Effect of Prior Chilling Period and Alga-Extract Packaging on the Quality of a Canned Underutilised Fish Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Initial Raw Fish and Chilling Storage
2.2. Preparation of Alga Extract
2.3. Canning Process and Sampling Procedure
2.4. Determination of Lipid Damage
2.5. Determination of Colour Changes and Trimethylamine Content
2.6. Statistical Analysis
3. Results and Discussion
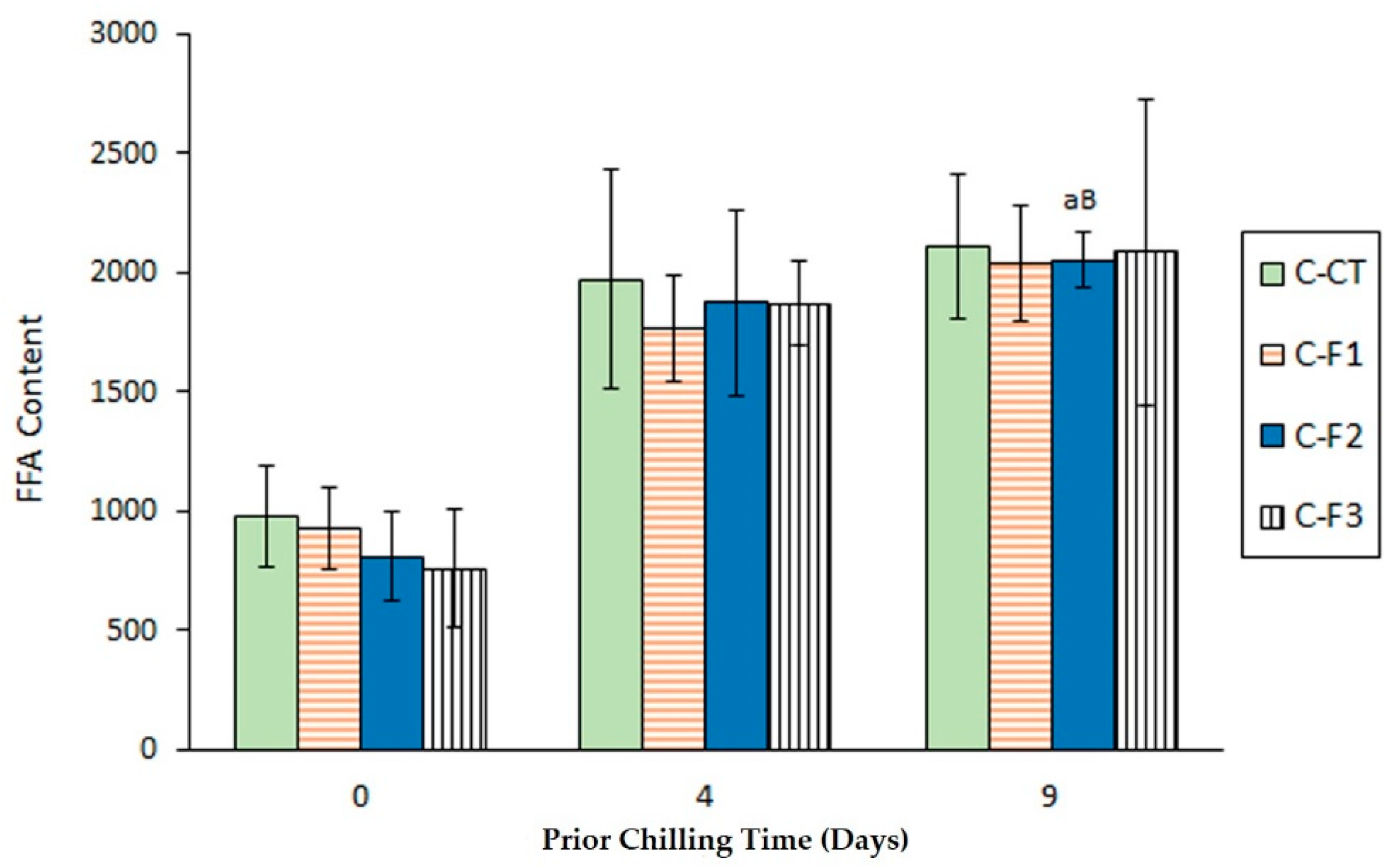
3.1. Determination of Lipid Hydrolysis Development
3.2. Determination of Lipid Oxidation Development
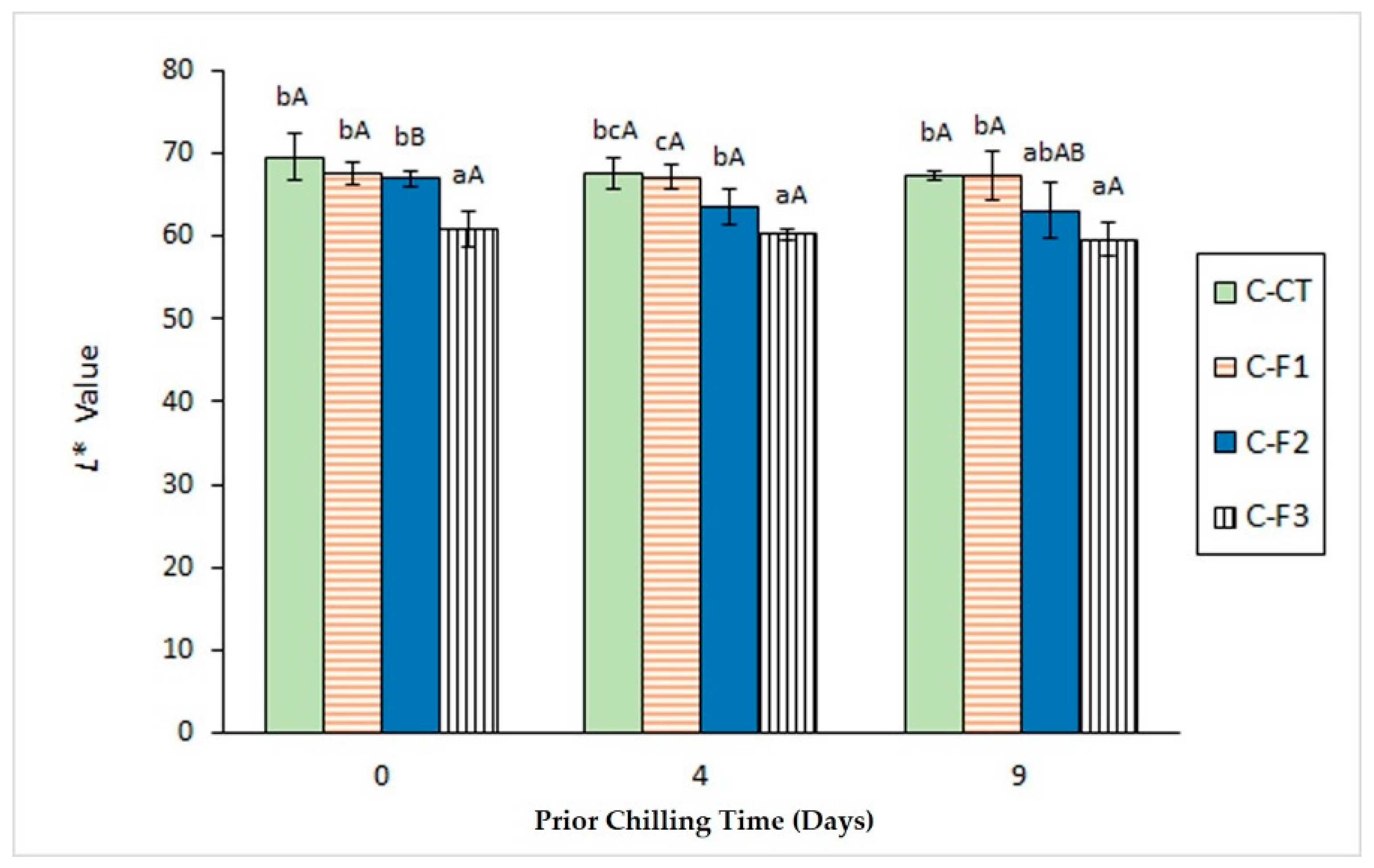
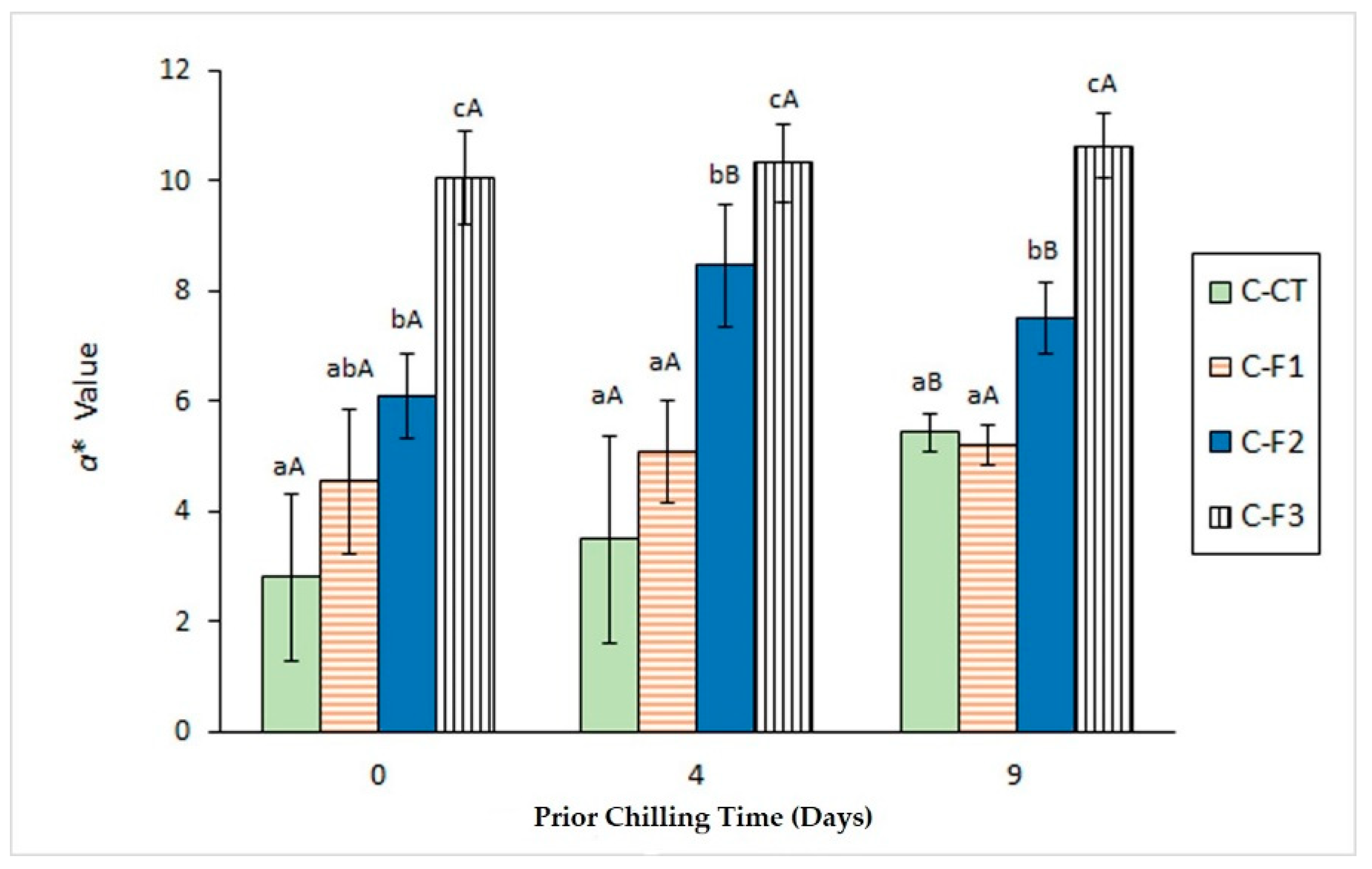
3.3. Determination of Colour Changes
3.4. Trimethylamine Content Assessment
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Horner, W. Canning fish and fish products. In Fish Processing Technology, 2nd ed.; Hall, G., Ed.; Blackie Academic & Professional An Imprint of Chapman & Hall: London, UK, 1997; pp. 119–159. [Google Scholar]
- Lukoshkina, M.; Odoeva, G. Kinetics of chemical reactions for prediction of quality of canned fish during storage. App. Biochem. Microb. 2003, 39, 321–327. [Google Scholar] [CrossRef]
- Pigott, G.M.; Tucker, B.W. Seafood. Effects of Technology on Nutrition; Marcel Dekker, Inc.: New York, NY, USA; Basel, Switzerland, 1990. [Google Scholar]
- García-Arias, T.; Sánchez-Muniz, J.; Castrillón, A.; Navarro, P. White tuna canning, total fat, and fatty acid changes during processing and storage. J. Food Comp. Anal. 1994, 7, 119–130. [Google Scholar] [CrossRef]
- Sikorski, Z.; Kolakowski, E. Endogenous enzyme activity and seafood quality: Influence of chilling, freezing, and other environmental factors. In Seafood Enzymes; Haard, N., Simpson, B., Eds.; Marcel Dekker: New York, NY, USA, 2000; pp. 451–487. [Google Scholar]
