Efficacy of Acetic Acid against Listeria monocytogenes Attached to Poultry Skin during Refrigerated Storage
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of Bacterial Inoculum
2.2. Inoculation of Poultry and Treatment
2.3. Sensorial Analysis
2.4. Microbiological Analyses and pH Determination
2.5. Statistical Analysis
3. Results and Discussion
3.1. Microbiological Quality
| Batch | Days of Storage | ||||
|---|---|---|---|---|---|
| 0 | 1 | 3 | 6 | 8 | |
| Control | 5.57 ± 0.13 a | 7.13 ± 0.04 a | 7.69 ± 0.44 a | 9.83 ± 0.03 a | 10.31 ± 0.01 a |
| 1% Acetic acid | 4.68 ± 0.01 b | 4.68 ± 0.06 b | 6.29 ± 0.03 b | 7.93 ± 0.03 b | 8.84 ± 0.03 b |
| 2% Acetic acid | 4.47 ± 0.01 b | 4.47 ± 0.02 b | 5.87 ± 0.02 b | 7.49 ± 0.01 c | 8.31 ± 0.01 c |
| Batch | Days of Storage | ||||
|---|---|---|---|---|---|
| 0 | 1 | 3 | 6 | 8 | |
| Control | 5.17 ± 0.03 a | 5.62 ± 0.01 a | 6.88 ± 0.01 a | 9.02 ± 0.03 a | 9.57 ± 0.01 a |
| 1% Acetic acid | 4.87 ± 0.01 b | 4.91 ± 0.01 b | 5.72 ± 0.03 b | 6.77 ± 0.03 b | 8.12 ± 0.04 b |
| 2% Acetic acid | 4.68 ± 0.02 c | 4.69 ± 0.03 b | 5.63 ± 0.01 b | 6.59 ± 0.01 c | 7.89 ± 0.01 c |
| Batch | Days of Storage | ||||
|---|---|---|---|---|---|
| 0 | 1 | 3 | 6 | 8 | |
| Control | 3.04 ± 0.04 a | 4.21 ± 0.06 a | 5.09 ± 0.01 a | 5.70 ± 0.07 a | 6.54 ± 0.08 a |
| 1% Acetic acid | 2.13 ± 0.01 b | 2.64 ± 0.01 b | 3.95 ± 0.01 b | 4.55 ± 0.01 b | 5.51 ± 0.04 b |
| 2% Acetic acid | 1.63 ± 0.04 c | 2.60 ± 0.01 b | 3.84 ± 0.11 b | 4.34 ± 0.07 b | 5.04 ± 0.03 c |
3.2. pH Evolution
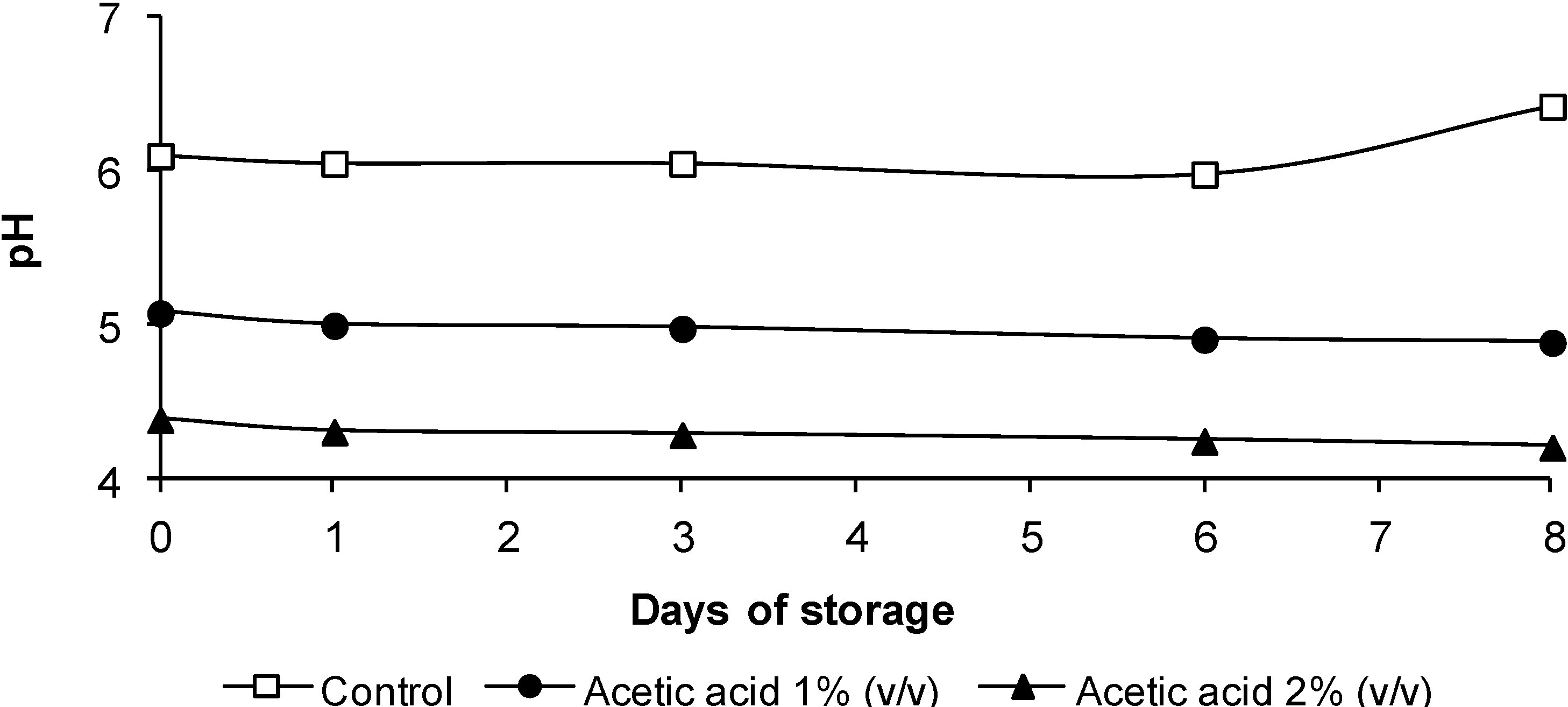
3.3. Listeria monocytogenes
| Batch | Days of Storage | ||||
|---|---|---|---|---|---|
| 0 | 1 | 3 | 6 | 8 | |
| Control | 4.90 ± 0.20 a | 5.53 ± 0.03 a | 7.38 ± 0.03 a | 7.74 ± 0.01 a | 8.77 ± 0.01 a |
