The Role of ccpA in Nitrogen Source-Induced Heat and Oxidative Stress Tolerance Changes in Lacticaseibacillus rhamnosus
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Plasmids, and Growth Conditions
| Strains | Genotype or Characteristics |
| L. rhamnosus hsryfm1301 | Chinese centenarian; CGMCC No. 8545 |
| L. rhamnosus ΔccpA | ccpA mutant of L. rhamnosus hsryfm 1301 |
| L. rhamnosus ∆ccpA/pccpA(L.r.)+ | L. rhamnosus ΔccpA with pccpA(L.r.)+ |
| L. rhamnosus ∆ccpA/pccpA(L.p.)+ | L. rhamnosus ΔccpA with pccpA(L.p.)+ |
| L. paracasei PC-01 | Commercially available drink Youyi C |
| E. coli XL1-Blue | SHBCC, Shanghai, China |
| Plasmids | Genotype or Characteristics |
| pUC19e | Ermr, pUC19 derivative [12] |
| pUC19e-ccpAUD | Ermr, pUC19e derivative with upstream and downstream sequences of ccpA gene |
| pMG36e | Ermr [23] |
| pccpA(L.r.)+ | Ermr, pMG36e derivative with ccpA gene of L. rhamnosus hsryfm 1301 |
| pccpA(L.p.)+ | Ermr, pMG36e derivative with ccpA gene of L. paracasei PC-01 |
2.2. Construction of Plasmids
2.3. Gene Deletion and Complementation
2.4. Growth Investigation of L. rhamnosus Strains
2.5. Detection of Heat Stress and Oxidative Stress Tolerance
2.6. RNA Isolation, and RNA Sequencing (RNA-seq)
2.7. Mapping Reads to the Reference Genome and Normalized Gene Expression
2.8. Differential Expression Analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) Enrichment Analysis
2.9. Nucleotide Sequence Accession Numbers
2.10. Statistical Analysis
3. Results
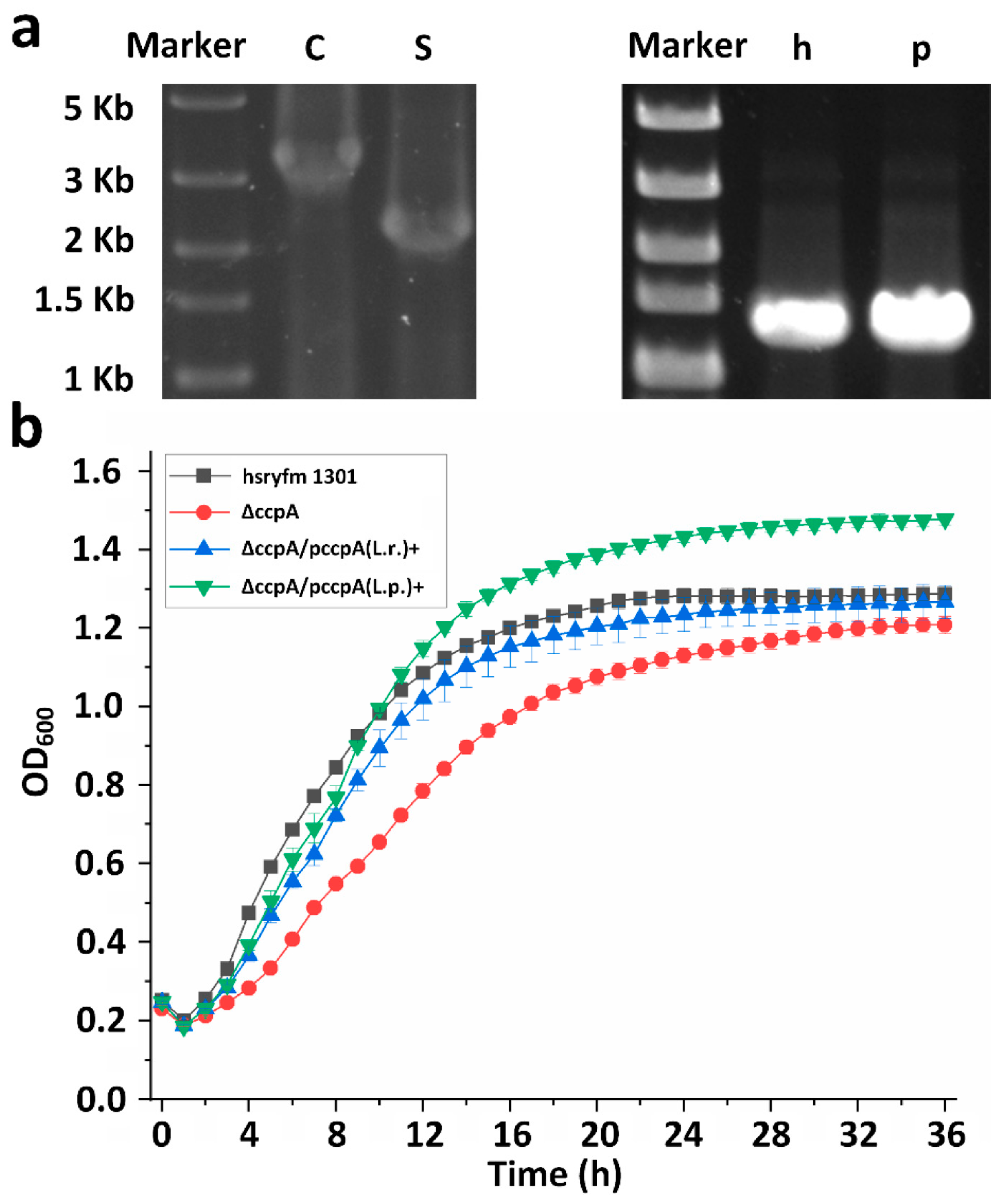
3.1. Deletion and Complementation of ccpA Gene in L. rhamnosus Hsryfm 1301
3.2. Impact of ccpA Gene Knockout on Heat and Oxidative Stress Tolerance in L. rhamnosus
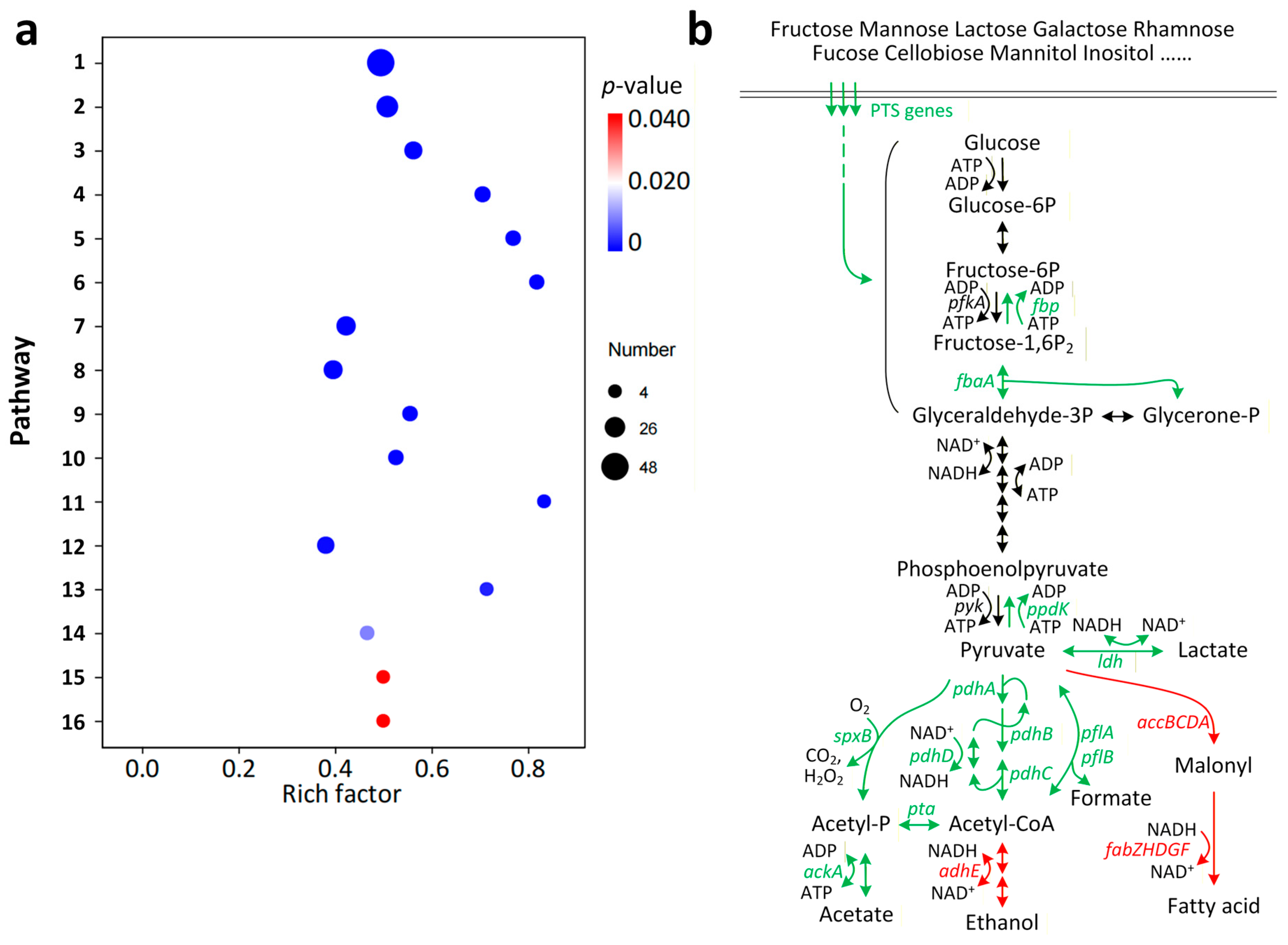
3.3. The Impact of ccpA Knockout on Gene Transcription in L. rhamnosus
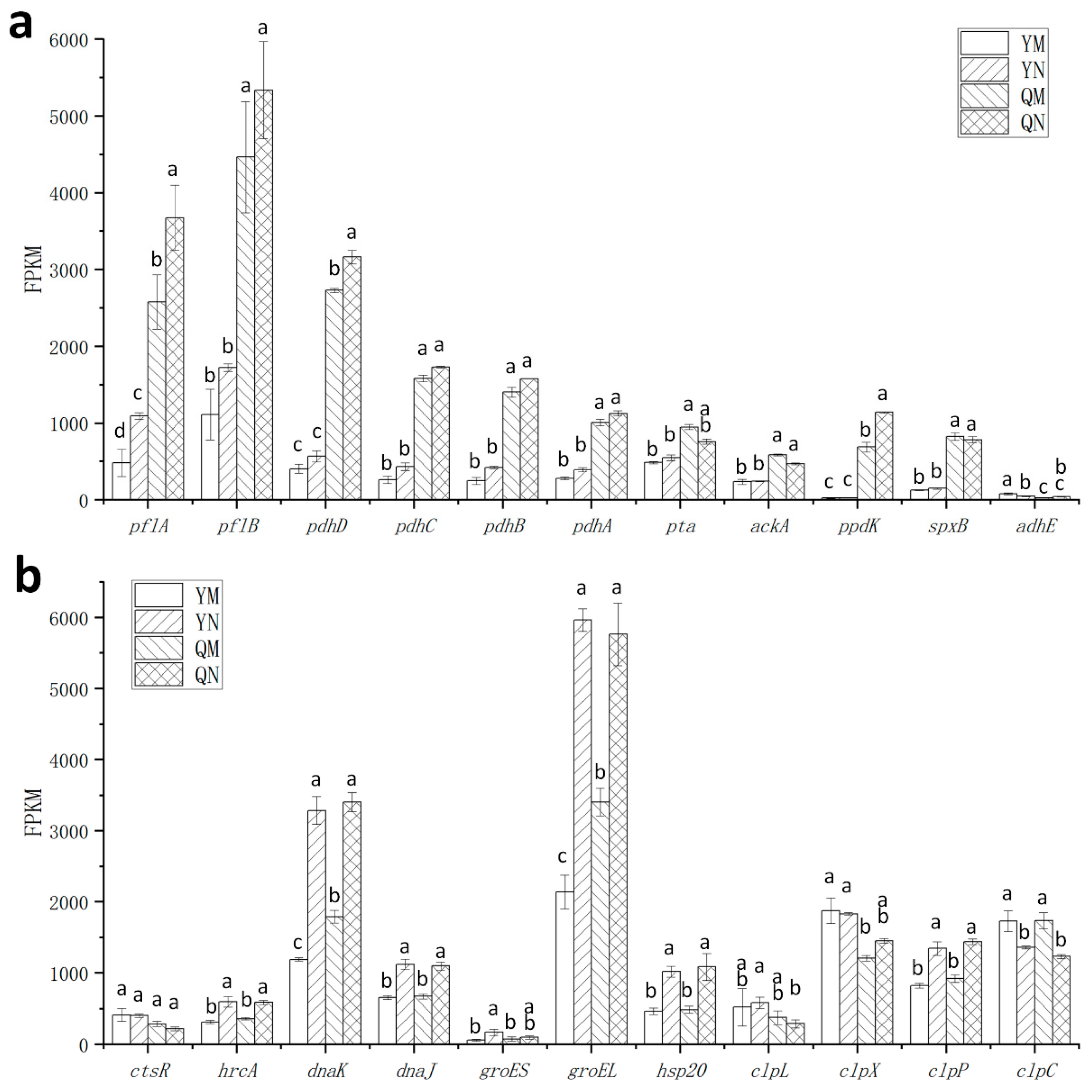
3.4. Changes in Heat and Oxidative Stress Tolerance of ΔccpA Under Different Nitrogen Conditions
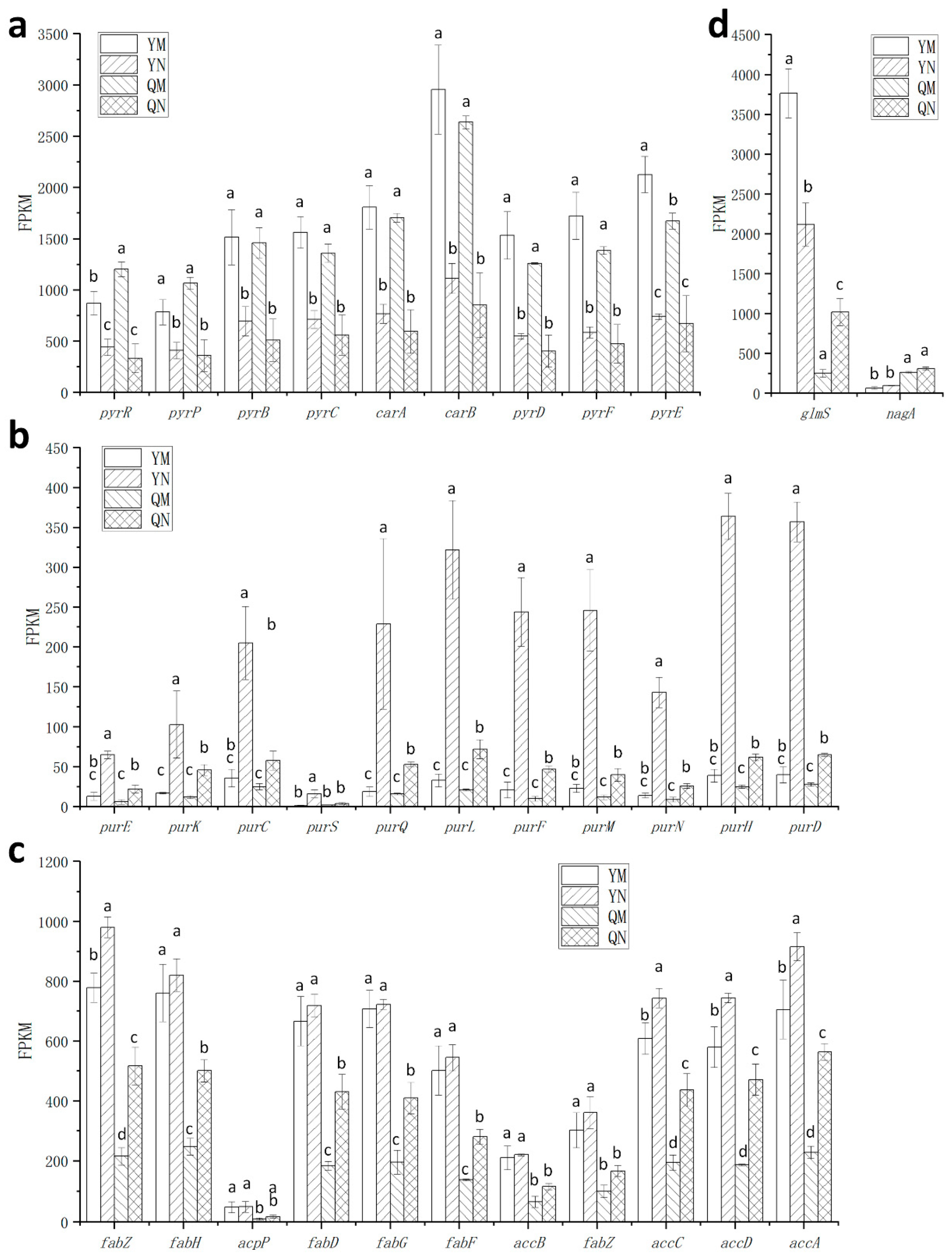
3.5. Effect of ccpA Gene Knockout on the Nitrogen Source Response in L. rhamnosus
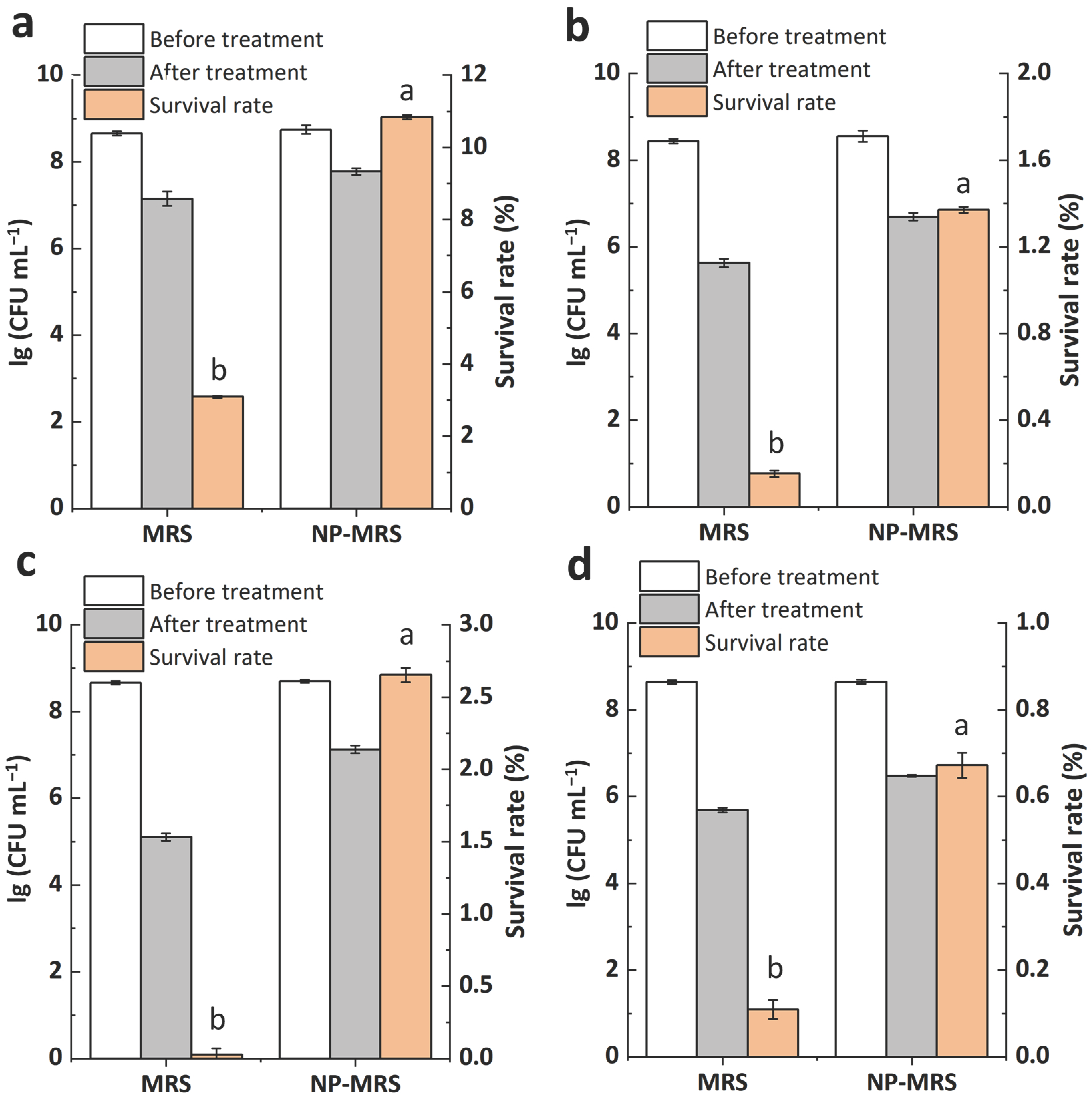
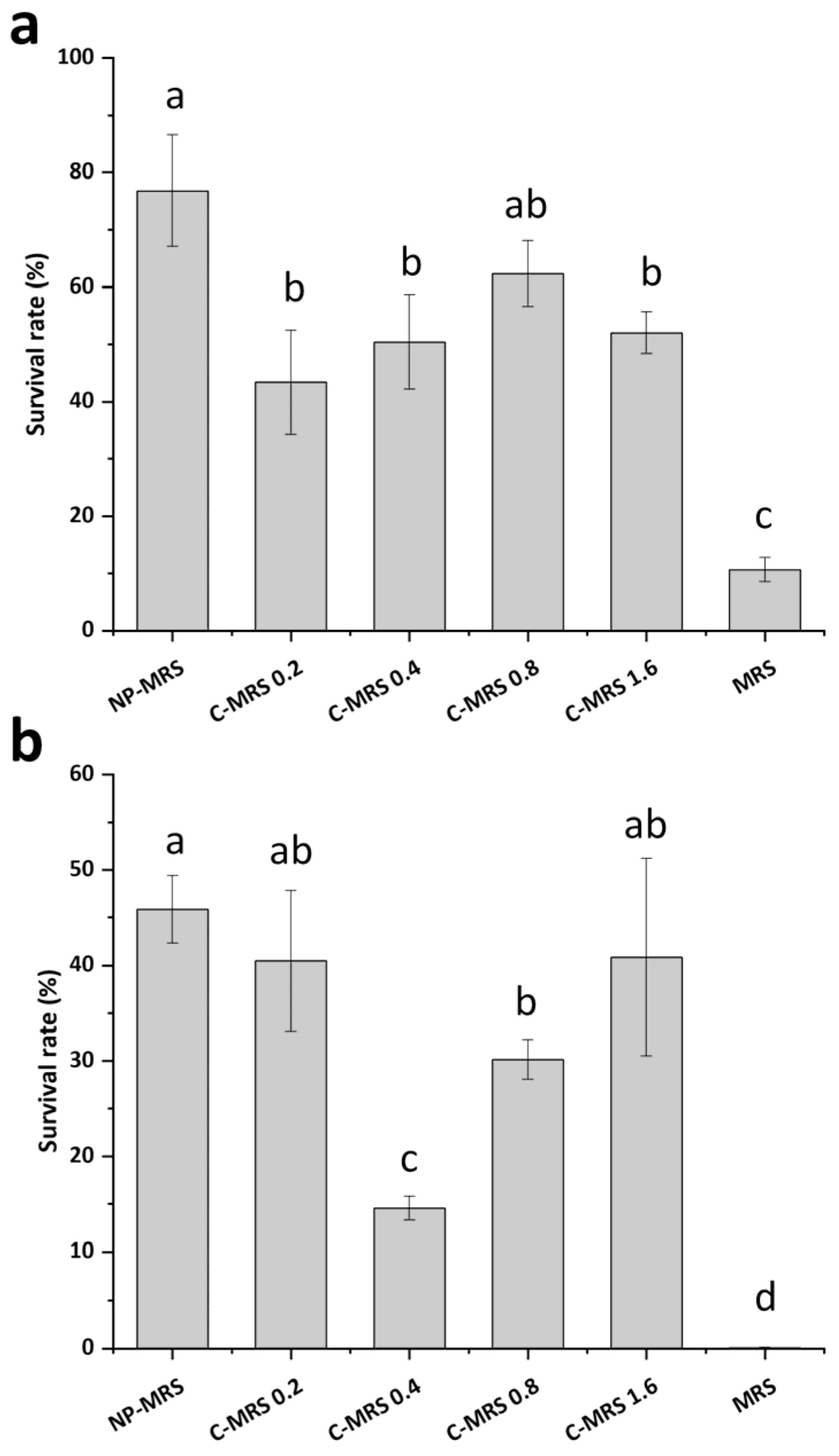
