Effects of Electron Beam Irradiation on the Storage Stability and Quality Characteristics of Chicken and Duck Meat
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Chicken and Duck Breast Meat
2.2. Preparation of Bacterial Strains and Inoculum Suspension
2.3. Bacterial Inoculation
2.4. Electron Beam Irradiation
2.5. Microbial Recovery Analysis
2.6. Thiobarbituric Acid-Reactive Substances (TBARS) Analysis
2.7. Volatile Basic Nitrogen (VBN) Analysis
2.8. Proximate Composition Analysis
2.9. pH
2.10. Water Holding Capacity (WHC) Analysis
2.11. Shear Force Analysis
2.12. Color Analysis
2.13. Statistical Analysis
3. Results and Discussion
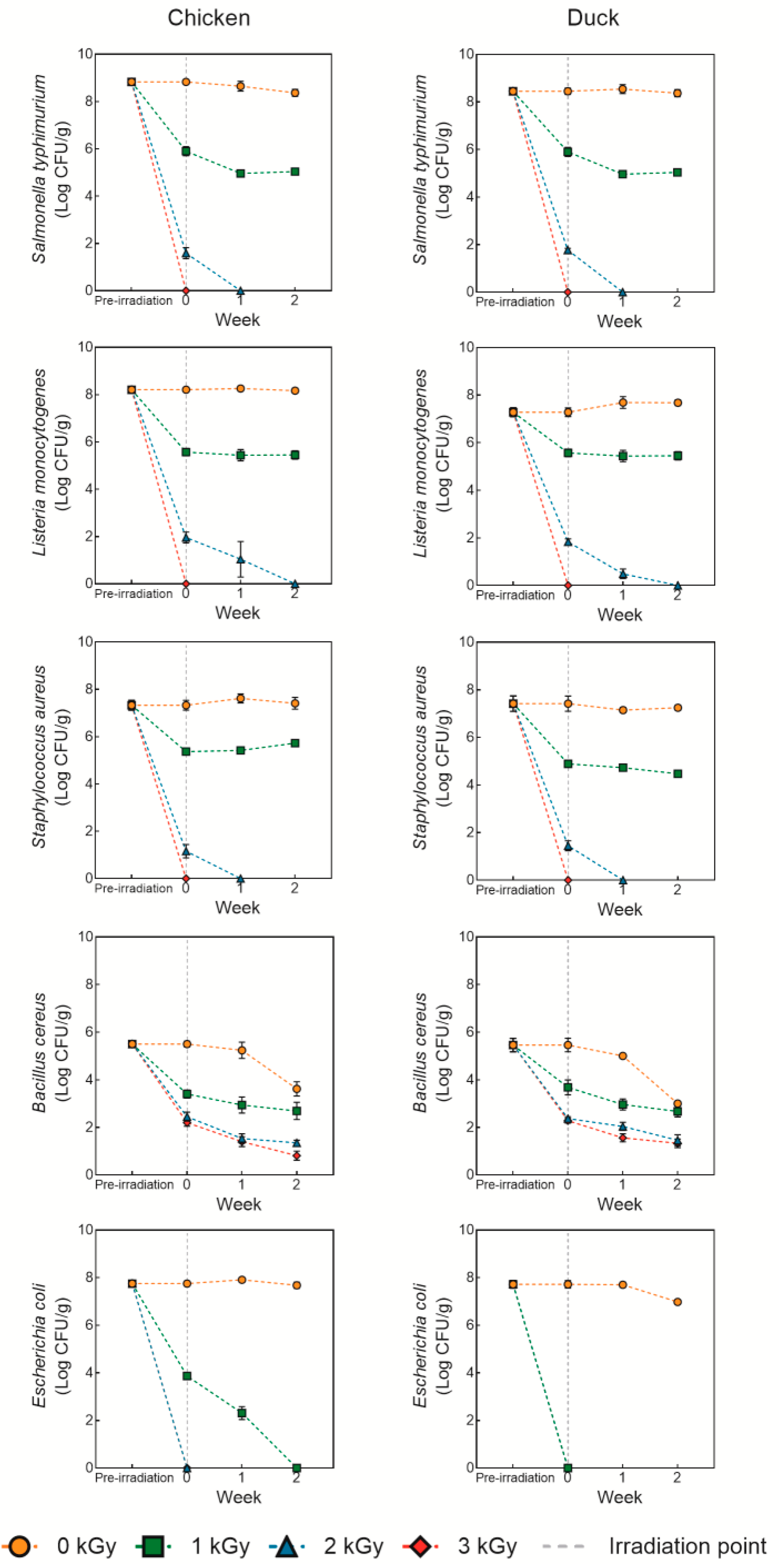
3.1. Microbial Recovery
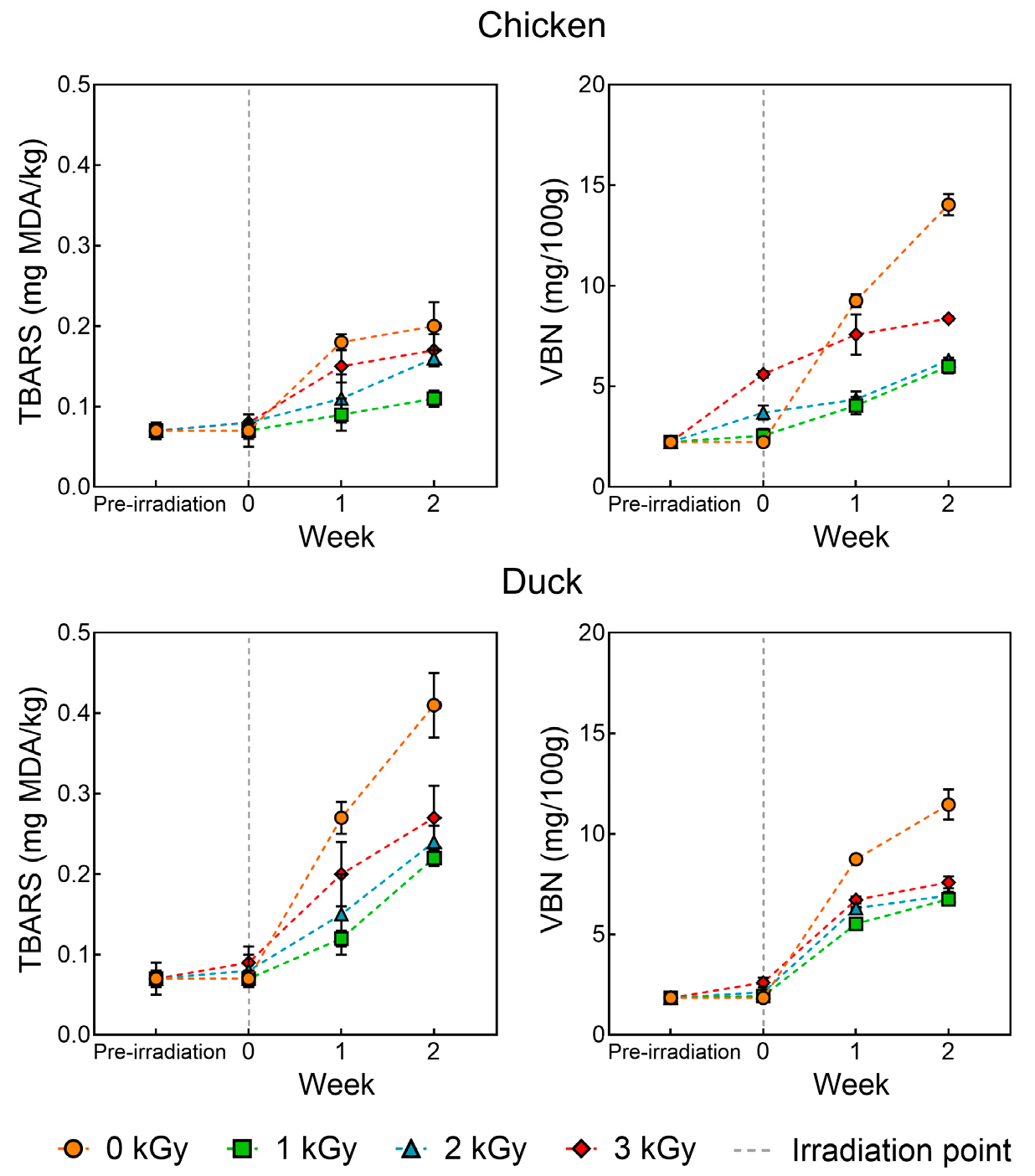
3.2. TBARS and VBN
3.3. Proximate Composition
3.4. pH, WHC, and Shear Force
3.5. Color
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Connolly, G.; Clark, C.M.; Campbell, R.E.; Byers, A.W.; Reed, J.B.; Campbell, W.W. Poultry Consumption and Human Health: How Much is Really Known? A Systematically Searched Scoping Review and Research Perspective. Adv. Nutr. 2022, 13, 2115–2124. [Google Scholar] [CrossRef]
- Petracci, M. Current Meat Quality Challenges for The Poultry Industry–A Review. Anim. Sci. Pap. Rep. 2022, 40, 253–261. [Google Scholar]
- Dal Bosco, A.; Cartoni Mancinelli, A.; Vaudo, G.; Cavallo, M.; Castellini, C.; Mattioli, S. Indexing of Fatty Acids in Poultry Meat for Its Characterization in Healthy Human Nutrition: A Comprehensive Application of the Scientific Literature and New Proposals. Nutrients 2022, 14, 3110. [Google Scholar] [CrossRef]
