Effect of Chitosan Coating on the Postharvest Quality and Antioxidant Enzyme System Response of Strawberry Fruit during Cold Storage
Abstract
:1. Introduction
2. Experimental Section
2.1. Fruit Samples
2.2. Weight Loss, Firmness and Surface Color
2.3. Total Soluble Solids Content and Titratable Acidity Content
2.4. Extraction and Measurements of Phenolic Compounds, Anthocyanins, Flavonoids and Total Antioxidant Capacity Assay
2.5. Ascorbic Acid Content
2.6. Enzyme Extraction and Activity Assays
2.6.1. Catalase, Ascorbate Peroxidase and Guaiacol Peroxidase Activity
2.6.2. Polyphenol Oxidase Activity
2.6.3. Lipoxygenase Activity
2.7. Malondialdehyde Content Determination
2.8. Statistical Analysis
3. Results and Discussion
3.1. Effect of Chitosan Treatment on Weight Loss and Firmness

3.2. Effect of Chitosan Treatment on TSS, TA and Surface Color of Strawberry Fruit
| Cultivar | Days of CS | L* | H | TSS (°Brix) | TA (mg Citric Acid/L Juice) | TSS/TA | |
|---|---|---|---|---|---|---|---|
| Sabrina | C | 0 | 28.53 ± 1.08 e | 30.8 ± 1.12 f | 8.37 ± 0.32 a | 7.93 ± 0.20 f | 1.05 ± 0.06 a |
| 3 | 27.25 ± 0.61 d,e | 27.71 ± 1.46 d,e | 9.93 ± 0.06 b | 6.60 ± 0.27 e | 1.50 ± 0.05 b | ||
| 6 | 24.29 ± 0.79 a | 26.7 ± 1.32 c,d | 11.93 ± 0.11 d,e | 5.10 ± 0.10 b | 2.34 ± 0.05 e | ||
| 9 | 24.50 ± 0.99 a | 23.03 ± 0.41 a | 13.07 ± 0.81 g | 4.17 ± 0.15 a | 3.14 ± 0.14 g | ||
| T1 | 0 | 28.53 ± 1.08 e | 30.08 ± 1.13 f | 8.37 ± 0.32 a | 7.93 ± 0.20 f | 1.05 ± 0.06 a | |
| 3 | 27.49 ± 1.01 e | 28.02 ± 0.05 d,e | 9.67 ± 0.25 b | 6.67 ± 0.25 e | 1.45 ± 0.09 b | ||
| 6 | 24.70 ± 0.79 a,b,c | 27.11 ± 0.56 c,d | 11.33 ± 0.15 d | 5.57 ± 0.15 c | 2.03 ± 0.09 d | ||
| 9 | 24.60 ± 0.82 a,b | 24.53 ± 0.56 b | 12.57 ± 0.59 f,g | 4.97 ± 0.15 b | 2.53 ± 0.04 f | ||
| T2 | 0 | 28.53 ± 1.08 e | 30.08 ± 1.13 f | 8.37 ± 0.32 a | 7.93 ± 0.20 f | 1.05 ± 0.06 a | |
| 3 | 27.68 ± 0.27 e | 29.08 ± 0.50 c,f | 9.33 ± 0.15 b | 6.77 ± 0.32 e | 1.38 ± 0.09 b | ||
| 6 | 25.89 ± 0.20 b,c,d | 27.61 ± 0.21 d | 10.67 ± 0.25 c | 6.10 ± 0.10 d | 1.75 ± 0.06 c | ||
| 9 | 26.04 ± 0.47 c,d | 25.97 ± 0.02 b,c | 12.07 ± 0.20 e,f | 5.27 ± 0.15 b,c | 1.80 ± 0.65 e | ||
| Candonga | C | 0 | 26.83 ± 0.68 e | 28.92 ± 0.12 f | 8.57 ± 0.15 a | 11.03 ± 0.20 g | 0.78 ± 0.01 a |
| 3 | 24.59 ± 0.42 d | 28.29 ± 0.36 e | 9.63 ± 0.20 c,d | 10.17 ± 0.15 e | 0.94 ± 0.03 c | ||
| 6 | 23.04 ± 0.09 c | 25.39 ± 0.35 b | 10.20 ± 0.20 e,f | 9.00 ± 0.20 c | 1.13 ± 0.04 e | ||
| 9 | 21.06 ± 0.06 a | 24.65 ± 0.17 a | 10.90 ± 0.60 g | 8.00 ± 0.10 a | 1.37 ± 0.09 g | ||