- Aubourg, S.P. Loss of quality during the manufacture of canned fish products. Food Sci. Technol. Int. 2001, 7, 199–215. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Rubio, MªE.; Pérez-Jiménez, J.; Saura-Calixto, F. Dietary fiber and antioxidant capacity in Fucus vesiculosus products. Int. J. Food Sci. Technol. 2009, 60, 23–34. [Google Scholar]
- Smit, A. Medicinal and pharmaceutical uses of seaweed natural products: A Review. J. App. Phycol. 1 2004, 16, 245–262. [Google Scholar] [CrossRef]
- Zubia, M.; Fabre, M.S.; Kerjean, V.; Le Lann, K.; Stiger-Pouvreau, V.; Fauchon, M.; Deslandes, E. Antioxidant and antitumoral activities of some Phaeophyta from Britanny coasts. Food Chem. 2009, 116, 693–701. [Google Scholar] [CrossRef]
- Gupta, S.; Abu-Ghannam, N. Bioactive potential and possible health effects of edible brown seaweeds. Trends Food Sci. Technol. 2011, 22, 315–326. [Google Scholar] [CrossRef] [Green Version]
- European Council Regulation (14/02/1997). European Community (EC), No 258/97, 27 January 1997. Concerning novel foods and novel food ingredients. CELEX-EUR Off. J. 1997, L-43, 1–7. [Google Scholar]
- Andrade, P.; Barbosa, M.; Pedro Matos, R.; Lopes, G.; Vinholes, J.; Mouga, T.; Valentão, P. Valuable compounds in macroalgae extracts. Food Chem. 2013, 138, 1819–1828. [Google Scholar] [CrossRef] [PubMed]
- Paiva, L.; Lima, E.; Ferreira Patarra, R.; Neto, A.; Baptista, J. Edible Azorean macroalgae as source of rich nutrients with impact on human health. Food Chem. 2014, 164, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Cérantola, S.; Breton, F.; Gall, E.; Deslandes, E. Co-occurrence and antioxidant activities of fucol and fucophlorethol classes of polymeric phenols in Fucus spiralis. Botanica Marina 2006, 49, 347–351. [Google Scholar] [CrossRef]
- Tierney, M.; Smyth, T.; Hayes, M.; Soler-Vila, A.; Croft, A.; Brunton, N. Influence of pressurised liquid extraction and solid-liquid extraction methods on the phenolic content and antioxidant activities of Irish macroalgae. Int. J. Food Sci. Technol. 2013, 48, 860–869. [Google Scholar] [CrossRef]
