Function and Potential ceRNA Identification of Circ_009773 in Neodymium Oxide Nanoparticle-Induced Lung Epithelial Mesenchymal Transition
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. NP-Nd2O3 Treatment
2.3. Animal Treatment
2.4. Cell Counting Kit-8 (CCK-8) Experiment
2.5. qRT-PCR Analysis
2.6. Western Blot (WB) Analysis
2.7. Cellular Transfection
2.8. Dual-Luciferase Reporter Gene Assay
2.9. Statistical Analysis
3. Results
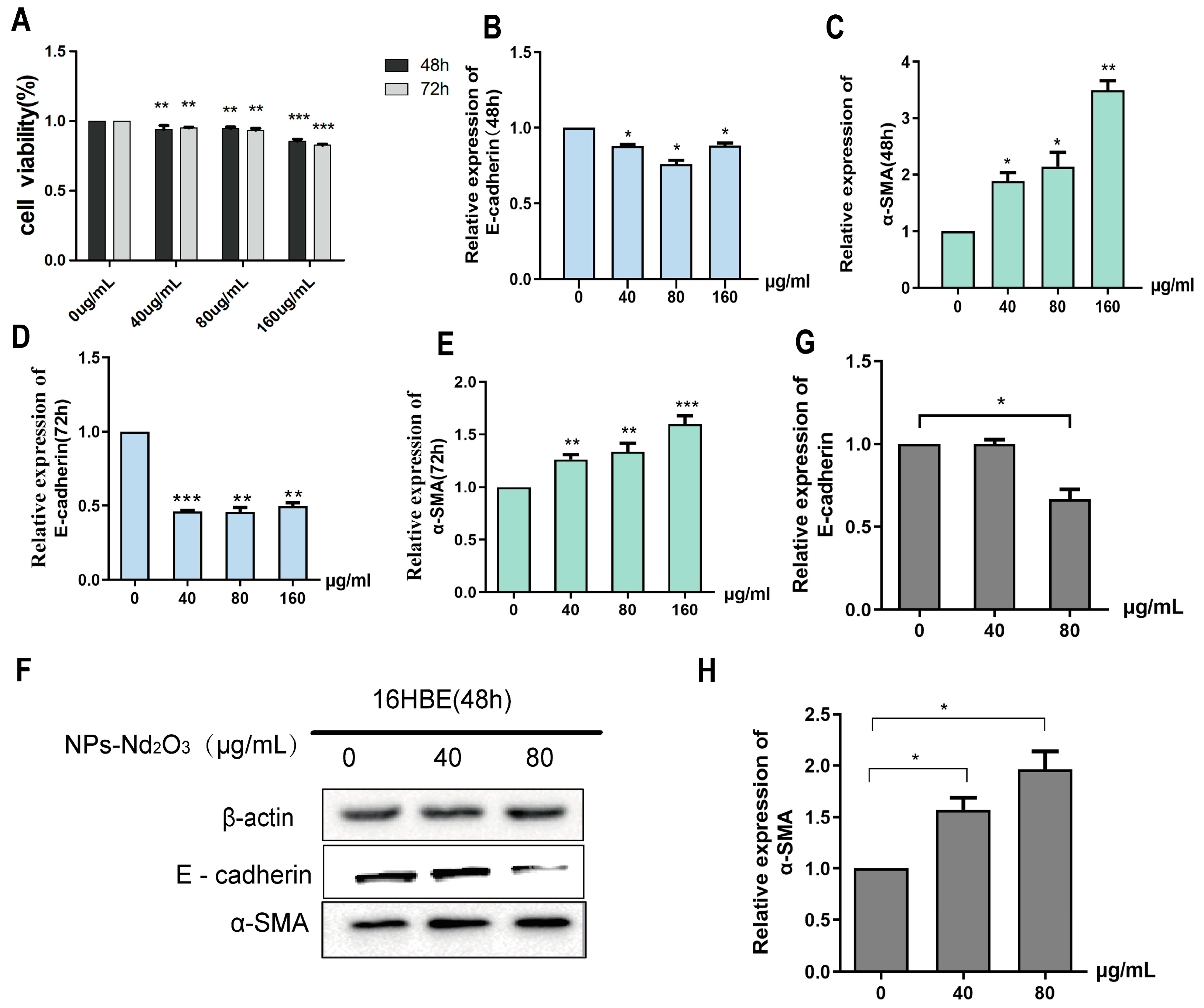
3.1. NPs-Nd2O3 Induces EMT in 16HBE Cells
3.2. NPs-Nd2O3 Exposure Induces EMT in Lung Tissue of Rat
3.3. circ_009773 Inhibition of NP-Nd2O3-Induced EMT in 16HBE Cells
3.4. circ_009773 Binds to and Regulates miR-135b-5p Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farouk, M.; El-Maboud, A.A.; Ibrahim, M.; Ratep, A.; Kashif, I. Optical properties of Lead bismuth borate glasses doped with neodymium oxide. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 149, 338–342. [Google Scholar] [CrossRef]
- Yu, F.; Zhang, X.; Gao, L.; Xue, H.; Liu, L.; Wang, S.; Chen, S.; Huang, L. LncRNA loc105377478 promotes NPs-Nd2O3-induced inflammation in human bronchial epithelial cells through the ADIPOR1/NF-κB axis. Ecotoxicol. Environ. Saf. 2021, 208, 111609. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Jia, Y.; Zhang, X.; Chen, S.; Wang, S.; Zhu, J.; Zheng, L.; Chen, Z.; Huang, L. Identification of the function and regulatory network of circ_009773 in DNA damage induced by nanoparticles of neodymium oxide. Toxicol. Vitr. 2022, 78, 105271. [Google Scholar] [CrossRef]
- Bu, N.; Wang, S.; Ma, Y.; Xia, H.; Zhao, Y.; Shi, X.; Liu, Q.; Wang, S.; Gao, Y. The lncRNA H19/miR-29a-3p/SNIP1/c-myc regulatory axis is involved in pulmonary fibrosis induced by Nd2O3. Toxicol. Sci. 2023, 197, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Enuka, Y.; Lauriola, M.; Feldman, M.E.; Sas-Chen, A.; Ulitsky, I.; Yarden, Y. Circular RNAs are long-lived and display only minimal early alterations in response to a growth factor. Nucleic Acids Res. 2016, 44, 1370–1383. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.B.; Jensen, T.I.; Clausen, B.H.; Bramsen, J.B.; Finsen, B.; Damgaard, C.K.; Kjems, J. Natural RNA circles function as efficient microRNA sponges. Nature 2013, 495, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.T.; Sun, F.Z.; Chen, J.-Y.; Liu, J.F.; Yan, Y.; Li, D.; Zhou, B.; Shan, H. The circular RNA circPTK2 inhibits EMT in hepatocellular carcinoma by acting as a ceRNA and sponging miR-92a to upregulate E-cadherin. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 9333–9342. [Google Scholar] [CrossRef]