| T1 | 0 | 26.83 ± 0.68 e | 28.92 ± 0.12 f | 8.57 ± 0.15 a | 11.03 ± 0.20 g | 0.78 ± 0.01 a | |
| 3 | 25.03 ± 0.05 d | 28.43 ± 0.27 e | 9.27 ± 0.11 b,c | 10.67 ± 0.15 f | 0.88 ± 0.01 b | ||
| 6 | 23.13 ± 0.02 c | 25.94 ± 0.61 c | 9.90 ± 0.10 d,e | 9.77 ± 0.15 d | 1.03 ± 0.005 d | ||
| 9 | 21.07 ± 0.25 b | 24.73 ± 0.26 a | 10.80 ± 0.45 g | 8.47 ± 0.45 b | 1.27 ± 0.01 f | ||
| T2 | 0 | 26.83 ± 0.68 e | 28.92 ± 0.13 f | 8.57 ± 0.15 a | 11.03 ± 0.20 g | 0.78 ± 0.01 a | |
| 3 | 25.15 ± 0.07 d | 28.54 ± 0.092 e,f | 9.10 ± 0.10 b | 10.87 ± 0.15 f,g | 0.83 ± 0.02 b | ||
| 6 | 24.63 ± 0.18 d | 26.78 ± 0.21 d | 9.57 ± 0.15 c,d | 10.00 ± 0.20 d,e | 0.95 ± 0.04 c,d | ||
| 9 | 21.85 ± 0.10 b | 25.53 ± 0.11 b,c | 10.57 ± 0.15 f,g | 9.17 ± 0.15 c | 1.15 ± 0.02 e | ||
| Jonica | C | 0 | 28.87 ± 0.32 e | 30.57 ± 0.15 e | 13.00 ± 0.10 a | 8.43 ± 0.15 f | 1.54 ± 0.04 a |
| 3 | 27.17 ± 0.06 d | 30.18 ± 0.13 e | 14.10 ± 0.10 c,d | 7.60 ± 0.26 d | 1.85 ± 0.07 c | ||
| 6 | 22.60 ± 0.10 b | 26.18 ± 0.29 a,b | 14.73 ± 0.11 e,f | 6.70 ± 0.26 b | 2.2 ± 0.07 e | ||
| 9 | 22.01 ± 0.02 a | 25.92 ± 0.47 a | 16.17 ± 0.29 g | 6.10 ± 0.10 a | 2.65 ± 0.08 g | ||
| T1 | 0 | 28.87 ± 0.32 e | 30.57 ± 0.15 e | 13.00 ± 0.10 a | 8.43 ± 0.15 f | 1.54 ± 0.03 a | |
| 3 | 27.41 ± 0.14 d | 30.47 ± 0.20 e | 13.87 ± 0.15 b,c | 7.97 ± 0.15 e | 1.74 ± 0.04 b | ||
| 6 | 22.93 ± 0.04 b | 27.63 ± 0.11 d | 14.43 ± 0.20 d,e | 7.10 ± 0.10 c | 2.03 ± 0.02 d | ||
| 9 | 22.92 ± 0.03 b | 26.63 ± 0.08 b,c | 15.43 ± 0.60 g | 6.63 ± 0.11 b | 2.32 ± 0.12 f | ||
| T2 | 0 | 28.87 ± 0.32 e | 30.57 ± 0.15 e | 13.00 ± 0.10 a | 8.43 ± 0.15 f | 1.54 ± 0.03 a | |
| 3 | 28.55 ± 0.50 e | 30.53 ± 0.41 e | 13.50 ± 0.27 b | 8.07 ± 0.15 e | 1.67 ± 0.01 b | ||
| 6 | 23.44 ± 0.16 c | 27.90 ± 0.56 d | 14.27 ± 0.16 c,d | 7.50 ± 0.10 d | 1.9 ± 0.04 c | ||
| 9 | 22.99 ± 0.02 b | 26.74 ± 1.94 c | 15.10 ± 0.37 f,g | 7.20 ± 0.10 c | 2.09 ± 0.03 d |
3.3. Effect of Chitosan Treatment on Nutraceutical Compounds and Antioxidant Activity
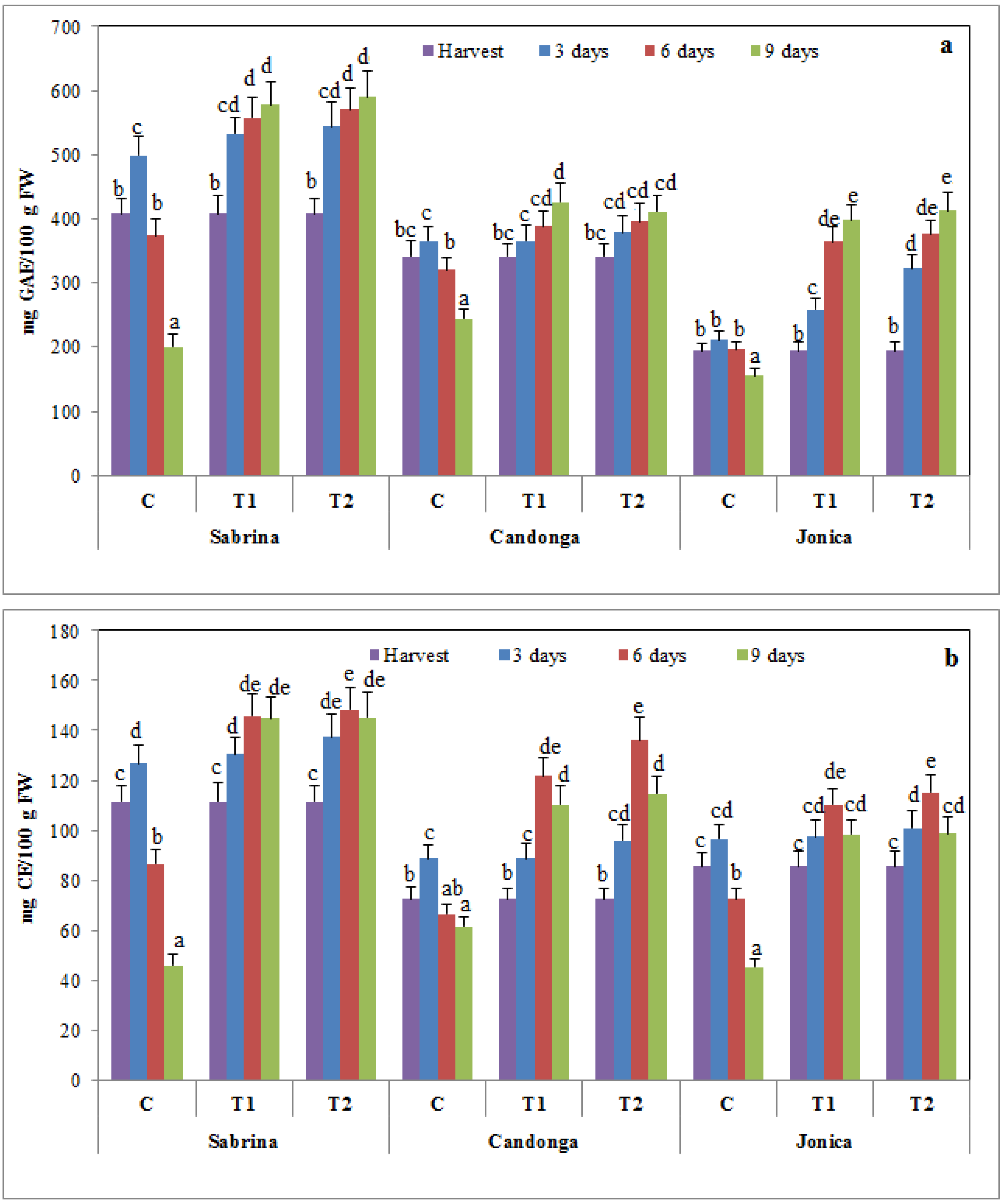
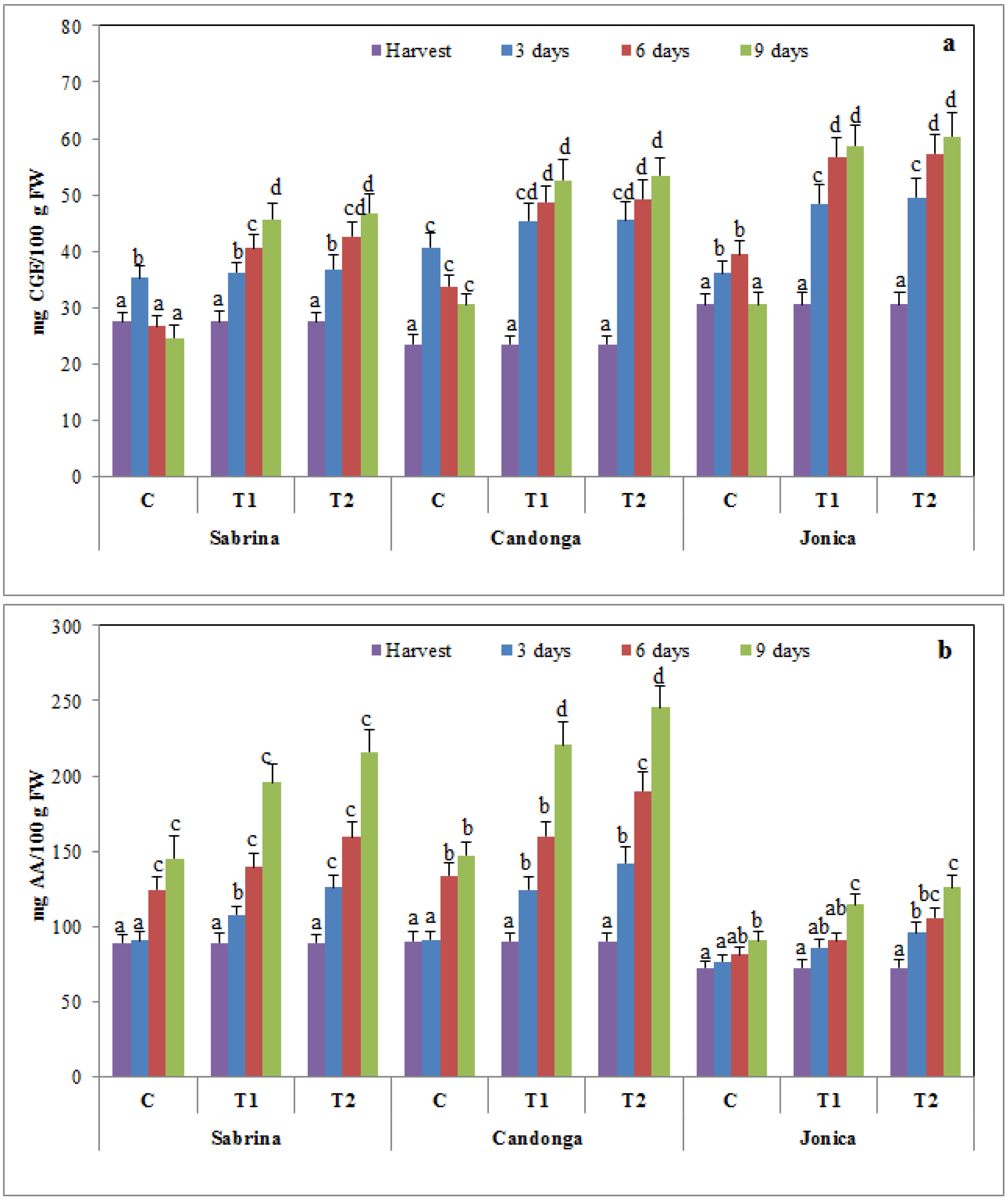
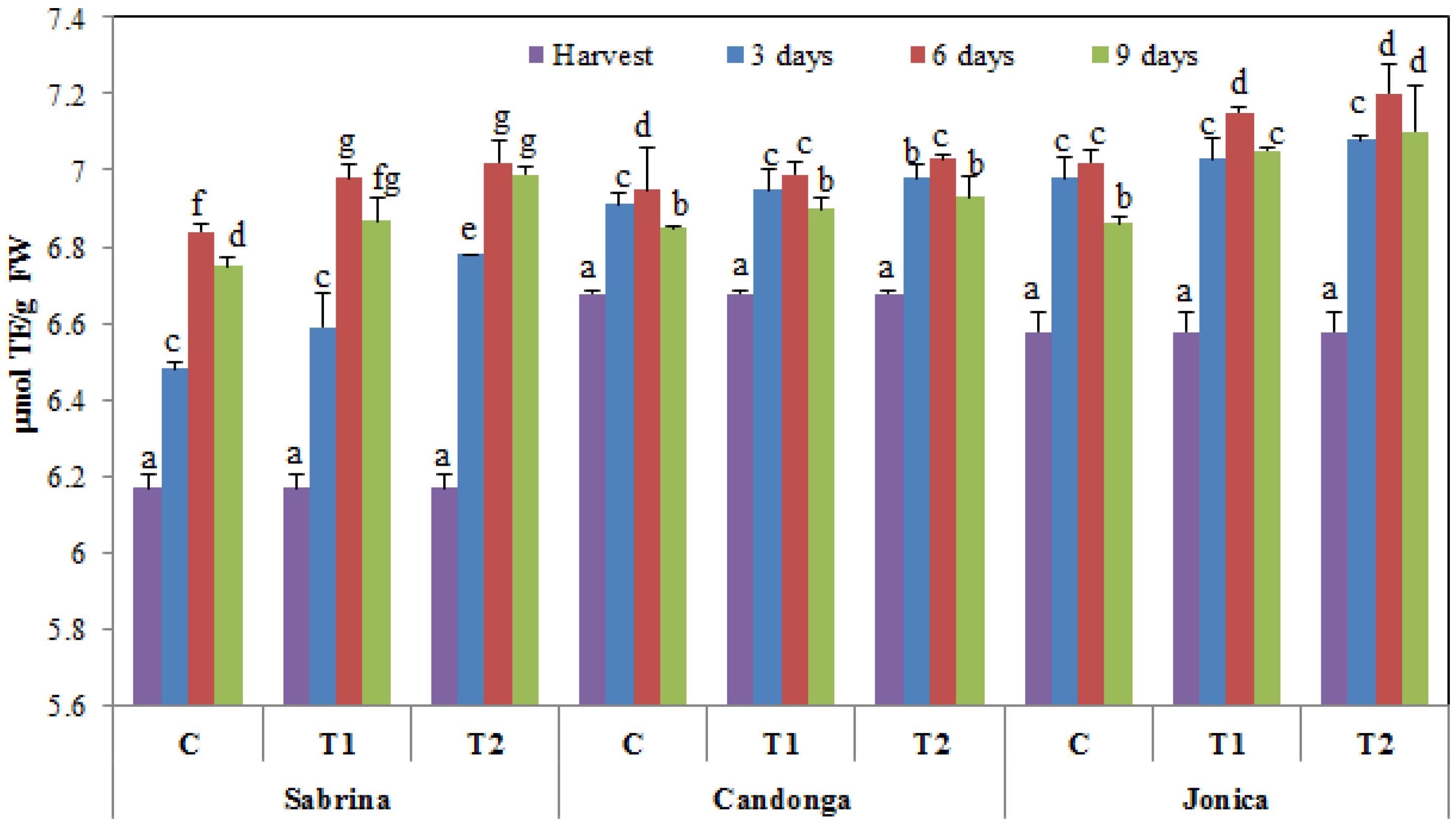
3.4. Effect of Chitosan Treatment on Antioxidant Enzymes
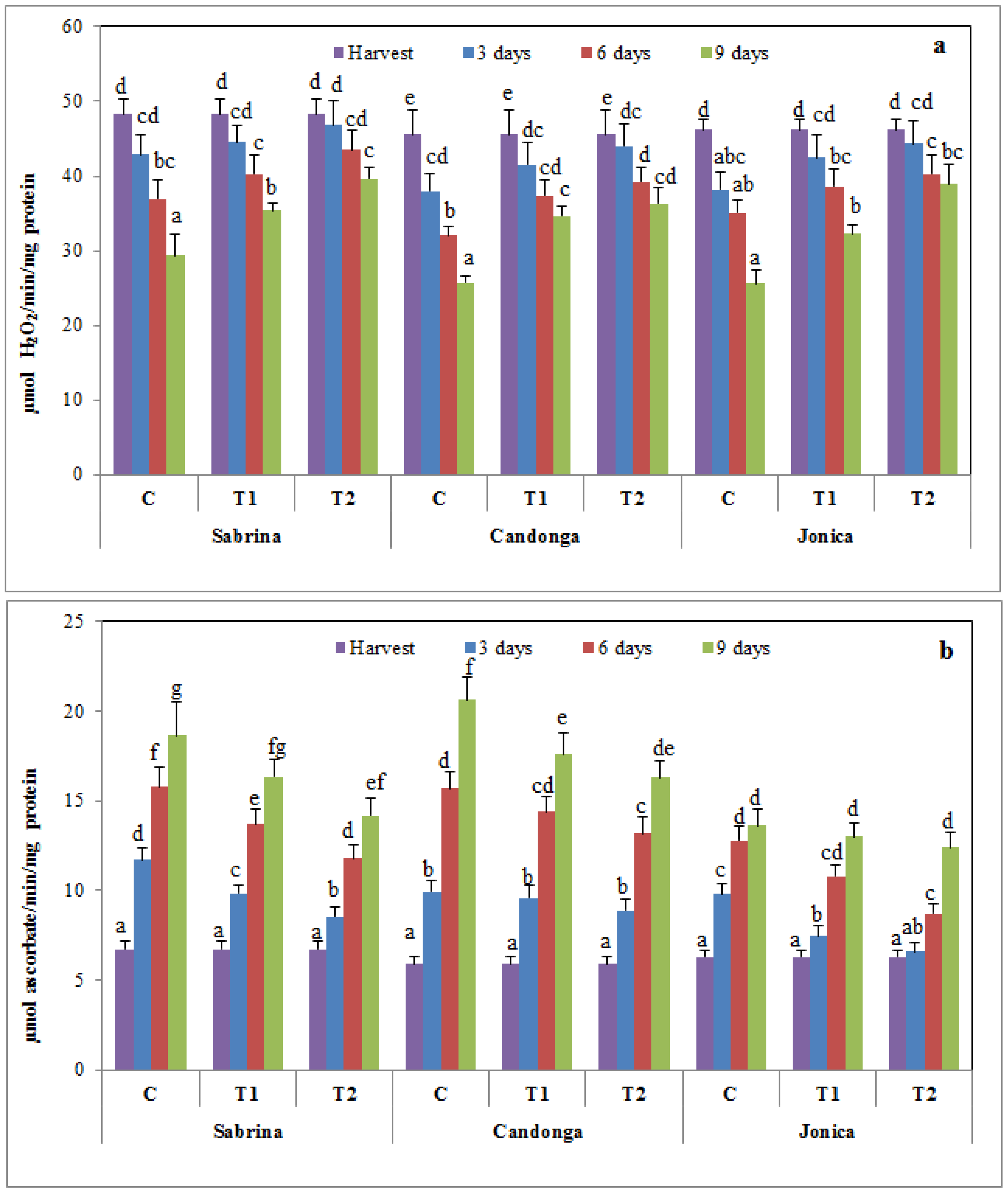
3.5. Effect of Chitosan Treatment on Membrane Damage
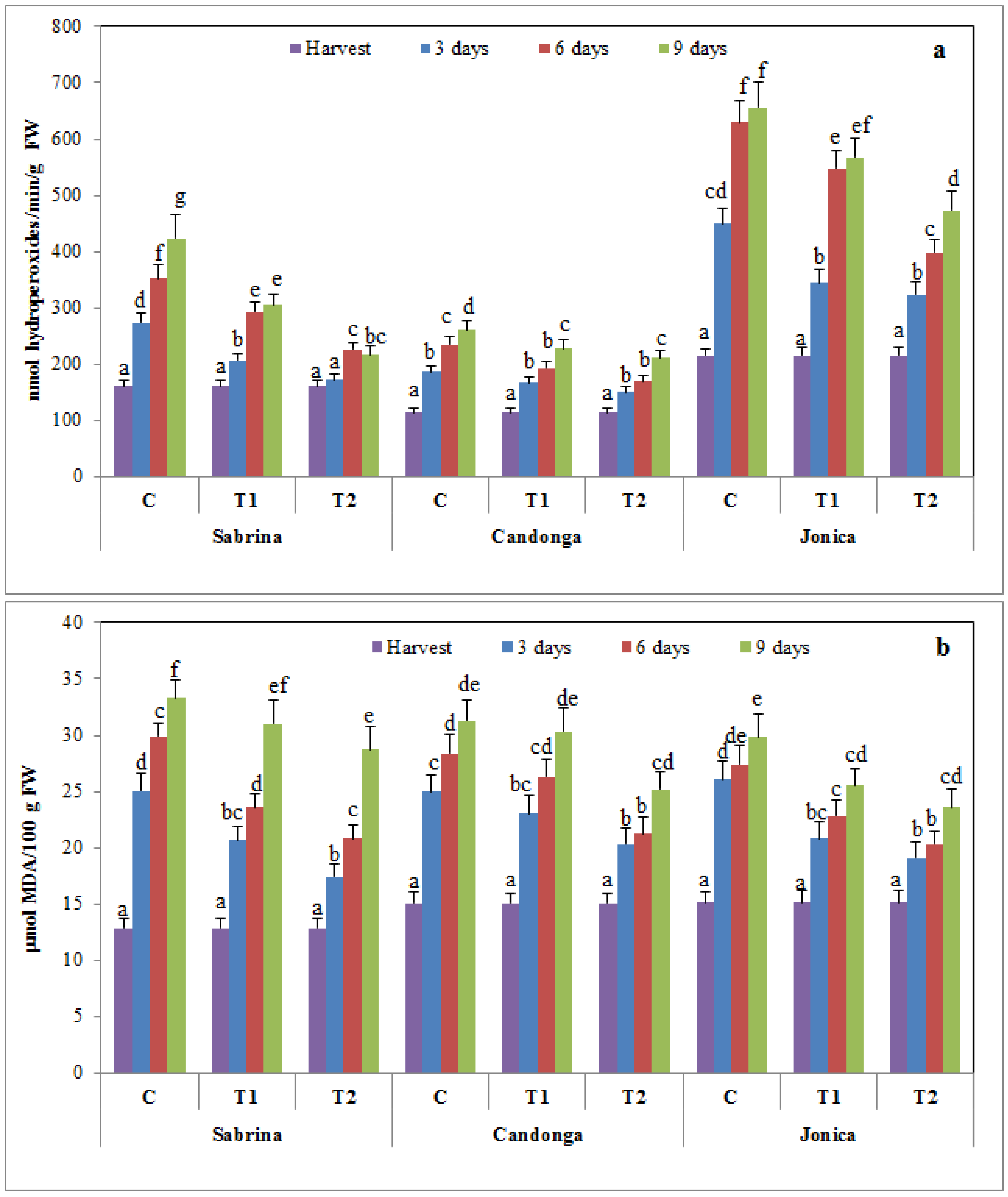
3.6. Effect of Chitosan Treatment on Enzymatic Browning
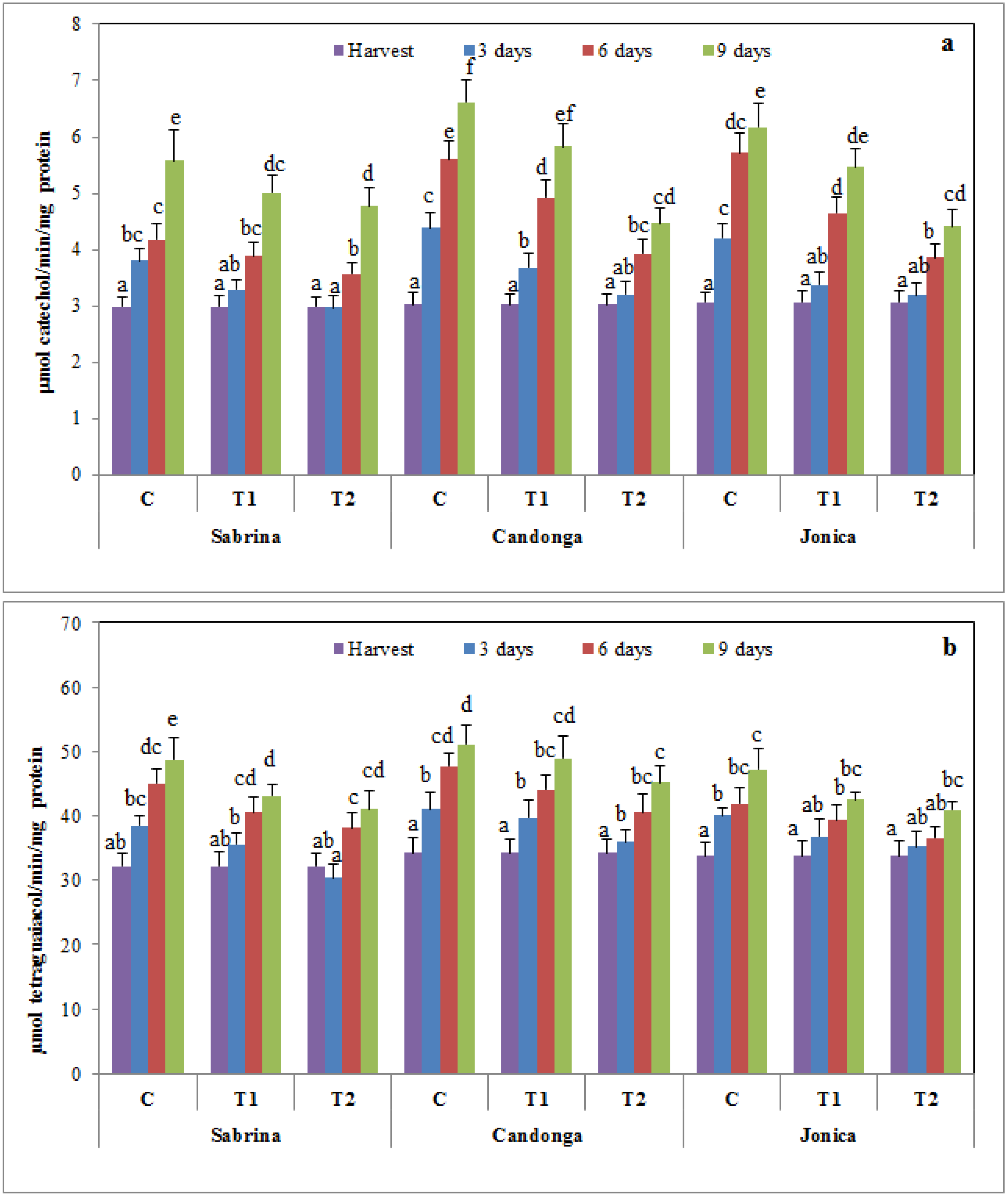
3.7. Principal Component Analysis
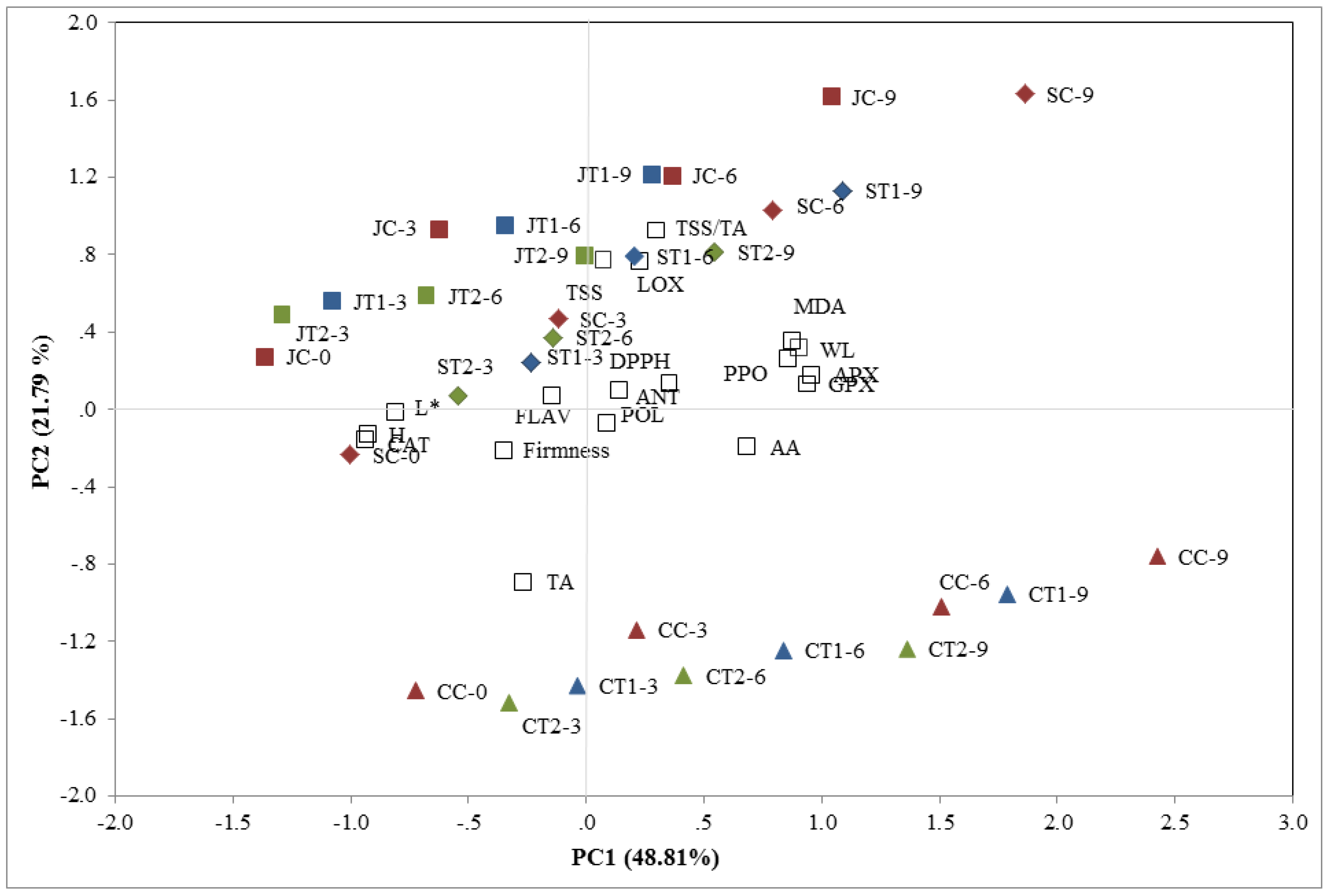
