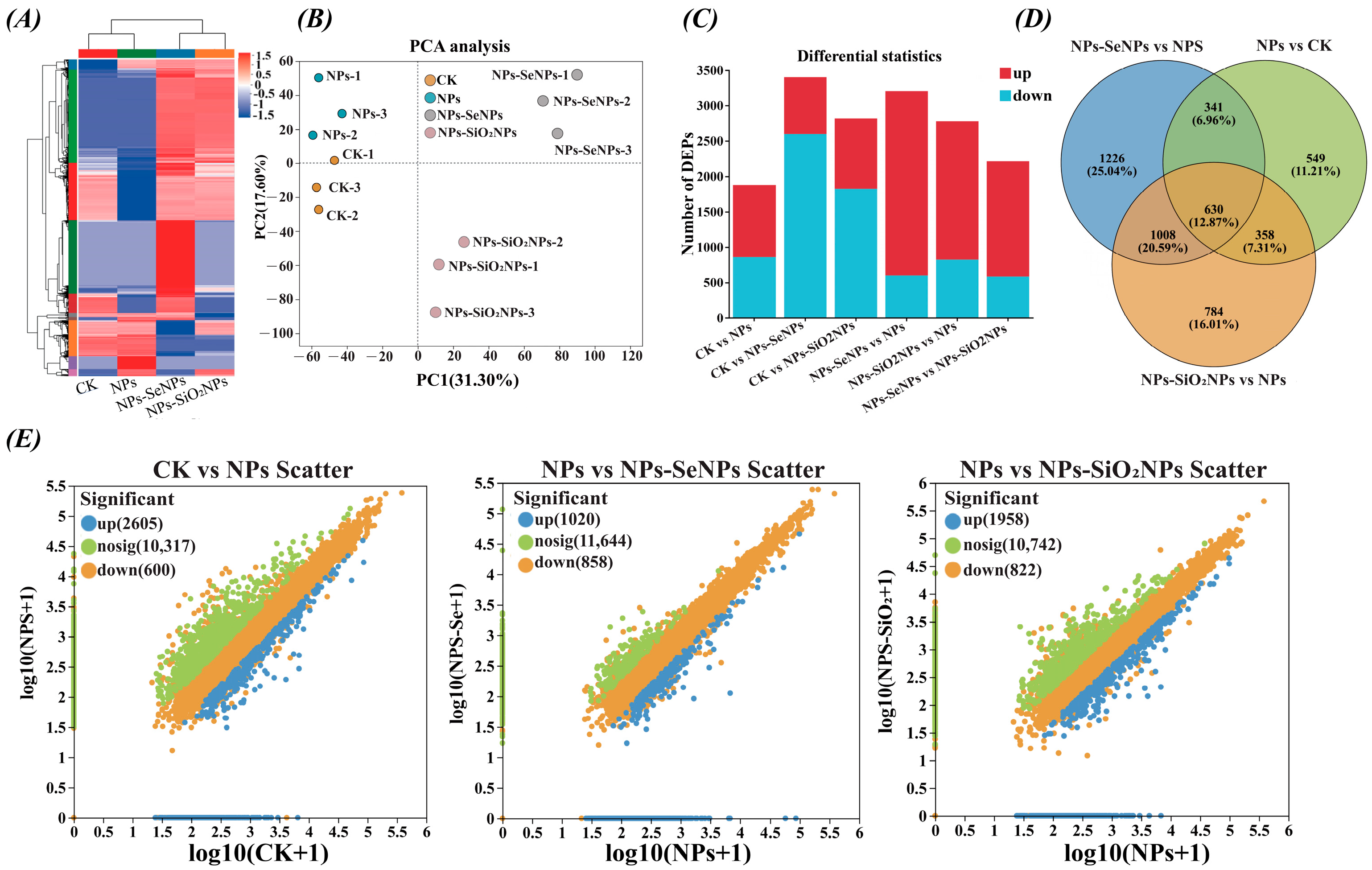
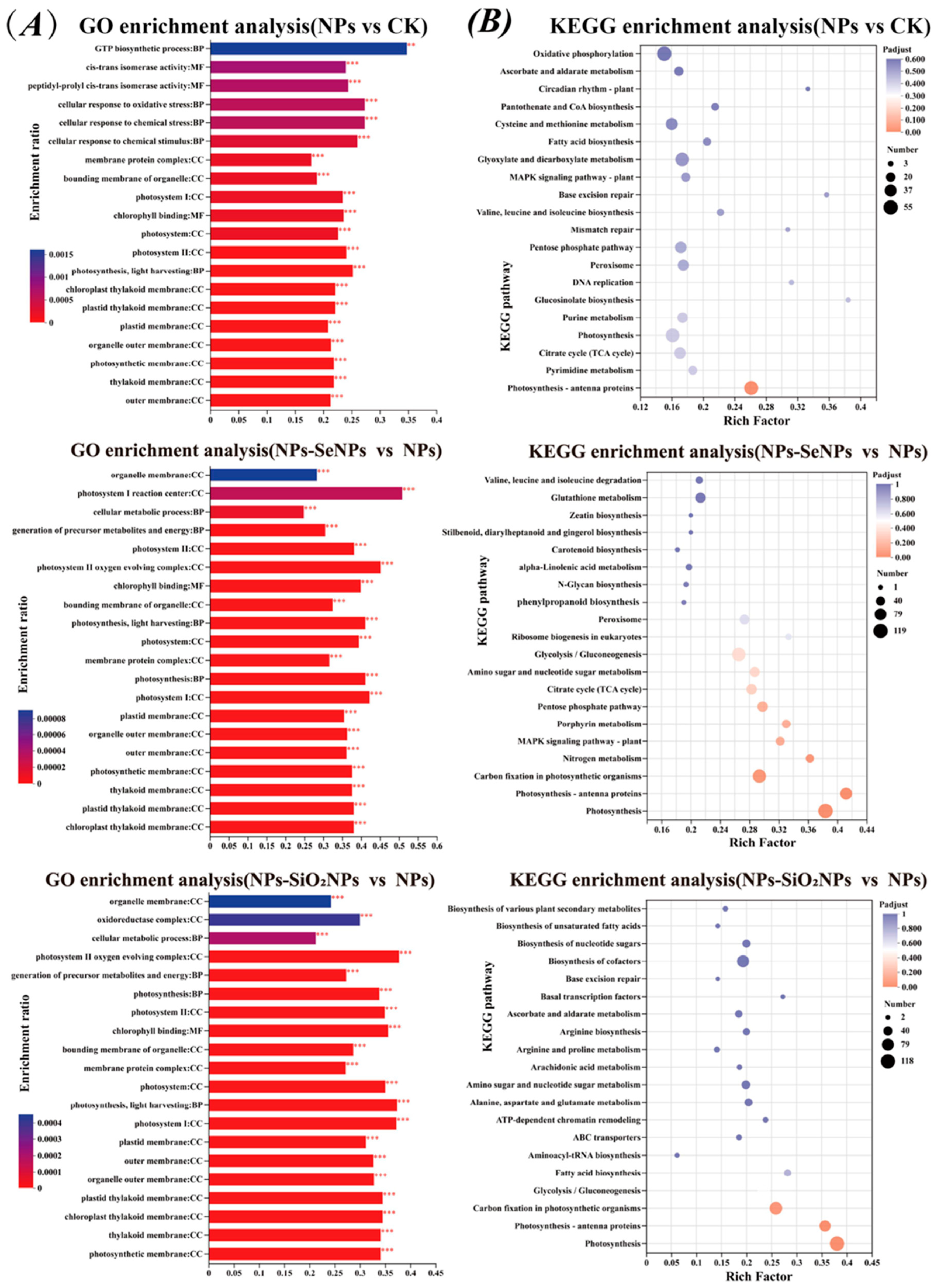
3.3. Protein Expression Profiling and Functional Enrichment Analysis
3.4. Protein Regulation and Conformational Activation of Key Defense Mechanisms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, Use, and Fate of All Plastics Ever Made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C.; Lehmann, A. Microplastics in Terrestrial Ecosystems. Science 2020, 368, 1430–1431. [Google Scholar] [CrossRef]
- Sun, Y.Z.; Ji, J.H.; Tao, J.G.; Yang, Y.Y.; Wu, D.; Han, L.F.; Li, S.; Wang, J. Current Advances in Interactions between Microplastics and Dissolved Organic Matter in Aquatic and Terrestrial Ecosystems. TrAC Trends Anal. Chem. 2023, 158, 116882. [Google Scholar] [CrossRef]
- He, P.; Chen, L.; Shao, L.; Zhang, H.; Lü, F. Municipal Solid Waste (MSW) Landfill: A Source of Microplastics?—Evidence of Microplastics in Landfill Leachate. Water Res. 2019, 159, 38–45. [Google Scholar] [CrossRef]
- Carr, S.A.; Liu, J.; Tesoro, A.G. Transport and Fate of Microplastic Particles in Wastewater Treatment Plants. Water Res. 2016, 91, 174–182. [Google Scholar] [CrossRef]
- Vivekanand, A.C.; Mohapatra, S.; Tyagi, V.K. Microplastics in aquatic environment: Challenges and perspectives. Chemosphere 2021, 282, 131151. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Liu, W.; Lian, Y.; Wang, Q.; Zeb, A.; Tang, J. Phytotoxicity of polystyrene, polyethylene, and polypropylene microplastics on tomato (Lycopersicon esculentum L.). J. Environ. Manag. 2022, 317, 115441. [Google Scholar] [CrossRef]
- Lian, J.; Liu, W.; Sun, Y.; Men, S.; Wu, J.; Zeb, A.; Yang, T.; Ma, L.Q.; Zhou, Q. Nanotoxicological effects and transcriptome mechanisms of wheat (Triticum aestivum L.) under stress of polystyrene nanoplastics. J. Hazard. Mater. 2022, 423, 127241. [Google Scholar] [CrossRef]
- Zhuang, H.; Qin, M.; Liu, B.; Li, R.; Li, Z. Combination of transcriptomics, metabolomics, and physiological traits reveals the effects of polystyrene microplastics on photosynthesis, carbon, and nitrogen metabolism in cucumber (Cucumis sativus L.). Plant Physiol. Biochem. 2023, 205, 108201. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Yuan, X.; Jia, Y.; Feng, L.; Zhu, F.; Dong, S.; Liu, J.; Kong, X.; Tian, H.; Duan, J.; et al. Differentially Charged Nanoplastics Demonstrate Distinct Accumulation in Arabidopsis thaliana. Nat. Nanotechnol. 2020, 15, 755–760. [Google Scholar] [CrossRef]