| 1% Acetic acid | 4.53 ± 0.01 ab | 4.38 ± 0.04 b | 6.25 ± 0.01 b | 7.18 ± 0.06 b | 7.88 ± 0.03 b |
| 2% Acetic acid | 4.25 ± 0.07 b | 4.28 ± 0.01 b | 5.72 ± 0.06 c | 6.60 ± 0.01 c | 7.46 ± 0.01 c |
3.4. Sensorial Quality
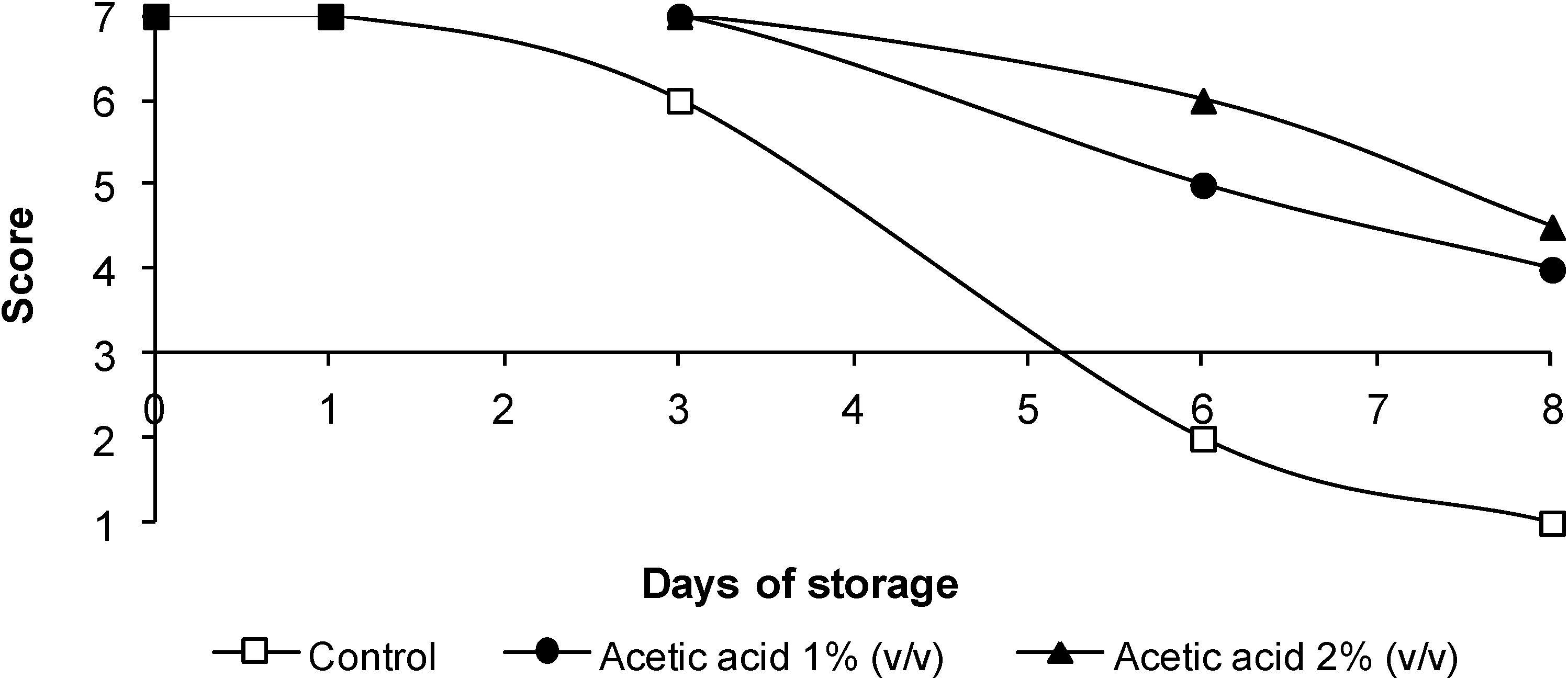
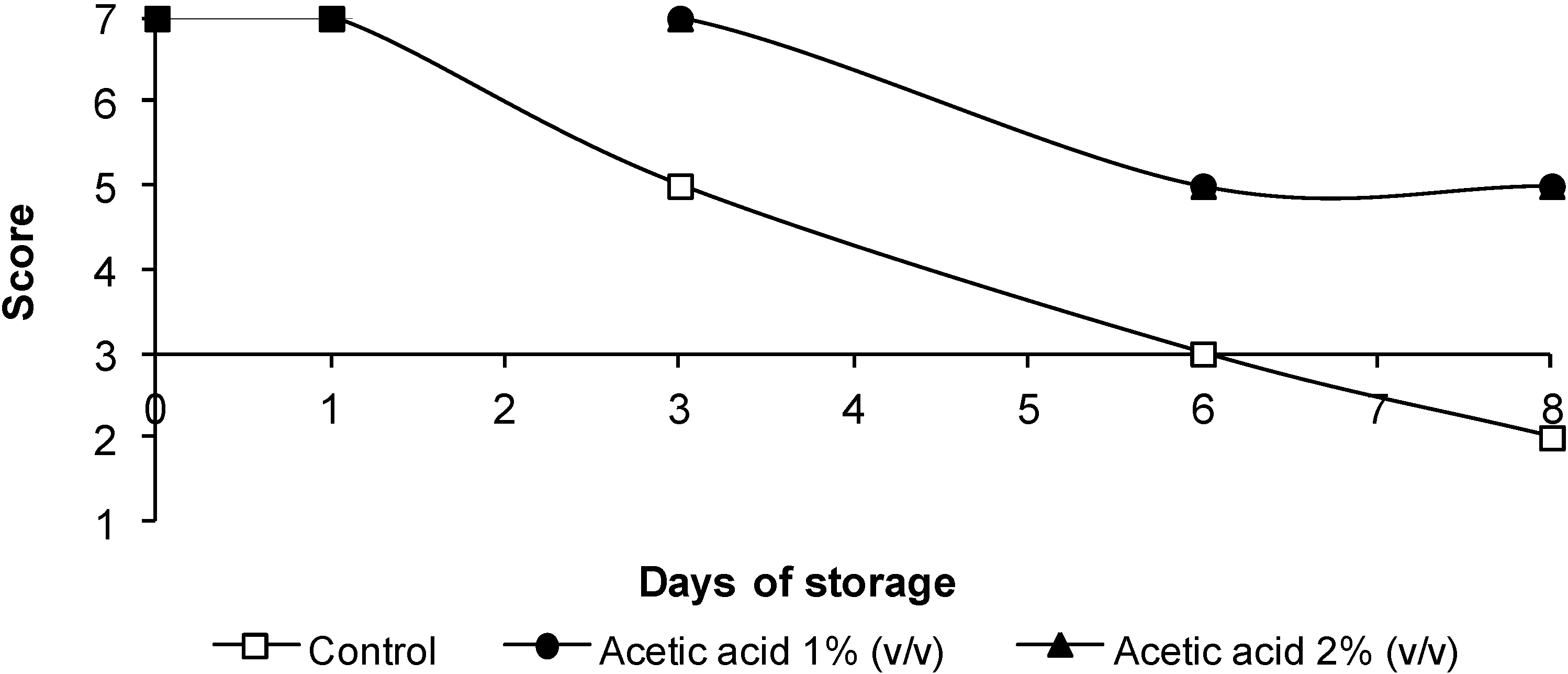
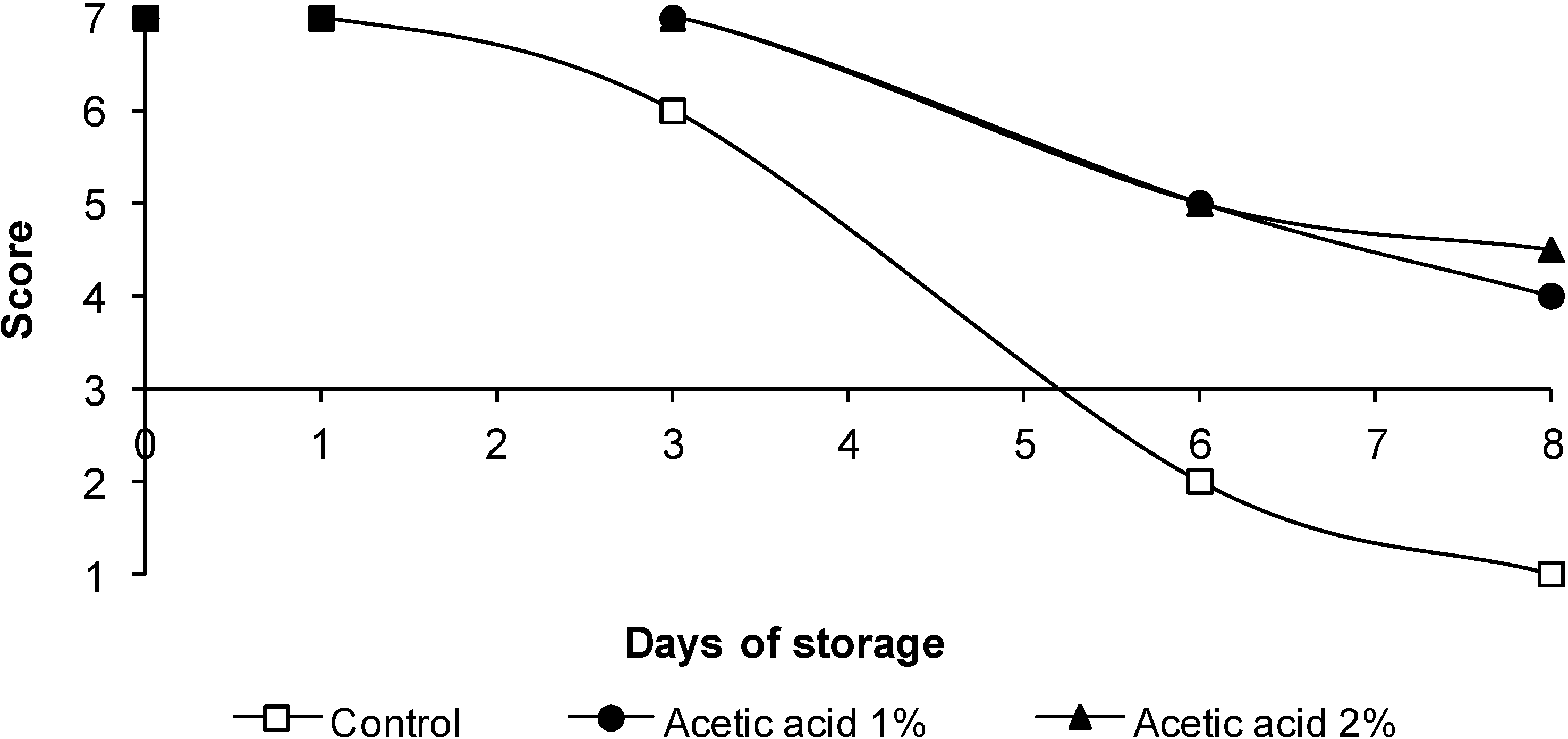
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- ICMSF (International Commission on Microbiological Specifications for Foods). Microorganisms in Foods. 6. Microbial Specifications of Food Commodities; Blackie Academic & Professional: London, UK, 1998. [Google Scholar]
- Bailey, J.S.; Fletcher, D.L.; Cox, N.A. Recovery and serotype distribution of Listeria monocytogenes from broiler chickens in the southeastern United States. J. Food Prot. 1989, 52, 148–150. [Google Scholar]
- Genigeorgis, C.A.; Dutulescu, D.; Garazabar, J.F. Prevalence of Listeria spp. in poultry meat at the supermarket and slaughterhouse level. J. Food Prot. 1989, 52, 618–624. [Google Scholar]
- Uyttendale, M.R.; Neyts, K.D.; Lips, R.M.; Devebere, J.M. Incidence of Listeria monocytogenes in poultry products obtained from Belgian and French abbatoirs. Food Microbiol. 1997, 14, 339–345. [Google Scholar]
- Schuchat, A.; Deaver, K.; Wenger, J.D.; Swaminathan, B.; Broome, C.V. Role of food in sporadic listeriosis: I. Case-control-study of dietary risk factors. J. Am. Med. Assoc. 1992, 267, 2041–2045. [Google Scholar]
- Carpenter, C.E.; Smith, J.V.; Broadbent, J.R. Efficacy of washing meat surfaces with 2% levulinic, acetic, or lactic acid for pathogen decontamination and residual growth inhibition. Meat Sci. 2011, 58, 256–260. [Google Scholar] [CrossRef]
- Gonzalez-Fandos, E.; Dominguez, J.L. Effect of potassium sorbate on the growth of Listeria monocytogenes on fresh poultry. Food Control 2007, 18, 842–846. [Google Scholar]