- Peinado, I.; Girón, J.; Koutsidis, G.; Ames, J.M. Chemical composition, antioxidant activity and sensory evaluation of five different species of brown edible seaweeds. Food Res. Int. 2014, 66, 36–44. [Google Scholar] [CrossRef] [Green Version]
- García-Soto, B.; Miranda, J.; Rodríguez-Bernaldo de Quirós, A.; Sendón, R.; Rodríguez-Martínez, A.; Barros-Velázquez, J.; Aubourg, S.P. Effect of biodegradable film (lyophilised alga Fucus spiralis and sorbic acid) on quality properties of refrigerated megrim (Lepidorhombus whiffiagonis). Int. J. Food Sci. Technol. 2015, 50, 1891–1900. [Google Scholar] [CrossRef]
- Miranda, J.; Trigo, M.; Barros-Velázquez, J.; Aubourg, S.P. Effect of an icing medium containing the alga Fucus spiralis on the microbiological activity and lipid oxidation in chilled megrim (Lepidorhombus whiffiagonis). Food Cont. 2016, 59, 290–297. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, R.G.; Trigo, M.; Campos, C.A.; Aubourg, S.P. Preservative effect of algae extracts on lipid composition and rancidity development in brine-canned Atlantic Chub mackerel (Scomber colias). Eur. J. Lipid Sci. Technol. 2019, 121, 1900129. [Google Scholar] [CrossRef]
- Venugopal, V.; Shahidi, F. Traditional methods to process underutilized fish species for human consumption. Food Rev. Int. 1998, 14, 35–97. [Google Scholar] [CrossRef]
- Blanco, M.; Sotelo, C.G.; Chapela, M.J.; Pérez-Martín, R.I. Towards sustainable and efficient use of fishery resources: Present and future trends. Trends Food Sci. Technol. 2007, 18, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Wituszynska, B. Determination of some group B vitamins in fresh and canned fish. Bromatologia i Chemia Toksykologiczna 1973, 6, 13–22. [Google Scholar]
- García-Moreno, P.; Pérez-Gálvez, R.; Espejo-Carpio, F.; Muñío, M.; Guadix, A.; Guadix, E. Lipid characterization and properties of protein hydrolysates obtained from discarded Mediterranean fish species. J. Sci. Food Agric. 2013, 93, 3777–3784. [Google Scholar] [CrossRef]