- Zhou, C.; Lu, C.; Mai, L.; Bao, L.; Liu, L.; Zeng, E. Response of rice (Oryza sativa L.) roots to nanoplastic treatment at the seedling stage. J. Hazard. Mater. 2021, 401, 123412. [Google Scholar] [CrossRef]
- Tang, N.; Li, X.; Gao, X.; Liu, X.; Xing, W. The adsorption of arsenic on micro- and nano-plastics intensifies the toxic effect on submerged macrophytes. Environ. Pollut. 2022, 311, 119896. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, H.; Liu, M.; Yu, H.; Peng, J.; Cao, X.; Wang, C.; Liu, R.; Kamali, M.; Qu, J. Press Perturbations of Microplastics and Antibiotics on Freshwater Micro-Ecosystem: Case Study for the Ecological Restoration of Submerged Plants. Water Res. 2022, 226, 119248. [Google Scholar] [CrossRef] [PubMed]
- Casella, C.; Ballaz, S.J. Genotoxic and Neurotoxic Potential of Intracellular Nanoplastics: A Review. J. Appl. Toxicol. 2024, 44, 1657–1678. [Google Scholar] [CrossRef]
- Reich, H.J.; Hondal, R.J. Why nature chose selenium. ACS Chem. Biol. 2016, 11, 821–841. [Google Scholar] [CrossRef]
- Tolu, J.; Bouchet, S.; Helfenstein, J.; Hausheer, O.; Chekifi, S.; Frossard, E.; Tamburini, F.; Chadwick, O.A.; Winkel, L.H.E. Understanding soil selenium accumulation and bioavailability through size-resolved and elemental characterization of soil extracts. Nat. Commun. 2022, 13, 6974. [Google Scholar] [CrossRef]
- Cheng, B.; Wang, C.; Chen, F.; Yue, L.; Cao, X.; Liu, X.; Yao, Y.; Wang, Z.; Xing, B. Multiomics understanding of improved quality in cherry radish (Raphanus sativus L. var. Radculus pers) after foliar application of selenium nanomaterials. Sci. Total Environ. 2022, 824, 153712. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, Y.X.; Ge, C.H.; Jing, F.; Wu, S.; Li, H.B.; Zhou, D.M. Foliar selenium nanoparticles application promotes the growth of maize (Zea mays L.) seedlings by regulating carbon, nitrogen, and oxidative stress metabolism. Sci. Hortic. 2023, 311, 111816. [Google Scholar] [CrossRef]
- Zhu, S.; Sun, S.; Zhao, W.; Yang, X.; Mao, H.; Sheng, L.; Chen, Z. Utilizing transcriptomics and proteomics to unravel key genes and proteins of Oryza sativa seedlings mediated by selenium in response to cadmium stress. BMC Plant Biol. 2024, 24, 360. [Google Scholar] [CrossRef] [PubMed]