- Gonzalez-Fandos, E.; Herrera, B. Efficacy of malic acid against Listeria monocytogenes attached in poultry skin during refrigerated storage. Poult. Sci. 2013, 92, 1936–1941. [Google Scholar]
- Gonzalez-Fandos, E.; Herrera, B. Efficacy of propionic acid against Listeria monocytogenes attached in poultry skin during refrigerated storage. Food Control 2013, 34, 601–606. [Google Scholar]
- Surekha, M.; Reddy, S.M. Preservatives. Classsification and properties. In Encyclopedia of Food Microbiology; Robinson, R.K., Batt, C.A., Patel, C., Eds.; Academic Press: New York, NY, USA, 2000; pp. 1710–1717. [Google Scholar]
- Dickens, J.A.; Whittemore, A.D. The effects of extended chilling times with acetic acid on the temperature and microbiological quality of processed poultry carcasses. Poult. Sci. 1995, 74, 1044–1048. [Google Scholar] [CrossRef] [PubMed]
- Dickens, J.A.; Whittemore, A.D. The effect of acetic acid and hydrogen peroxide application during defeathering on the microbiological quality of broiler carcasses. Poult. Sci. 1997, 76, 657–660. [Google Scholar] [CrossRef] [PubMed]
- Dorsa, W.J.; Cutter, C.N.; Siragusa, G. Effects of acetic acid, lactic acid and trisodium phosphate on the microflora of refrigerated beef carcass surface tissue inoculated with Escherichia coli O157:H7, Listeria innocua and Clostridium sporogenes. J. Food Prot. 1997, 60, 619–624. [Google Scholar]
- Jiménez, S.M.; Caliusco, M.F.; Tiburzi, M.C.; Salsi, M.S.; Pirovani, M.E. Predictive models for reduction of Salmonella Hadar on chicken skin during single and double sequential spraying treatments with acetic acid. J. Appl. Microbiol. 2007, 103, 528–535. [Google Scholar]