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Seeram, N.P.; Adams, L.S.; Zhang, Y.; Lee, R.; Sand, D.; Scheuller, H.S.; Heber, D. Blackberry, black raspberry, blueberry, cranberry, red raspberry, and strawberry extracts inhibit growth and stimulate apoptosis of human cancer cells in vitro. J. Agric. Food Chem. 2006, 54, 9329–9339. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Pinto, M.; Lajolo, M.F.; Genovese, M.I. Bioactive compoundsand quantification of total ellagic acid in strawberries (Bioactive compoundsand quantification of total ellagic acid in strawberries (Fragaria × ananassa Duch.). Food Chem. 2008, 107, 1629–1635. [Google Scholar] [CrossRef]
- Pineli, L.L.O.; Moretti, C.L.; dos Santos, M.S.; Campos, A.B.; Brasileiro, A.V.; Córdova, A.C.; Chiarello, M.D. Antioxidants and other chemical and physical characteristics of two strawberry cultivars at different ripeness stages. J. Food Comp. Anal. 2001, 24, 11–16. [Google Scholar] [CrossRef]
- Vu, K.D.; Hollingsworth, R.G.; Leroux, E.; Salmieri, S.; Lacroix, M. Development of edible bioactive coating based on modified chitosan for increasing the shelf life of strawberries. Food Res. Int. 2001, 44, 198–203. [Google Scholar] [CrossRef]
- Castelló, M.L.; Fito, P.J.; Chiralt, A. Changes in respiration rate and physical properties of strawberries due to osmotic dehydration and storage. J. Food Eng. 2010, 97, 64–71. [Google Scholar] [CrossRef]
- Harker, F.R.; Elgar, H.J.; Watkins, C.B.; Jackson, P.J.; Hallett, I.C. Physical and mechanical changes in strawberry fruit after high carbon dioxide treatments. Postharvest Biol. Technol. 2000, 19, 139–146. [Google Scholar] [CrossRef]
- Vicente, A.R.; Costa, M.L.; Martínez, G.A.; Chaves, A.R.; Civello, P.M. Effect of heat treatments on cell wall degradation and softening in strawberry fruit. Postharvest Biol. Technol. 2005, 38, 213–222. [Google Scholar] [CrossRef]
- Wszelaki, A.L.; Mitcham, E.J. Effects of superatmospheric oxygen on strawberry fruit quality and decay. Postharvest Biol. Technol. 2000, 20, 125–133. [Google Scholar] [CrossRef]
- Zhu, S.; Zhou, J. Effect of NO on ethylene production in strawberry fruit during storage. Food Chem. 2007, 100, 1517–1522. [Google Scholar] [CrossRef]
- Wang, S.Y.; Gao, H. Effect of chitosan-based edible coating on antioxidants, antioxidant enzyme system, and postharvest fruit quality of strawberries (Fragaria × ananassa Duch.). LWT-Food Sci. Technol. 2013, 52, 71–79. [Google Scholar] [CrossRef]