- Schiavon, M.; Pilon-Smits, E.A.H. The Fascinating Facets of Plant Selenium Accumulation—Biochemistry, Physiology, Evolution, and Ecology. New Phytol. 2017, 213, 1582–1596. [Google Scholar] [CrossRef]
- Jin, J.; Ghouri, F.; Xia, W.; Wang, J.; Shahid, M.Q. Alleviation of Nanoplastic Stress in Rice: Evidence from Biochemical, Cytological, Physiological, and Transcriptome Analysis. J. Agric. Food Chem. 2025, 73, 16612–16626. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Chen, Z.B.; Yang, X.Q.; Sheng, L.Y.; Mao, H.; Zhu, S.X. Integrated transcriptomics and metabolomics reveal key metabolic pathway responses in Pistia stratiotes under Cd stress. J. Hazard. Mater. 2023, 452, 131214. [Google Scholar] [CrossRef]
- Chen, H.; Jin, J.; Hu, S.; Shen, L.; Zhang, P.; Li, Z.; Fang, Z.; Liu, H. Metabolomics and proteomics reveal the toxicological mechanisms of florfenicol stress on wheat (Triticum aestivum L.) seedlings. J. Hazard. Mater. 2023, 443, 130264. [Google Scholar] [CrossRef]
- Li, X.; Hu, N.; Li, Y.; Tang, H.; Huang, X.; Yang, T.; Xu, J. Integrated ultrastructural, physiological, transcriptomic, and metabolomic analysis uncovers the mechanisms by which nicotinamide alleviates cadmium toxicity in Pistia stratiotes L. J. Hazard. Mater. 2024, 467, 133702. [Google Scholar] [CrossRef]
- Zhao, Y.; Gong, J.; Shi, R.; Wu, Z.; Liu, S.; Chen, S.; Tao, Y.; Li, S.; Tian, J. Application of proteomics in investigating the responses of plant to abiotic stresses. Planta 2025, 261, 128. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, Q.; Wan, Y.; Huang, Q.; Li, H. Transcriptome analysis reveals different mechanisms of selenite and selenate regulation of cadmium translocation in Brassica rapa. J. Hazard. Mater. 2023, 452, 131218. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Xu, E.G.; Li, L.; Zhou, A.; Peijnenburg, W.J.G.M.; Grossart, H.-P.; Liu, W.; Yang, Y. Tracing and trapping micro- and nanoplastics: Untapped mitigation potential of aquatic plants? Water Res. 2023, 242, 120249. [Google Scholar] [CrossRef]