3.6. Effects of Cytosine on the Heat and Oxidative Stress Tolerance of L. rhamnosus
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LAB | Lactic acid bacteria |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| DEGs | differentially expressed genes |
| FPKM | fragments per kilobase of exon model per million mapped fragments |
| PTS | Phosphotransferase system |
| CCR | carbon catabolite repression |
| ROS | reactive oxygen species |
References
- Hu, Y.; Zhang, L.; Wen, R.; Chen, Q.; Kong, B. Role of lactic acid bacteria in flavor development in traditional Chinese fermented foods: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 2741–2755. [Google Scholar] [CrossRef] [PubMed]
- Smolinska, S.; Popescu, F.D.; Zemelka-Wiacek, M. A Review of the Influence of Prebiotics, Probiotics, Synbiotics, and Postbiotics on the Human Gut Microbiome and Intestinal Integrity. J. Clin. Med. 2025, 14, 3673. [Google Scholar] [CrossRef]
- Parlindungan, E.; Jones, O.A.H. Using metabolomics to understand stress responses in Lactic Acid Bacteria and their applications in the food industry. Metabolomics 2023, 19, 99. [Google Scholar] [CrossRef]
- Rossi, F.; Zotta, T.; Iacumin, L.; Reale, A. Theoretical insight into the heat shock response (HSR) regulation in Lactobacillus casei and L. rhamnosus. J. Theor. Biol. 2016, 402, 21–37. [Google Scholar] [CrossRef]
- Kelley, W.L. Lex marks the spot: The virulent side of SOS and a closer look at the LexA regulon. Mol. Microbiol. 2006, 62, 1228–1238. [Google Scholar] [CrossRef]
- Starosta, A.L.; Lassak, J.; Jung, K.; Wilson, D.N. The bacterial translation stress response. FEMS Microbiol. Rev. 2014, 38, 1172–1201. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, K.; Alegría, Á.; Bron, P.A.; de Angelis, M.; Gobbetti, M.; Kleerebezem, M.; Lemos, J.A.; Linares, D.M.; Ross, P.; Stanton, C.; et al. Stress Physiology of Lactic Acid Bacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 837–890. [Google Scholar] [CrossRef] [PubMed]
- Basu Thakur, P.; Long, A.R.; Nelson, B.J.; Kumar, R.; Rosenberg, A.F.; Gray, M.J. Complex Responses to Hydrogen Peroxide and Hypochlorous Acid by the Probiotic Bacterium Lactobacillus reuteri. mSystems 2019, 4, e00453-19. [Google Scholar] [CrossRef]