- Velickova, E.; Winkelhausen, E.; Kuzmanova, S.; Alves, V.D.; Moldão-Martins, M. Impact of chitosan-beeswax edible coatings on the quality of fresh strawberries (Fragaria ananassa cv Camarosa) under commercial storage conditions. LWT-Food Sci. Technol. 2013, 52, 80–92. [Google Scholar] [CrossRef]
- Shiekh, R.A.; Malik, M.A.; Al-Thabaiti, S.A.; Shiekh, M.A. Chitosan as a novel edible coating for fresh fruits. Food Sci. Technol. Res. 2013, 19, 139–155. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Hirano, S.; Ohe, Y.; Ono, H. Selective N-acylation of chitosan. Carbohyd. Res. 1976, 47, 315–320. [Google Scholar] [CrossRef]
- Fan, Y.; Ying, X.; Dongfeng, W.; Zhang, L.; Sun, J.; Sun, L.; Zhang, B. Effect of alginate coating combined with yeast antagonist on strawberry (Fragaria ananassa) preservation quality. Postharvest Biol. Technol. 2009, 53, 84–90. [Google Scholar] [CrossRef]
- Romanazzi, G.; Feliziani, E.; Bautista Banos, S.; Sivakumar, D. Shelf life extension of fresh fruit and vegetables by chitosan treatment. Crit. Rev. Food Sci. Nutr. 2015. [Google Scholar] [CrossRef] [PubMed]
- Kerch, G.; Sabovics, M.; Kruma, Z.; Kampuse, S.; Straumite, E. Effect of chitosan and chitooligosaccharide on vitamin C and polyphenols contents in cherries and strawberries during refrigerated storage. Eur. Food Res. Technol. 2001, 233, 351–358. [Google Scholar] [CrossRef]
- Hernández-Muñoz, P.; Almenar, E.; Ocio, M.J.; Gavara, R. Effect of calcium dips and chitosan coatings on postharvest life of strawberries (Fragaria × ananassa). Postharvest Biol. Technol. 2006, 39, 247–253. [Google Scholar] [CrossRef]
- Gol, N.B.; Patel, P.R.; Ramana Rao, T.V. Improvement of quality and shelf-life of strawberries with edible coatings enriched with chitosan. Postharvest Biol. Technol. 2013, 85, 185–195. [Google Scholar] [CrossRef]
- Petriccione, M.; de Sanctis, F.; Pasquariello, M.S.; Mastrobuoni, F.; Rega, P.; Scortichini, M.; Mencarelli, F. The effect of chitosan coating on the quality and nutraceutical traits of sweet cherry during postharvest life. Food Bioprocess Technol. 2015, 8, 394–408. [Google Scholar] [CrossRef]
- McGuire, R.G. Reporting of objective colour measurements. HortScience 1992, 27, 1254–1255. [Google Scholar]
- Tomás-Barberán, F.A.; Espín, J.C. Phenolic compounds and related enzymes as determinants of quality in fruits and vegetables. J. Agric. Food Chem. 2001, 49, 4748–4760. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colourimetry of total phenolics with phosphomolybdic- phosphotungstic acid reagents. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar]