- Vidacek, S.; Medic, H.; Marusic, N.; Tonkovic, S.; Petrak, T. Influence of different freezing regimes on bioelectrical properties of Atlantic chub mackerel (Scomber colias). J. Food Proc. Eng. 2012, 35, 735–741. [Google Scholar] [CrossRef]
- Alberio, G.; Barbagallo, R.; Todaro, A.; Bono, G.; Spagna, G. Effect of freezing/thawing process in different sizes of blue fish in the Mediterranean through lysosomal enzymatic tests. Food Chem. 2014, 148, 47–53. [Google Scholar] [CrossRef]
- Stamatis, N.; Arkoudelos, J. Quality assessment of Scomber colias japonicus under modified atmosphere and vacuum packaging. Food Cont. 2007, 18, 292–300. [Google Scholar] [CrossRef]
- Miranda, J.M.; Carrera, M.; Pastén, A.; Vega-Gálvez, A.; Barros-Velázquez, J.; Aubourg, S.P. The impact of quinoa (Chenopodium quinoa Willd.) ethanolic extracts in the icing medium on quality loss of Atlantic chub mackerel (Scomber colias) under chilling storage. Eur. J. Lipid Sci. Technol. 2018, 120, 1800280. [Google Scholar] [CrossRef] [Green Version]
- Bligh, E.G.; Dyer, W.J. A rapid method of total extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [Green Version]
- Lowry, R.; Tinsley, I. Rapid colorimetric determination of free fatty acids. J. Am. Oil Chem. Soc. 1976, 53, 470–472. [Google Scholar] [CrossRef]
- Chapman, R.; McKay, J. The estimation of peroxides in fats and oils by the ferric thiocyanate method. J. Am. Oil Chem. Soc. 1949, 26, 360–363. [Google Scholar] [CrossRef]
- Vyncke, W. Direct determination of the thiobarbituric acid value in trichloracetic acid extracts of fish as a measure of oxidative rancidity. Fette, Seifen, Anstrichm. 1970, 72, 1084–1087. [Google Scholar] [CrossRef]
- Aubourg, S.P.; Medina, I. Quality differences assessment in canned sardine (Sardina pilchardus) by fluorescence detection. J. Agric. Food Chem. 1997, 45, 3617–3621. [Google Scholar] [CrossRef] [Green Version]