- Wang, L.; Tong, X.; Zhou, Z.; Wang, S.; Lei, Z.; Zhang, T.; Liu, Z.; Zeng, Y.; Li, C.; Zhao, J.; et al. Circular RNA hsa_circ_0008305 (circPTK2) inhibits TGF-β-induced epithelial-mesenchymal transition and metastasis by controlling TIF1γ in non-small cell lung cancer. Mol. Cancer 2018, 17, 140. [Google Scholar] [CrossRef]
- Li, X.Y.; Liu, Y.R.; Zhou, J.H.; Li, W.; Guo, H.H.; Ma, H.P. Enhanced expression of circular RNA hsa_circ_000984 promotes cells proliferation and metastasis in non-small cell lung cancer by modulating Wnt/β-catenin pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 3366–3374. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhang, X.; Cui, J.; Liu, L.; Tai, D.; Wang, S.; Huang, L. Transcription factor TP63 mediates LncRNA CNTFR-AS1 to promote DNA damage induced by neodymium oxide nanoparticles via homologous recombination repair. Environ. Pollut. 2023, 334, 122191. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Mao, C.; Peng, J.; Xie, S.; Yang, J.; Xie, W.; Li, W.; Yang, H.; Guo, H.; Zhu, Z.; et al. Improved lung cancer classification by employing diverse molecular features of microRNAs. Heliyon 2024, 10, e26081. [Google Scholar] [CrossRef]
- Abdipourbozorgbaghi, M.; Vancura, A.; Radpour, R.; Haefliger, S. Circulating miRNA panels as a novel non-invasive diagnostic, prognostic, and potential predictive biomarkers in non-small cell lung cancer (NSCLC). Br. J. Cancer 2024, 131, 1350–1362. [Google Scholar] [CrossRef] [PubMed]
- O’Farrell, H.E.; Bowman, R.V.; Fong, K.M.; Yang, I.A. Plasma Extracellular Vesicle miRNAs Can Identify Lung Cancer, Current Smoking Status, and Stable COPD. Int. J. Mol. Sci. 2021, 22, 5803. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; Sun, S.; Chen, Y.; Xu, C.; Chen, Q.; Li, M.; Pei, Y.; Li, Q. MiR-3130-5p is an intermediate modulator of 2q33 and influences the invasiveness of lung adenocarcinoma by targeting NDUFS1. Cancer Med. 2021, 10, 3700–3714. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Che, Q.; Xie, C. NORAD regulates epithelial-mesenchymal transition of non-small cell lung cancer cells via miR-422a. Mol. Med. Rep. 2020, 23, 111. [Google Scholar] [CrossRef]
- Yin, C.; Bai, Q.; Feng, J. MiR-216a-5p protects 16HBE cells from H2O2-induced oxidative stress through targeting HMGB1/NF-kB pathway. Biochem. Biophys. Res. Commun. 2019, 508, 416–420. [Google Scholar] [CrossRef]