- Giusti, M.M.; Wrolstad, R.E. Unit F1.2.1-13. Anthocyanins. Characterization and measurement with UV- visible spectroscopy. In Current Protocols in Food Analytical Chemistry; Wrolstad, R.S., Ed.; Wiley: New York, NY, USA, 2001. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Malik, A.U.; Singh, Z. Pre-storage application of polyamines improves shelf-life and fruit quality in mango. J. Hortic. Sci. Biotech. 2005, 80, 363–369. [Google Scholar]
- Bradford, M.M. A dye binding assay for protein. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Garcìa-Limones, C.; Hervàs, A.; Navas-Cortés, J.A.; Jiménez-Dìaz, R.M.; Tena, M. Induction of an antioxidant enzyme system and other oxidative stress markers associated with compatible and incompatible interactions between chickpea (Cicer arietinum L.) and Fusarium oxysporum f. sp. ciceris. Physiol. Mol. Plant Pathol. 2002, 61, 325–337. [Google Scholar] [CrossRef]
- Rao, M.V.; Paliyath, G.; Ormrod, D.P. Ultraviolet-B- and ozone-induced biochemical changes in antioxidant enzymes of Arabidopsis thaliana. Plant Physiol. 1996, 110, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Zheng, Y.; Wang, K.; Jin, P.; Rui, H. Methyl jasmonate reduces chilling injury and enhances antioxidant enzyme activity in postharvest loquat fruit. Food Chem. 2009, 115, 1458–1463. [Google Scholar] [CrossRef]
- Pérez, A.G.; Sanz, C.; Olı́as, R.; Olı́as, J.M. Lipoxygenase and hydroperoxidelyase activities in ripening strawberry fruits. J. Agric. Food Chem. 1999, 47, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Peever, T.L.; Higgins, V.J. Electrolyte leakage lipoxygenase, and lipid peroxidation induced in tomato leaf tissue by specific and nonspecific elicitors from Cladosporium fulvum. Plant Physiol. 1989, 90, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Bao, A.K.; Wang, S.M.; Wu, G.Q.; Xi, J.J.; Zhang, J.L.; Wang, C.M. Over expression of the Arabidopsis H+-PPase enhanced resistance to salt and drought stress in transgenic alfalfa (Medicago sativa L.). Plant Sci. 2009, 176, 232–240. [Google Scholar]
- El Ghaouth, A.E.; Arul, J.; Ponnampalam, R.; Boulet, M. Chitosan coating effect on storability and quality of fresh strawberries. J. Food Sci. 1991, 56, 1618–1620. [Google Scholar] [CrossRef]
- Pasquariello, M.S.; Rega, P.; Migliozzi, T.; Capuano, L.R.; Scortichini, M.; Petriccione, M. Effect of cold storage and shelf life on physiological and quality traits of early ripening pear cultivars. Sci. Hortic. 2013, 162, 341–350. [Google Scholar] [CrossRef]