- Sakhare, P.Z.; Sachindra, N.M.; Yashoda, K.P.; Rao, D.N. Efficacy of intermittent decontamination treatments during processing in reducing the microbial load on broiler chicken carcass. Food Control 1999, 10, 189–194. [Google Scholar] [CrossRef]
- Greer, G.C.; Dilts, B. Factors affecting the susceptiblility of meatborne pathogens and spoilage bacteria to organic acids. Food Res. Int. 1992, 25, 355–364. [Google Scholar] [CrossRef]
- Ahamad, N.; Marth, E.H. Behaviour of Listeria monocytogenes at 7, 13, 21 and 35 °C in tryptose broth acidified with acetic, citric, or lactic acid. J. Food Prot. 1989, 52, 688–695. [Google Scholar]
- Cunningham, E.; O’Byrne, C.; Oliver, J.D. Effect of weak acids on Listeria monocytogenes survival: Evidence for a viable but nonculturable state in response to low pH. Food Control 2009, 20, 1141–1144. [Google Scholar] [CrossRef]
- George, S.M.; Richardson, L.C.C.; Peck, M.W. Predictive models of the effect of temperature, pH and acetic and lactic acids on the growth of Listeria monocytogenes. Food Microbiol. 1996, 32, 73–90. [Google Scholar] [CrossRef]
- Vermeulen, A.; Gysemans, K.; Bernaerts, K.; Geeraerd, A.H.; van Impe, J.; Debevere, J.; Devlieghere, F. Influence of pH, water activity and acetic acid concentration on Listeria monocytogenes at 7 °C: Data collection for the development of a growth/no growth model. Int. J. Food Microbiol. 2007, 114, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Dickson, J.S.; Siragusa, G.R. Survival of Salmonella typhimurium, Escherichia coli O157:H7 and Listeria monocytogenes during storage on beef sanitized with organic acids. J. Food Saf. 1994, 14, 313–327. [Google Scholar] [CrossRef]
- Anzaldúa-Morales, A. La Evaluación Sensorial de los Alimentos en la Teoría y en la Práctica; (in Spanish). Acribia: Zaragoza, Spain, 1994. [Google Scholar]