- Zhang, C.; Cheng, H.; Han, Y.; Wa, Y.; Chen, D.; Guan, C.; Huang, Y.; Gu, R. Transcriptome-phenotype matching analysis of how nitrogen sources influence Lacticaseibacillus rhamnosus tolerance to heat stress and oxidative stress. Microb. Cell Fact. 2022, 21, 257. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, X.; Wang, D.; Gui, Y.; Wang, C.; Li, Q.; Wang, J.; Yin, B.; Pan, Z.; Gu, R. Rapid strain-specific identification of two Lactobacillus rhamnosus strains using PCR based on gene family analysis. LWT 2021, 146, 111395. [Google Scholar] [CrossRef]
- Kang, W.; Pan, L.; Peng, C.; Dong, L.; Cao, S.; Cheng, H.; Wang, Y.; Zhang, C.; Gu, R.; Wang, J.; et al. Isolation and characterization of lactic acid bacteria from human milk. J. Dairy Sci. 2020, 103, 9980–9991. [Google Scholar] [CrossRef]
- Zhang, C.; Han, Y.; Gui, Y.; Wa, Y.; Chen, D.; Huang, Y.; Yin, B.; Gu, R. Influence of nitrogen sources on the tolerance of Lacticaseibacillus rhamnosus to heat stress and oxidative stress. J. Ind. Microbiol. Biotechnol. 2022, 49, kuac020. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, H.; Han, Y.; Sun, Y.; Cheng, H.; Wa, Y.; Chen, D.; Guan, C.; Gu, R. The role of glnR gene in heat and oxidative stress cross-adaptation in Lacticaseibacillus rhamnosus. LWT 2024, 201, 116278. [Google Scholar] [CrossRef]
- Görke, B.; Stülke, J. Carbon catabolite repression in bacteria: Many ways to make the most out of nutrients. Nat. Rev. Microbiol. 2008, 6, 613–624. [Google Scholar] [CrossRef]
- Lu, Y.; Song, S.; Tian, H.; Yu, H.; Zhao, J.; Chen, C. Functional analysis of the role of CcpA in Lactobacillus plantarum grown on fructooligosaccharides or glucose: A transcriptomic perspective. Microb. Cell Fact. 2018, 17, 201. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, L.; Li, C. Effects of ccpA gene deficiency in Lactobacillus delbrueckii subsp. bulgaricus under aerobic conditions as assessed by proteomic analysis. Microb. Cell Fact. 2020, 19, 9. [Google Scholar] [CrossRef] [PubMed]