- You, Z.; Bai, Y.; Bo, D.; Feng, Y.; Shen, J.; Wang, Y.; Li, J.; Bai, Y. A Review of Taste-Active Compounds in Meat: Identification, Influencing Factors, and Taste Transduction Mechanism. J. Food Sci. 2024, 89, 8128–8155. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Guo, X.; Chai, Y.; Li, X.; Chen, L.; Feng, X. Superabsorbent Whey Protein Isolates/Chitosan-based Antibacterial Aerogels: Preparation, Characterization and Application in Chicken Meat Preservation. Int. J. Biol. Macromol. 2024, 259, 128961. [Google Scholar] [CrossRef]
- Salama, Y.; Chennaoui, M. Understanding Microbial Contamination in Meat and Poultry Production. J. Res. Agric. Food Sci. 2024, 1, 87–107. [Google Scholar] [CrossRef]
- Antunes, P.; Novais, C.; Peixe, L.; Freitas, A.R. Pet Food Safety: Emerging Bacterial Hazards and Implications for Public Health. Curr. Opin. Food Sci. 2024, 57, 101165. [Google Scholar] [CrossRef]
- Popa, S.A.; Herman, V.; Tîrziu, E.; Morar, A.; Ban-Cucerzan, A.; Imre, M.; Pătrînjan, R.; Imre, K. Public Health Risk of Campylobacter spp. Isolated from Slaughterhouse and Retail Poultry Meat: Prevalence and Antimicrobial Resistance Profiles. Pathogens 2025, 14, 316. [Google Scholar] [CrossRef]
- Uazhanova, R.; Moldakhmetova, Z.; Tungyshbayeva, U.; Izteliyeva, R.; Amanova, S.; Baimakhanov, G.; Seksenbay, S.; Sabraly, S. Ensuring Quality and Safety in the Production and Storage of Poultry Meat. Casp. J. Environ. Sci. 2024, 22, 1271–1277. [Google Scholar]
- Hassoun, A.; Aït-Kaddour, A.; Sahar, A.; Cozzolino, D. Monitoring Thermal Treatments Applied to Meat Using Traditional Methods and Spectroscopic Techniques: A Review of Advances over the Last Decade. Food Bioprocess Technol. 2021, 14, 195–208. [Google Scholar] [CrossRef]