- ICMSF (International Commission on Microbiological Specifications for Foods). Microorganisms in Foods. 1: Their Significance and Methods of Enumeration, 2nd ed.; University of Toronto Press: Toronto, ON, Canada, 1978. [Google Scholar]
- Mossel, D.A.A.; Corry, J.E.L.; Struijk, C.B.; Baird, R.M. Essentials of the Microbiology of Foods. A Textbook for Advanced Studies; John Wiley and Sons Ltd.: Chichester, UK, 1995. [Google Scholar]
- Seeliger, H.P.R.; Jones, D. Listeria . In Bergey’s Manual of Systematic Bacteriology; Sneath, P.H.A., Nair, N.S., Sharpe, M.E., Holt, J.G., Eds.; Williams and Wilkins: Baltimore, MD, USA, 1986; Volume 2, pp. 1235–1245. [Google Scholar]
- Fabrizio, K.A.; Sharma, R.R.; Demirci, A.; Cutter, C.N. Comparison of electrolyzed oxidizing water with various antimicrobial interventions to reduce Salmonella species on poultry. Poult. Sci. 2002, 81, 1598–1605. [Google Scholar] [CrossRef] [PubMed]
- Dickens, J.A.; Lyon, B.G.; Whittemore, A.D.; Lyon, C.E. The effect of an acetic acid dip on carcass appearance, microbiological quality, and cooked breast meat texture and flavor. Poult. Sci. 1994, 73, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Eggenberger, L.; Niebuhr, S.E.; Acuff, G.R.; Dickson, J.S. Hot water and organic acid interventions to control microbiological contamination on hog carcasses during processing. J. Food Prot. 2002, 65, 1248–1252. [Google Scholar] [PubMed]
- Fu, A.H.; Sebranek, J.G.; Murano, E.A. Microbial and quality characteristics of pork cuts from carcasses treated with sanitizing sprays. J. Food Sci. 1994, 59, 306–309. [Google Scholar] [CrossRef]
- Hardin, M.D.; Acuff, G.R.; Lucia, L.M.; Oman, J.S.; Savell, J.W. Comparison of methods for decontamination from beef carcass surfaces. J. Food Prot. 1995, 58, 368–374. [Google Scholar]