- Duan, Z.P.; Yu, X.J.; Wei, H.L. Circular RNA Sec61 subunit alpha isoform 1 by competitive absorption of microRNA-513a-5p mediates peroxisomal biogenesis factor 5 expression and promotes the malignant phenotype of non-small cell lung cancer. Kaohsiung J. Med. Sci. 2023, 39, 326–336. [Google Scholar] [CrossRef]
- Ma, H.; Lu, L.; Xia, H.; Xiang, Q.; Sun, J.; Xue, J.; Xiao, T.; Cheng, C.; Liu, Q.; Shi, A. Circ0061052 regulation of FoxC1/Snail pathway via miR-515-5p is involved in the epithelial-mesenchymal transition of epithelial cells during cigarette smoke-induced airway remodeling. Sci. Total Environ. 2020, 746, 141181. [Google Scholar] [CrossRef]
- Fedele, M.; Sgarra, R.; Battista, S.; Cerchia, L.; Manfioletti, G. The Epithelial-Mesenchymal Transition at the Crossroads between Metabolism and Tumor Progression. Int. J. Mol. Sci. 2022, 23, 800. [Google Scholar] [CrossRef] [PubMed]
- Bracken, C.P.; Goodall, G.J. The many regulators of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2022, 23, 89–90. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liang, J.; Cao, N.; Gao, J.; Song, L.; Tang, X. Coal dust nanoparticles induced pulmonary fibrosis by promoting inflammation and epithelial-mesenchymal transition via the NF-κB/NLRP3 pathway driven by IGF1/ROS-mediated AKT/GSK3β signals. Cell Death Discov. 2022, 8, 500. [Google Scholar] [CrossRef]
- Huang, X.; Li, C.; Wei, T.; Liu, N.; Zou, L.; Bai, C.; Yao, Y.; Wang, Z.; Xue, Y.; Wu, T.; et al. Ag/TiO2 nanohybrids induce fibrosis-related epithelial-mesenchymal transition in lung epithelial cells and the influences of silver content and silver particle size. Sci. Total Environ. 2023, 901, 165875. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xia, L.; Huang, P.; Wang, Z.; Guo, Q.; Huang, C.; Leng, W.; Qin, S. Heterogeneity and plasticity of epithelial-mesenchymal transition (EMT) in cancer metastasis: Focusing on partial EMT and regulatory mechanisms. Cell Prolif. 2023, 56, e13423. [Google Scholar] [CrossRef] [PubMed]
- Cannito, S.; Novo, E.; di Bonzo, L.V.; Busletta, C.; Colombatto, S.; Parola, M. Epithelial-mesenchymal transition: From molecular mechanisms, redox regulation to implications in human health and disease. Antioxid. Redox Signal 2010, 12, 1383–1430. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gao, Y.; Huang, L.; Zheng, S.; Wang, C.; Yu, Y.; Xie, K. Activation of NF-κB signaling in rare earth neodymium oxide particle-induced acute lung injury. Toxicol. Res. 2015, 4, 1587–1596. [Google Scholar] [CrossRef]