- Zomer, A.L.; Buist, G.; Larsen, R.; Kok, J.; Kuipers, O.P. Time-resolved determination of the CcpA regulon of Lactococcus lactis subsp. cremoris MG1363. J. Bacteriol. 2007, 189, 1366–1381. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.N.; Burne, R.A. CcpA and CodY Coordinate Acetate Metabolism in Streptococcus mutans. Appl. Environ. Microbiol. 2017, 83, e03274. [Google Scholar] [CrossRef] [PubMed]
- Zotta, T.; Ricciardi, A.; Guidone, A.; Sacco, M.; Muscariello, L.; Mazzeo, M.F.; Cacace, G.; Parente, E. Inactivation of ccpA and aeration affect growth, metabolite production and stress tolerance in Lactobacillus plantarum WCFS1. Int. J. Food Microbiol. 2012, 155, 51–59. [Google Scholar] [CrossRef]
- Chen, D.; Liang, Y.; Liang, J.; Shen, F.; Cheng, Y.; Qu, H.; Wa, Y.; Guo, C.; Gu, R.; Qian, J.; et al. Beneficial effects of Lactobacillus rhamnosus hsryfm 1301 fermented milk on rats with nonalcoholic fatty liver disease. J. Dairy Sci. 2023, 106, 1533–1548. [Google Scholar] [CrossRef]
- Qu, H.; Zong, L.; Sang, J.; Wa, Y.; Chen, D.; Huang, Y.; Chen, X.; Gu, R. Effect of Lactobacillus rhamnosus hsryfm 1301 Fermented Milk on Lipid Metabolism Disorders in High-Fat-Diet Rats. Nutrients 2022, 14, 4850. [Google Scholar] [CrossRef]
- Zhang, C.; Gui, Y.; Chen, X.; Chen, D.; Guan, C.; Yin, B.; Pan, Z.; Gu, R. Transcriptional homogenization of Lactobacillus rhamnosus hsryfm 1301 under heat stress and oxidative stress. Appl. Microbiol. Biotechnol. 2020, 104, 2611–2621. [Google Scholar] [CrossRef]
- van de Guchte, M.; van der Vossen, J.M.; Kok, J.; Venema, G. Construction of a lactococcal expression vector: Expression of hen egg white lysozyme in Lactococcus lactis subsp. lactis. Appl. Environ. Microbiol. 1989, 55, 224–228. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, L.; Ding, Z.; Yin, B.; Chen, D.; Guan, C.; Gu, R. New selective media for isolation and enumeration of Lactobacillus rhamnosus and Streptococcus thermophilus. J. Food Meas. Charact. 2019, 13, 1431–1439. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods. 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Kanehisa, M.; Araki, M.; Goto, S.; Hattori, M.; Hirakawa, M.; Itoh, M.; Katayama, T.; Kawashima, S.; Okuda, S.; Tokimatsu, T.; et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2008, 36, D480–D484. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Chen, X.; Zhang, S.; Zhu, J.; Tang, B.; Wang, A.; Dong, L.; Zhang, Z.; Yu, C.; Sun, Y.; et al. The Genome Sequence Archive Family: Toward Explosive Data Growth and Diverse Data Types. Genom. Proteom. Bioinf. 2021, 19, 578–583. [Google Scholar] [CrossRef]
- CNCB-NGDC Members and Partners. Database Resources of the National Genomics Data Center, China National Center for Bioinformation in 2025. Nuclei Acid Res. 2025, 53, D30–D44. [Google Scholar] [CrossRef]
- Li, C.; Sun, J.W.; Zhang, G.F.; Liu, L.B. Effect of the absence of the CcpA gene on growth, metabolic production, and stress tolerance in Lactobacillus delbrueckii ssp. bulgaricus. J. Dairy Sci. 2016, 99, 104–111. [Google Scholar] [CrossRef]
- Zhang, C.; Guo, T.; Xin, Y.; Gao, X.; Kong, J. Catabolite responsive element deficiency of xyl operon resulting in carbon catabolite derepression in Lactobacillus fermentum 1001. J. Appl. Microbiol. 2016, 120, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Reichenbach, B.; Maes, A.; Kalamorz, F.; Hajnsdorf, E.; Görke, B. The small RNA GlmY acts upstream of the sRNA GlmZ in the activation of glmS expression and is subject to regulation by polyadenylation in Escherichia coli. Nucleic Acids Res. 2008, 36, 2570–2580. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Huang, K.; Li, X.; Tian, H.; Yu, H.; Huang, J.; Yuan, H.; Zhao, S.; Shao, L. Effects of CcpA against salt stress in Lactiplantibacillus plantarum as assessed by comparative transcriptional analysis. Appl. Microbiol. Biotechnol. 2021, 105, 3691–3704. [Google Scholar] [CrossRef]
- Castaldo, C.; Siciliano, R.A.; Muscariello, L.; Marasco, R.; Sacco, M. CcpA affects expression of the groESL and dnaK operons in Lactobacillus plantarum. Microb. Cell Fact. 2006, 5, 35. [Google Scholar] [CrossRef] [PubMed]
- Mazzeo, M.F.; Cacace, G.; Peluso, A.; Zotta, T.; Muscariello, L.; Vastano, V.; Parente, E.; Siciliano, R.A. Effect of inactivation of ccpA and aerobic growth in Lactobacillus plantarum: A proteomic perspective. J. Proteom. 2012, 75, 4050–4061. [Google Scholar] [CrossRef]