- Jiménez, S.M.; Salsi, M.S.; Tiburzi, M.C.; Rafaghelli, R.C.; Pirovani, M.E. Combined use of acetic acid treatment and modified atmosphere packaging for extending the shelf-life of chilled chicken breast portions. J. Appl. Microbiol. 1999, 87, 339–344. [Google Scholar]
- Gill, C.O.; Landers, C. Microbiological effects of carcass decontaminating treatments at four beef packing plants. Meat Sci. 2003, 65, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Fandos, E.; Dominguez, J.L. Efficacy of lactic acid against Listeria monocytogenes attached to poultry skin during refrigerated storage. J. Appl. Microbiol. 2006, 101, 1331–1339. [Google Scholar]
- Gonzalez-Fandos, E.; Herrera, B.; Maya, N. Efficacy of citric acid against Listeria monocytogenes attached to poultry skin during refrigerated storage. Int. J. Food Sci. Technol. 2009, 44, 262–268. [Google Scholar]
- Doores, S. Organic acids. In Antimicrobials in Foods; Branen, A.L., Davidson, P.M., Eds.; Marcel Dekker Inc.: New York, NY, USA, 1983; pp. 75–108. [Google Scholar]
- Cherrington, C.A.; Hinton, A.M.; Pearson, G.R.; Copra, I. Inhibition of Escherichia coli K12 by short-chain organic acids: Lack of evidence for induction of the SOS response. J. Appl. Bacteriol. 1991, 70, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Farber, J.M.; Sanders, G.W.; Dunfield, S.; Prescott, R. The effect of various acidulants on the growth of Listeria monocytogenes. Lett. Appl. Microbiol. 1989, 9, 181–183. [Google Scholar] [CrossRef]
- Thomas, L.V. Preservatives. Sorbic acid. In Encyclopedia of Food Microbiology; Robinson, R.K., Batt, C.A., Patel, C., Eds.; Academic Press: New York, NY, USA, 2000; pp. 1769–1776. [Google Scholar]
- Glass, K.P.; Doyle, M.P. Listeria monocytogenes in processed meat products during refrigerated storage. Appl. Environ. Microbiol. 1989, 55, 1565–1569. [Google Scholar] [PubMed]