- Perkins-Veazie, P. Growth and ripening of strawberry fruit. Hortic. Rev. 1995, 17, 267–297. [Google Scholar]
- Del-Valle, V.; Hernández-Muñoz, P.; Guarda, A.; Galotto, M.J. Development of a cactus-mucilage edible coating (Opuntia ficus indica) and its application to extend strawberry (Fragaria ananassa) shel-life. Food Chem. 2005, 91, 751–756. [Google Scholar] [CrossRef]
- Vargas, M.; Albors, A.; Chiralt, A.; González-Martínez, C. Quality of cold stored strawberries as affected by chitosan-oleic acid edible coatings. Postharvest Biol. Technol. 2006, 41, 164–171. [Google Scholar] [CrossRef]
- Kittur, F.S.; Saroja, N.; Tharanathan, H.R.N. Polysaccharide-based composite coating formulations for shelf-life extension of fresh banana and mango. Eur. Food Res. Technol. 2001, 213, 306–311. [Google Scholar] [CrossRef]
- Ali, A.; Muhammad, M.T.M.; Sijam, K.; Siddiqui, Y. Effect of chitosan coatings on the physicochemical characteristics of Eksotika II papaya (Carica papaya L.) fruit during cold storage. Food Chem. 2011, 124, 620–626. [Google Scholar] [CrossRef] [Green Version]
- Hong, K.; Xie, J.; Zhang, L.; Sun, D.; Gong, D. Effects of chitosan coating on postharvest life and quality of guava (Psidium guajava L.) fruit during cold storage. Sci. Hortic. 2012, 144, 172–178. [Google Scholar] [CrossRef]
- Cordenusi, B.R.; Genovese, M.I.; do Nascimento, J.R.O.; Hassimoto, N.M.A.; dos Santos, R.J.; Lajolo, F.M. Effects of temperature on the chemical composition and antioxidant activity of three strawberry cultivars. Food Chem. 2005, 91, 113–121. [Google Scholar] [CrossRef]
- Dris, R.; Niskanen, R. Quality changes of “Lobo” apples during cold storage. Acta Hortic. 1999, 485, 125–133. [Google Scholar]
- Ghasemnezhad, M.; Nezhad, M.A.; Gerailoo, S. Changes in postharvest quality of loquat (Eriobotrya japonica) fruits influenced by chitosan. Hortic. Environ. Biotechnol. 2011, 54, 40–45. [Google Scholar] [CrossRef]
- Kallio, H.; Hakala, M.; Pelkkikangas, A.M.; Lapvetelainen, A. Sugars and acids of strawberry varieties. Eur. Food Res. Tech. 2000, 212, 81–85. [Google Scholar] [CrossRef]
- Díaz-Mula, H.M.; Zapata, P.J.; Guillén, F.; Martínez-Romero, D.; Castillo, S.; Serrano, M.; Valero, D. Changes in hydrophilic and lipophilic antioxidant activity and related bioactive compounds during postharvest storage of yellow and purple plum cultivars. Postharvest Biol. Technol. 2009, 51, 354–363. [Google Scholar] [CrossRef]