- Sirima, S.J.; Pachamon, P.; Ratsadakorn, Y.M.; Cheng, K.C.; Kaemwich, J. Transcriptional analysis of oxidative-tolerant and temperature-sensitive genes of Bifidobacterium animalis BF052 during freeze-drying process and development of its soymilk-synbiotic product containing banana and jicama powders. Food Res. Int. 2025, 221, 117600. [Google Scholar] [CrossRef]
- An, H.; Zhai, Z.; Yin, S.; Luo, Y.; Han, B.; Hao, Y. Coexpression of the superoxide dismutase and the catalase provides remarkable oxidative stress resistance in Lactobacillus rhamnosus. J. Agric. Food Chem. 2011, 59, 3851–3856. [Google Scholar] [CrossRef]
- Dinarieva, T.Y.; Klimko, A.I.; Kahnt, J.; Cherdyntseva, T.A.; Netrusov, A.I. Adaptation of Lacticaseibacillus rhamnosus CM MSU 529 to Aerobic Growth: A Proteomic Approach. Microorganisms 2023, 11, 313. [Google Scholar] [CrossRef] [PubMed]
- Quatravaux, S.; Remize, F.; Bryckaert, E.; Colavizza, D.; Guzzo, J. Examination of Lactobacillus plantarum lactate metabolism side effects in relation to the modulation of aeration parameters. J. Appl. Microbiol. 2006, 101, 903–912. [Google Scholar] [CrossRef]
- Zhai, Z.; Yang, Y.; Wang, H.; Wang, G.; Ren, F.; Li, Z.; Hao, Y. Global transcriptomic analysis of Lactobacillus plantarum CAUH2 in response to hydrogen peroxide stress. Food Microbiol. 2020, 87, 103389. [Google Scholar] [CrossRef]
- Sun, J.; Chen, H.; Qiao, Y.; Liu, G.; Leng, C.; Zhang, Y.; Lv, X.; Feng, Z. The nutrient requirements of Lactobacillus rhamnosus GG and their application to fermented milk. J. Dairy Sci. 2019, 102, 5971–5978. [Google Scholar] [CrossRef]
- Yang, H.; He, M.; Wu, C. Cross protection of lactic acid bacteria during environmental stresses: Stress responses and underlying mechanisms. LWT 2021, 144, 111203. [Google Scholar] [CrossRef]
- Randazzo, P.; Aucouturier, A.; Delumeau, O.; Auger, S. Revisiting the in vivo GlnR-binding sites at the genome scale in Bacillus subtilis. BMC Res. Notes 2017, 10, 422. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Ren, C.; Xu, Y. GlnR Negatively Regulates Glutamate-Dependent Acid Resistance in Lactobacillus brevis. Appl. Environ. Microbiol. 2020, 86, e02615-19. [Google Scholar] [CrossRef] [PubMed]