- Tozawa, H.; Erokibara, K.; Amano, K. Proposed modification of Dyer’s method for trimethylamine determination in codfish. In Fish Inspection and Quality Control; Kreuzer, R., Ed.; Fishing News Books Ltd.: London, UK, 1971. [Google Scholar]
- Ashie, I.; Smith, J.; Simpson, B. Spoilage and shelf-life extension of fresh fish and shellfish. Crit. Rev. Food Sci. Nutr. 1996, 36, 87–121. [Google Scholar] [CrossRef]
- Miyashita, K.; Tagagi, T. Study on the oxidative rate and prooxidant activity of free fatty acids. J. Am. Oil Chem. Soc. 1986, 63, 1380–1384. [Google Scholar] [CrossRef]
- Naseri, M.; Rezaei, M. Lipid changes during long-term storage of canned sprat. J. Aquat. Food Prod. Technol. 2012, 21, 48–58. [Google Scholar] [CrossRef]
- Aubourg, S.P.; Gallardo, J.M.; Medina, I. Changes in lipids during different sterilising conditions in canning albacore (Thunnus alalunga) in oil. Int. J. Food Sci. Technol. 1997, 32, 427–431. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, A.; Carriles, N.; Aubourg, S.P. Effect of chill storage under different icing conditions on sensory and physical properties of canned farmed salmon (Oncorhynchus kisutch). Int. J. Food Sci. Technol. 2010, 45, 295–304. [Google Scholar] [CrossRef]
- Barbosa, R.G.; Trigo, M.; Fett, R.; Aubourg, S.P. Impact of a packing medium with alga Bifurcaria bifurcata extract on canned Atlantic mackerel (Scomber scombrus) quality. J. Sci. Food Agric. 2018, 98, 3462–3467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tironi, V.A.; Tomás, M.C.; Añón, M.C. Structural and functional changes in myofibrillar proteins on sea salmon (Pseudopercis semifasciata) by interaction with malondialdehyde (RI). J. Food Sci. 2002, 67, 930–935. [Google Scholar] [CrossRef]
- Selmi, S.; Monser, L.; Sadok, S. The influence of local canning process and storage on pelagic fish from Tunisia: Fatty acids profile and quality indicators. J. Food Proc. Preserv. 2008, 32, 443–457. [Google Scholar] [CrossRef]