- Diaz-Mula, H.M.; Serrano, M.; Valero, D. Alginate coatings preserve fruit quality and bioactive compounds during storage sweet cherry fruit. Food Bioprocess Tech. 2012, 5, 2990–2997. [Google Scholar] [CrossRef]
- Hernandez-Munoz, P.; Almenar, E.; del Valle, V.; Gavara, R. Effect of chitosan coating combined with postharvest calcium treatment on strawberry (Fragaria × ananassa) quality during refrigerated storage. Food Chem. 2008, 110, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yu, T. Effect of chitosan on incidence of brown rot, quality and physiological attributes of postharvest peach fruit. J. Sci. Food Agric. 2001, 81, 269–274. [Google Scholar] [CrossRef]
- Dong, H.; Cheng, L.; Tan, J.; Zheng, K.; Jiang, Y. Effects of chitosan coating on quality and shelf life of peeled litchi fruit. J. Food Eng. 2004, 64, 355–358. [Google Scholar] [CrossRef]
- Sturm, K.; Koron, D.; Stampar, F. The composition of fruit of different strawberry varieties depending on maturity stage. Food Chem. 2003, 83, 417–422. [Google Scholar] [CrossRef]
- Clifford, M.N.; Brown, J.E. Dietary flavonoids and health–broadening the perspective. In Flavonoids, Chemistry, Biochemistry and Applications; Andersen, Ø.M., Markham, K.R., Eds.; CRC Press: Boca Raton, FL, USA, 2005; pp. 319–370. [Google Scholar]
- Buendia, B.; Gil, M.I.; Tudela, J.A.; Gady, A.L.; Medina, J.J.; Soria, C.; López, J.M.; Tomás-Barberán, F.A. HPLC-MS analysis of proanthocyanidin oligomers and other phenolics in 15 strawberry cultivars. J. Agric. Food Chem. 2010, 58, 3916–3926. [Google Scholar] [CrossRef] [PubMed]
- Määttä-Riihinen, K.R.; Kamal-Eldin, A.; Törrönen, A.R. Identification and quantification of phenolic compounds in berries of Fragaria and Rubus species (Family Rosaceae). J. Agric. Food Chem. 2004, 52, 6178–6187. [Google Scholar] [CrossRef] [PubMed]
- Van de Velde, F.; Tarola, A.M.; Guemes, D.; Pirovani, M.E. Bioactive compounds and antioxidant capacity of Camarosa and Selva strawberries (Fragaria × ananassa Duch.). Foods 2013, 2, 120–131. [Google Scholar] [CrossRef]
- Dang, Q.F.; Yan, J.Q.; Li, Y.; Cheng, X.J.; Liu, C.S.; Chen, X.G. Chitosan acetate as an active coating material and its effects on the storing of Prunus avium L. J. Food Sci. 2010, 75, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Zeng, K.; Deng, Y.; Ming, J.; Deng, L. Induction of disease resistance and ROS metabolism in navel oranges by chitosan. Sci. Hortic. 2010, 126, 223–228. [Google Scholar] [CrossRef]