- Janßen, H.J.; Steinbüchel, A. Fatty acid synthesis in Escherichia coli and its applications towards the production of fatty acid based biofuels. Biotechnol. Biofuels 2014, 7, 7. [Google Scholar] [CrossRef] [PubMed]


| Primer | Sequence (5′-3′) | Restriction Site |
|---|---|---|
| Eco-ccpaupF | TTTGAATTCCATATACCCATGATTGTCGGTGC | EcoR I |
| ccpaupR | TTTATTTTCTCCTTAGTCGTGAAAA | |
| ccpadownF | TTTTCACGACTAAGGAGAAAATAAAACAGAAGTAACACGATATTCTGGC | |
| hind-ccpadownR | AAGAAGCTTGTCATCAAGTCAAAAAGACCAAG | Hind III |
| ccpaTestF | GTCCATCGCGGTTAAGTTAGCC | |
| ccpaTestR | CAACATTCGCCAATCAAGGTG | |
| M13c-F | CCCAGTCACGACGTTGTAAAACG | |
| RV-M | GAGCGGATAACAATTTCACACAGG | |
| Eco-1301ccpAF | GGAATTCGGCTACTCCTTAAAACTCGCTG | EcoR I |
| Hind-1301ccpAR | ATTAAGCTTCTACTTGGTTGAACCACGCTTC | Hind III |
| pMG36eF | TAATTCGAGCTCGCCCGG | |
| pMG36eR | ACCGAATTCGATCGACCCATA | |
| 36e-FGL-ccpAF | TATGGGTCGATCGAATTCGGTCAATCAAGCATCGTGGTAAAATAG | |
| 36e-FGL-ccpAR | CCGGGCGAGCTCGAATTATTATTTCGTTGAACCACGCTTC |
| YM vs. QM | YN vs. QN | YM vs. YN | QM vs. QN | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pathways | U * | D * | Pathways | U * | D * | Pathways | U * | D * | Pathways | U * | D * |
| Phosphotransferase system (PTS) | 46 | 2 | Phosphotransferase system (PTS) | 33 | 2 | Purine metabolism | 13 | 1 | Fatty acid biosynthesis | 9 | 0 |
| Fructose and mannose metabolism | 28 | 2 | Fructose and mannose metabolism | 20 | 2 | Pyrimidine metabolism | 2 | 9 | Pyrimidine metabolism | 3 | 9 |
| Pyruvate metabolism | 12 | 6 | Inositol phosphate metabolism | 8 | 0 | beta-Lactam resistance | 5 | 0 | Purine metabolism | 13 | 0 |
| Fatty acid biosynthesis | 0 | 12 | Ascorbate and aldarate metabolism | 10 | 0 | Alanine, aspartate and glutamate metabolism | 1 | 4 | beta-Lactam resistance | 5 | 1 |
| Propanoate metabolism | 6 | 4 | Staphylococcus aureus infection | 5 | 0 | Tuberculosis | 2 | 0 | Quorum sensing | 9 | 0 |
| Inositol phosphate metabolism | 9 | 0 | Galactose metabolism | 17 | 0 | RNA degradation | 2 | 1 | Alanine, aspartate and glutamate metabolism | 2 | 3 |