- Losada, V.; Rodríguez, A.; Ortiz, J.; Aubourg, S.P. Quality enhancement of canned sardine (Sardina pilchardus) by a preliminary chilling treatment. Eur. J. Lipid Sci. Technol. 2006, 108, 598–605. [Google Scholar] [CrossRef] [Green Version]
- Farvin, K.H.S.; Jacobsen, C. Phenolic compounds and antioxidant activities of selected species of seaweeds from Danish coast. Food Chem. 2013, 138, 1670–1681. [Google Scholar] [CrossRef]
- Kuda, T.; Ikemori, T. Minerals, polysaccharides and antioxidant properties of aqueous solutions obtained from macroalgal beach-casts in the Noto Peninsula, Ishikawa, Japan. Food Chem. 2009, 112, 575–581. [Google Scholar] [CrossRef]
- Pereira, L.; Amado, A.; Critchley, A.; van de Velde, F.; Ribeiro-Claro, P. Identification of selected seaweed polysaccharides (phycocolloides) by vibrational spectroscopy (FTIR-ATR and FT-Raman). Food Hydroc. 2009, 23, 1903–1909. [Google Scholar] [CrossRef] [Green Version]
- Little, A. Effect of pre- and post-mortem handling on reflectance characteristics of skipjack tuna. J. Food Sci. 1972, 37, 502. [Google Scholar] [CrossRef]
- Lee, S.; Joo, S.T.; Alderton, A.L.; Hill, D.W.; Faustman, C. Oxymioglobin and lipid oxidation in yellowfin tuna (Thunnus albacares) loins. J. Food Sci. 2003, 68, 1664–1668. [Google Scholar] [CrossRef]
- Wetterskog, D.; Undeland, I. Loss of redness (a*) as a tool to follow hemoglobin-mediated lipid oxidation in washed cod mince. J. Agric. Food Chem. 2004, 52, 7214–7221. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, J.M.; Pérez-Martín, R.; Franco, J.M.; Aubourg, S.P.; Sotelo, C.G. Changes in volatile bases and trimethylamine oxide during the canning of albacore (Thunnus alalunga). Int. J. Food Sci. Technol. 1990, 25, 78–81. [Google Scholar] [CrossRef]
| Quality Index | Alga Extract Concentration | Prior Chilling Time (Days) | ||
|---|---|---|---|---|
| 0 | 4 | 9 | ||
| PV (meq. active oxygen·kg−1 lipids) | C-CT | a 1.01 ± 0.24 A | a 1.31 ± 0.81 AB | a 2.14 ± 0.55 B |