- Zhu, S.; Sun, S.; Zhao, W.; Yang, X.; Chen, Z.; Mao, H.; Sheng, L. Comprehensive physiology and proteomics analysis revealed the resistance mechanism of rice (Oryza sativa L.) to cadmium stress. Ecotoxicol. Environ. Saf. 2024, 278, 116413. [Google Scholar] [CrossRef]
- Eitzen, L.; Ruhl, A.S.; Jekel, M. Particle Size and Pre-Treatment Effects on Polystyrene Microplastic Settlement in Water: Implications for Environmental Behavior and Ecotoxicological Tests. Water 2020, 12, 3436. [Google Scholar] [CrossRef]
- Sixi, Z.; Sun, S.; Zhao, W.; Yang, X.; Mao, H.; Sheng, L. Comprehensive physiology and proteomics analysis revealed the molecular toxicological mechanism of Se stress on indica and japonica rice. Chemosphere 2024, 358, 142190. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, S.; Zeng, Y.; Chen, Y.; Liu, H.; Yan, X.; Pu, S. A new quantitative insight: Interaction of polyethylene microplastics with soil–microbiome–crop. J. Hazard. Mater. 2023, 460, 132302. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liu, C.; Ren, Y.; Huang, Y.; Liu, Y.; Feng, S.; Zhong, X.; Fu, D.; Zhou, X.; Wang, J.; et al. Nanoplastic toxicity induces metabolic shifts in Populus × euramericana cv. ‘74/76′ revealed by multi-omics analysis. J. Hazard. Mater. 2024, 470, 134148. [Google Scholar] [CrossRef]
- Wang, M.; Li, H.; Dang, F.; Cheng, B.; Cheng, C.; Ge, C.; Zhou, D. Common metabolism and transcription responses of low-cadmium-accumulative wheat (Triticum aestivum L.) cultivars sprayed with nano-selenium. Sci. Total. Environ. 2024, 948, 174936. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Luo, J.; Du, Y.; Li, J.; Liu, Y.; Liang, Y.; Li, T. Coordination between root cell wall thickening and pectin modification is involved in cadmium accumulation in Sedum alfredii. Environ. Pollut. 2021, 268, 115665. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, X.; Chu, S.; You, Y.; Chi, Y.; Wang, R.; Yang, X.; Hayat, K.; Zhang, D.; Zhou, P. Comparative cytology combined with transcriptomic and metabolomic analyses of Solanum nigrum L. in response to Cd toxicity. J. Hazard. Mater. 2022, 423, 127168. [Google Scholar] [CrossRef]
- Krämer, U. Metal hyperaccumulation in plants. Annu. Rev. Plant Biol. 2010, 61, 517–534. [Google Scholar] [CrossRef]