| Galactose metabolism | 22 | 0 | Starch and sucrose metabolism | 17 | 0 | Quorum sensing | 5 | 0 | Biotin metabolism | 3 | 0 |
| Starch and sucrose metabolism | 21 | 0 | Cationic antimicrobial peptide (CAMP) resistance | 5 | 0 | ABC transporters | 10 | 1 | |||
| Pentose and glucuronate interconversions | 10 | 0 | Pyruvate metabolism | 11 | 0 | Prodigiosin biosynthesis | 2 | 0 | |||
| Ascorbate and aldarate metabolism | 10 | 0 | Glycolysis/Gluconeogenesis | 13 | 0 | Propanoate metabolism | 3 | 0 | |||
| Staphylococcus aureus infection | 5 | 0 | Purine metabolism | 0 | 13 | ||||||
| Glycolysis/Gluconeogenesis | 15 | 1 | Pentose and glucuronate interconversions | 7 | 0 | ||||||
| Cationic antimicrobial peptide (CAMP) resistance | 5 | 0 | Citrate cycle (TCA cycle) | 4 | 0 | ||||||
| Carbon fixation pathways in prokaryotes | 3 | 4 | Propanoate metabolism | 5 | 0 | ||||||
| Citrate cycle (TCA cycle) | 4 | 0 | |||||||||
| Biotin metabolism | 0 | 4 | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Cheng, H.; Li, Q.; Sun, Y.; Wu, Y.; Wang, H.; Wa, Y.; Chen, D.; Guan, C.; Huang, Y.; et al. The Role of ccpA in Nitrogen Source-Induced Heat and Oxidative Stress Tolerance Changes in Lacticaseibacillus rhamnosus. Foods 2025, 14, 3894. https://doi.org/10.3390/foods14223894
Li M, Cheng H, Li Q, Sun Y, Wu Y, Wang H, Wa Y, Chen D, Guan C, Huang Y, et al. The Role of ccpA in Nitrogen Source-Induced Heat and Oxidative Stress Tolerance Changes in Lacticaseibacillus rhamnosus. Foods. 2025; 14(22):3894. https://doi.org/10.3390/foods14223894
Chicago/Turabian StyleLi, Mengting, Haohao Cheng, Qiming Li, Yue Sun, You Wu, Haikang Wang, Yunchao Wa, Dawei Chen, Chengran Guan, Yujun Huang, and et al. 2025. "The Role of ccpA in Nitrogen Source-Induced Heat and Oxidative Stress Tolerance Changes in Lacticaseibacillus rhamnosus" Foods 14, no. 22: 3894. https://doi.org/10.3390/foods14223894
APA StyleLi, M., Cheng, H., Li, Q., Sun, Y., Wu, Y., Wang, H., Wa, Y., Chen, D., Guan, C., Huang, Y., Gu, R., & Zhang, C. (2025). The Role of ccpA in Nitrogen Source-Induced Heat and Oxidative Stress Tolerance Changes in Lacticaseibacillus rhamnosus. Foods, 14(22), 3894. https://doi.org/10.3390/foods14223894