| C-F1 | b 3.08 ± 0.91 A | ab 2.08 ± 0.72 A | ab 2.89 ± 0.57 A | |
| C-F2 | b 3.68 ± 0.61 A | bc 3.01 ± 0.55 A | b 3.63 ± 0.53 A | |
| C-F3 | c 6.88 ± 1.60 A | d 6.84 ± 1.47 A | c 6.50 ± 1.24 A | |
| TBA-i (mg malondialdehyde·kg−1 muscle) | C-CT | a 0.73 ± 0.22 A | a 1.22 ± 0.40 AB | a 1.39 ± 0.40 B |
| C-F1 | a 0.51 ± 0.20 A | a 1.77 ± 0.65 B | a 1.28 ± 0.33 B | |
| C-F2 | a 0.44 ± 0.07 A | a 1.69 ± 0.35 B | a 1.27 ± 0.45 B | |
| C-F3 | a 0.48 ± 0.09 A | a 1.36 ± 0.39 B | a 1.02 ± 0.14 B | |
| FR | C-CT | c 4.34 ± 0.57 A | a 5.27 ± 0.55 A | b 6.08 ± 0.14 B |
| C-F1 | ab 3.30 ± 0.16 A | a 5.06 ± 0.50 B | b 6.28 ± 0.31 C | |
| C-F2 | b 3.56 ± 0.14 A | a 4.73 ± 0.10 B | b 6.03 ± 0.25 B | |
| C-F3 | a 2.96 ± 0.33 A | a 4.99 ± 0.12 B | a 5.01 ± 0.36 B | |
| Quality Index | Alga Extract Concentration | Prior Chilling Time (Days) | ||
|---|---|---|---|---|
| 0 | 4 | 9 | ||
| TMA-N (g·kg−1 muscle) | C-CT | a 25.41 ± 2.64 A | b 31.40 ± 3.79 A | ab 28.12 ± 6.10 A |
| C-F1 | a 23.78 ± 5.65 A | a 18.06 ± 3.26 A | b 33.58 ± 2.77 B | |
| C-F2 | a 21.21 ± 4.22 A | a 18.55 ± 3.32 A | a 24.96 ± 5.11 A | |
| C-F3 | a 21.70 ± 1.87 A | b 28.71 ± 2.05 B | a 26.40 ± 2.64 B | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aubourg, S.P.; Trigo, M.; Martínez, B.; Rodríguez, A. Effect of Prior Chilling Period and Alga-Extract Packaging on the Quality of a Canned Underutilised Fish Species. Foods 2020, 9, 1333. https://doi.org/10.3390/foods9091333
Aubourg SP, Trigo M, Martínez B, Rodríguez A. Effect of Prior Chilling Period and Alga-Extract Packaging on the Quality of a Canned Underutilised Fish Species. Foods. 2020; 9(9):1333. https://doi.org/10.3390/foods9091333
Chicago/Turabian StyleAubourg, Santiago P., Marcos Trigo, Beatriz Martínez, and Alicia Rodríguez. 2020. "Effect of Prior Chilling Period and Alga-Extract Packaging on the Quality of a Canned Underutilised Fish Species" Foods 9, no. 9: 1333. https://doi.org/10.3390/foods9091333