- Yadav, V.; Arif, N.; Kovac, J.; Singh, V.P.; Tripathi, D.K.; Chauhan, D.K.; Vaculik, M. Structural modifications of plant organs and tissues by metals and metalloids in the environment: A review. Plant Physiol. Biochem. 2021, 159, 100–112. [Google Scholar] [CrossRef]
- Zhu, C.Q.; Cao, X.C.; Zhu, L.F.; Hu, W.J.; Hu, A.Y.; Bai, Z.G.; Zhong, C.; Sun, L.M.; Liang, Q.D.; Huang, J.; et al. Ammonium mitigates Cd toxicity in rice (Oryza sativa) via putrescine-dependent alterations of cell wall composition. Plant Physiol. Biochem. 2018, 132, 189–201. [Google Scholar] [CrossRef]
- Di, X.; Jing, R.; Qin, X.; Wei, Y.; Liang, X.; Wang, L.; Xu, Y.; Sun, Y.; Huang, Q. Transcriptome analysis reveals the molecular mechanism of different forms of selenium in reducing cadmium uptake and accumulation in wheat seedlings. Chemosphere 2023, 340, 139888. [Google Scholar] [CrossRef]
- Agarwal, S.; Kumari, S.; Singh, N.; Khan, S. Fate of plastic nanoparticles (PNPs) in soil and plant systems: Current status & research gaps. J. Hazard. Mater. Adv. 2023, 11, 100345. [Google Scholar] [CrossRef]
- Tang, N.; Huang, W.; Li, X.; Gao, X.; Liu, X.; Wang, L.; Xing, W. Drilling into the physiology, transcriptomics, and metabolomics to enhance insight on Vallisneria denseserrulata responses to nanoplastics and metalloid co-stress. J. Clean. Prod. 2024, 448, 141653. [Google Scholar] [CrossRef]
- Wang, M.; Mu, C.; Lin, X.; Ma, W.; Wu, H.; Si, D.; Ge, C.; Cheng, C.; Zhao, L.; Li, H.; et al. Foliar Application of Nanoparticles Reduced Cadmium Content in Wheat (Triticum aestivum L.) Grains via Long-Distance “Leaf–Root–Microorganism” Regulation. Environ. Sci. Technol. 2024, 58, 6900–6912. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, K.; Liu, Z.; Yu, Y.; Wang, Q.; Li, H. Effect of selenium on the subcellular distribution of cadmium and oxidative stress induced by cadmium in rice (Oryza sativa L.). Environ. Sci. Pollut. Res. 2019, 26, 16220–16228. [Google Scholar] [CrossRef]
- Cui, J.; Liu, T.; Li, Y.; Li, F. Selenium Reduces Cadmium Uptake into Rice Suspension Cells by Regulating the Expression of Lignin Synthesis and Cadmium-Related Genes. Sci. Total. Environ. 2018, 644, 602–610. [Google Scholar] [CrossRef]
- Baryla, A.; Laborde, C.; Montillet, J.L.; Triantaphylidès, C.; Chagvardieff, P. Evaluation of lipid peroxidation as a toxicity bioassay for plants exposed to copper. Environ. Pollut. 2000, 109, 131–135. [Google Scholar] [CrossRef]