- Ma, J.; Bishoff, B.; Mercer, R.R.; Barger, M.; Schwegler-Berry, D.; Castranova, V. Role of epithelial-mesenchymal transition (EMT) and fibroblast function in cerium oxide nanoparticles-induced lung fibrosis. Toxicol. Appl. Pharmacol. 2017, 323, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ding, W.; Wang, J.; Ao, X.; Xue, J. Non-coding RNAs in lung cancer: Molecular mechanisms and clinical applications. Front. Oncol. 2023, 13, 1256537. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Wen, Z.J.; Xu, H.M.; Zhang, Y.; Zhang, Y.F. Exosomal noncoding RNAs in central nervous system diseases: Biological functions and potential clinical applications. Front. Mol. Neurosci. 2022, 15, 1004221. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Tan, Z.; Zhou, J.; Wu, Y.; Hu, Q.; Ling, Q.; Ling, J.; Liu, M.; Ma, J.; Zhang, D.; et al. The regulation of circRNA and lncRNAprotein binding in cardiovascular diseases: Emerging therapeutic targets. Biomed. Pharmacother. 2023, 165, 115067. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Yu, F.; Zhang, X.; Liu, L.; Huang, L. circ_0000638 inhibits neodymium oxide-induced bronchial epithelial cell inflammation through the miR-498-5p/NF-κB axis. Ecotoxicol. Environ. Saf. 2020, 195, 110455. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Guo, H.; Cheng, Y.; Zhou, Z.; Zhang, W.; Han, B.; Luo, W.; Wang, J.; Xie, W.; Chao, J. circHECTD1 promotes the silica-induced pulmonary endothelial-mesenchymal transition via HECTD1. Cell Death Dis. 2018, 9, 396. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, L.; Juan, J.; Zheng, Z.; Huang, L. Function and Potential ceRNA Identification of Circ_009773 in Neodymium Oxide Nanoparticle-Induced Lung Epithelial Mesenchymal Transition. Toxics 2024, 12, 917. https://doi.org/10.3390/toxics12120917
Gao L, Juan J, Zheng Z, Huang L. Function and Potential ceRNA Identification of Circ_009773 in Neodymium Oxide Nanoparticle-Induced Lung Epithelial Mesenchymal Transition. Toxics. 2024; 12(12):917. https://doi.org/10.3390/toxics12120917
Chicago/Turabian StyleGao, Lei, Juan Juan, Zimeng Zheng, and Lihua Huang. 2024. "Function and Potential ceRNA Identification of Circ_009773 in Neodymium Oxide Nanoparticle-Induced Lung Epithelial Mesenchymal Transition" Toxics 12, no. 12: 917. https://doi.org/10.3390/toxics12120917
APA StyleGao, L., Juan, J., Zheng, Z., & Huang, L. (2024). Function and Potential ceRNA Identification of Circ_009773 in Neodymium Oxide Nanoparticle-Induced Lung Epithelial Mesenchymal Transition. Toxics, 12(12), 917. https://doi.org/10.3390/toxics12120917