- Chelikani, P.; Fita, I.; Loewen, P.C. Diversity of structures and properties among catalases. Cell. Mol. Life Sci. 2004, 61, 192–208. [Google Scholar] [CrossRef] [PubMed]
- Pasquariello, M.S.; di Patre, D.; Mastrobuoni, F.; Zampella, L.; Scortichini, M.; Petriccione, M. Influence of postharvest chitosan treatment on enzymatic browning and antioxidant enzyme activity in sweet cherry fruit. Postharvest Biol. Technol. 2015, 109, 45–56. [Google Scholar] [CrossRef]
- Davletova, S.; Rizhsky, L.; Liang, H.; Shengqiang, D.; Oliver, D.; Coutu, J.; Shulaev, V.; Schlauch, K.; Mittler, R. Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis. Plant Cell 2004, 17, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.P.; Jiang, A.L.; Xu, Y.; Wang, Y.S. Responses of physiology and quality of sweet cherry fruit to different atmosphere in storage. Food Chem. 2004, 87, 43–49. [Google Scholar] [CrossRef]
- Jiang, Y.; Duan, X.; Joyce, D.; Li, Z.; Zhang, J. Advances in understanding of enzymatic browning in harvested litchi fruit. Food Chem. 2004, 88, 443–446. [Google Scholar] [CrossRef]
- Jin, P.; Shang, H.; Chen, J.; Zhu, H.; Zhao, Y.; Zheng, Y. Effect of 1-Methylcyclopropene on chilling injury and quality of peach fruit during cold storage. J. Food Sci. 2011, 76, S485–S491. [Google Scholar] [CrossRef] [PubMed]
- Finnegan, E.; O’Beirne, D. Characterising and tracking deterioration patterns of fresh-cut fruit using principal component analysis-Part I. Postharvest Biol. Technol. 2015, 100, 73–80. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petriccione, M.; Mastrobuoni, F.; Pasquariello, M.S.; Zampella, L.; Nobis, E.; Capriolo, G.; Scortichini, M. Effect of Chitosan Coating on the Postharvest Quality and Antioxidant Enzyme System Response of Strawberry Fruit during Cold Storage. Foods 2015, 4, 501-523. https://doi.org/10.3390/foods4040501
Petriccione M, Mastrobuoni F, Pasquariello MS, Zampella L, Nobis E, Capriolo G, Scortichini M. Effect of Chitosan Coating on the Postharvest Quality and Antioxidant Enzyme System Response of Strawberry Fruit during Cold Storage. Foods. 2015; 4(4):501-523. https://doi.org/10.3390/foods4040501
Chicago/Turabian StylePetriccione, Milena, Francesco Mastrobuoni, Maria Silvia Pasquariello, Luigi Zampella, Elvira Nobis, Giuseppe Capriolo, and Marco Scortichini. 2015. "Effect of Chitosan Coating on the Postharvest Quality and Antioxidant Enzyme System Response of Strawberry Fruit during Cold Storage" Foods 4, no. 4: 501-523. https://doi.org/10.3390/foods4040501