- Kang, Y.; Qin, H.; Wang, G.; Lei, B.; Yang, X.; Zhong, M. Selenium nanoparticles mitigate cadmium stress in tomato through enhanced accumulation and transport of sulfate/selenite and polyamines. J. Agric. Food Chem. 2024, 72, 1473–1486. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, T.; Gao, J.; Li, B.; Han, L.; Ge, W.; Wang, Z. The accumulation of cadmium and lead in wheat grains is primarily determined by the soil-reducible cadmium level during wheat tillering. Chemosphere 2024, 361, 142509. [Google Scholar] [CrossRef] [PubMed]
- Handa, N.; Kohli, S.K.; Sharma, A.; Thukral, A.K.; Bhardwaj, R.; Abd, E.F.; Alqarawi, A.A.; Ahmad, P. Dynamics of antioxidative defence expression, photosynthetic attributes, and secondary metabolites to mitigate chromium toxicity in Brassica juncea L. plants. Environ. Exp. Bot. 2019, 161, 180–192. [Google Scholar] [CrossRef]
- Taylor, S.S.; Kornev, A.P. Protein Kinases: Evolution of Dynamic Regulatory Proteins. Trends Biochem. Sci. 2011, 36, 65–77. [Google Scholar] [CrossRef]
- Brigelius-Flohé, R.; Flohé, L. Selenium and Redox Signaling. Arch. Biochem. Biophys. 2017, 617, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Wu, J.; Jiang, M.; Wang, Y. Plant Mitogen-Activated Protein Kinase Cascades in Environmental Stresses. Int. J. Mol. Sci. 2021, 22, 1543. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Wei, C.; Tu, S. The Roles of Selenium in Protecting Plants against Abiotic Stresses. Environ. Exp. Bot. 2013, 87, 58–68. [Google Scholar] [CrossRef]
- Galant, A.; Preuss, M.L.; Cameron, J.C.; Jez, J.M. Plant glutathione biosynthesis: Diversity in biochemical regulation and reaction products. Front. Plant Sci. 2011, 2, 45. [Google Scholar] [CrossRef]
- Song, W.-Y.; Park, J.; Mendoza-Cózatl, D.G.; Suter-Grotemeyer, M.; Shim, D.; Hörtensteiner, S.; Geisler, M.; Weder, B.; Rea, P.A.; Rentsch, D.; et al. Arsenic tolerance in Arabidopsis is mediated by two ABCC-type phytochelatin transporters. Proc. Natl. Acad. Sci. USA 2010, 107, 21187–21192. [Google Scholar] [CrossRef]
- Abdalla, M.A.; Lentz, C.; Mühling, K.H. Crosstalk between selenium and sulfur is associated with changes in primary metabolism in lettuce plants grown under Se and S enrichment. Plants 2022, 11, 927. [Google Scholar] [CrossRef]