- Barnes, E.M. Microbiological problems of poultry at refrigerator temperatures. A review. J. Sci. Food Agric. 1976, 27, 777–782. [Google Scholar] [CrossRef]
- Waterman, S.R.; Small, P.L.C. Acid-sensitive enteric pathogens are protected from killing under extremely acidic conditions of pH 2.5 when they are inoculated onto certain solid food sources. Appl. Environ. Microbiol. 1998, 64, 3882–3886. [Google Scholar]
- Smulders, F.J.M.; Greer, G.G. Integrating microbial decontamination with organic acids in HACCP programmes for muscle foods: Prospects and controversies. Int. J. Food Microbiol. 1998, 44, 149–169. [Google Scholar] [CrossRef] [PubMed]
- Elliot, P.H.; Tomlins, R.J.; Gray, R.J.H. Control of microbial spoilage on fresh poultry using a combiantion potassium sorbate/carbon dioxide packaging system. J. Food Sci. 1985, 50, 1360–1363. [Google Scholar] [CrossRef]
- Studer, P.; Schmidt, R.E.; Gallo, L.; Schmidt, W. Microbial spoilage of refrigerated fresh broilers. II. Effect of packaging on microbial association of poultry carcasses. Lebensm. Wiss. Technol. 1988, 21, 224–228. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gonzalez-Fandos, E.; Herrera, B. Efficacy of Acetic Acid against Listeria monocytogenes Attached to Poultry Skin during Refrigerated Storage. Foods 2014, 3, 527-540. https://doi.org/10.3390/foods3030527
Gonzalez-Fandos E, Herrera B. Efficacy of Acetic Acid against Listeria monocytogenes Attached to Poultry Skin during Refrigerated Storage. Foods. 2014; 3(3):527-540. https://doi.org/10.3390/foods3030527
Chicago/Turabian StyleGonzalez-Fandos, Elena, and Barbara Herrera. 2014. "Efficacy of Acetic Acid against Listeria monocytogenes Attached to Poultry Skin during Refrigerated Storage" Foods 3, no. 3: 527-540. https://doi.org/10.3390/foods3030527