- Kang, L.; Wu, Y.; Jia, Y.; Chen, Z.; Kang, D.; Zhang, L.; Pan, C. Nano-selenium enhances melon resistance to Podosphaera xanthii by enhancing the antioxidant capacity and promoting alterations in the polyamine, phenylpropanoid and hormone signaling pathways. J. Nanobiotechnol. 2023, 21, 377. [Google Scholar] [CrossRef]
- Becker, Y.; Eaton, C.J.; Brasell, E.; May, K.J.; Becker, M.; Hassing, B.; Cartwright, G.M.; Reinhold, L.; Scott, B. The Fungal Cell-Wall Integrity MAPK Cascade Is Crucial for Hyphal Network Formation and Maintenance of Restrictive Growth of Epichloë festucae in Symbiosis with Lolium perenne. Mol. Plant-Microbe Interact. 2015, 28, 69–85. [Google Scholar] [CrossRef]
- Maidment, J.H.R.; Franceschetti, M.; Maqbool, A.; Saitoh, H.; Jantasuriyarat, C.; Kamoun, S.; Terauchi, R.; Banfield, M.J. Multiple variants of the fungal effector AVR-Pik bind the HMA domain of the rice protein OsHIPP19, providing a foundation to engineer plant defense. J. Biol. Chem. 2021, 296, 100371. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, S.; Yang, H.; Lv, Y.; Sun, S.; Zhao, W.; Chen, Z. Ultrastructural and Proteomic Analyses Revealed the Mechanism by Which Foliar Spraying of Se Nanoparticles Alleviated the Toxicity of Microplastics in Pistia stratiotes L. Toxics 2025, 13, 938. https://doi.org/10.3390/toxics13110938
Zhu S, Yang H, Lv Y, Sun S, Zhao W, Chen Z. Ultrastructural and Proteomic Analyses Revealed the Mechanism by Which Foliar Spraying of Se Nanoparticles Alleviated the Toxicity of Microplastics in Pistia stratiotes L. Toxics. 2025; 13(11):938. https://doi.org/10.3390/toxics13110938
Chicago/Turabian StyleZhu, Sixi, Haobin Yang, Yutian Lv, Suxia Sun, Wei Zhao, and Zhongbing Chen. 2025. "Ultrastructural and Proteomic Analyses Revealed the Mechanism by Which Foliar Spraying of Se Nanoparticles Alleviated the Toxicity of Microplastics in Pistia stratiotes L." Toxics 13, no. 11: 938. https://doi.org/10.3390/toxics13110938
APA StyleZhu, S., Yang, H., Lv, Y., Sun, S., Zhao, W., & Chen, Z. (2025). Ultrastructural and Proteomic Analyses Revealed the Mechanism by Which Foliar Spraying of Se Nanoparticles Alleviated the Toxicity of Microplastics in Pistia stratiotes L. Toxics, 13(11), 938. https://doi.org/10.3390/toxics13110938