- Vinnikova, L.; Synytsia, O.; Kyshenia, A. The Problems of Meat Products Thermal Treatment. Food Sci. Technol. 2019, 13, 44–57. [Google Scholar] [CrossRef]
- Chang, S.; Zhang, Z.; Liu, Q.; Wu, H.; Dong, A. An Innovative Food Processing Technology: Microwave Electrodeless Ultraviolet, Luminescence Mechanism, Microbial Inactivation, and Food Application. Foods 2024, 13, 4110. [Google Scholar] [CrossRef]
- Chacha, J.S.; Zhang, L.; Ofoedu, C.E.; Suleiman, R.A.; Dotto, J.M.; Roobab, U.; Agunbiade, A.O.; Duguma, H.T.; Mkojera, B.T.; Hossaini, S.M.; et al. Revisiting Non-Thermal Food Processing and Preservation Methods—Action Mechanisms, Pros and Cons: A Technological Update (2016–2021). Foods 2021, 10, 1430. [Google Scholar] [CrossRef]
- Li, C.; Zhang, R.; Sogore, T.; Feng, J.; Liao, X.; Wang, X.; Zhang, Z.; Ding, T. Electron Beam Irradiation in Food Processing: Current Applications and Strategies for Commercial Scale Implementation. Sustain. Food Technol. 2025, 3, 875–893. [Google Scholar] [CrossRef]
- Wei, Q.; Mei, J.; Xie, J. Application of Electron Beam Irradiation as a Non-Thermal Technology in Seafood Preservation. Lwt 2022, 169, 113994. [Google Scholar] [CrossRef]
- Liang, W.; Zhao, W.; Liu, X.; Zheng, J.; Sun, Z.; Ge, X.; Shen, H.; Ospankulova, G.; Muratkhan, M.; Li, W. Investigating the Role and Mechanism of Water in E-beam Modified Sweet Potato Starch: Multi-scale Structure, Physicochemical Properties, and in vitro Digestibility. Food Hydrocoll. 2023, 137, 108433. [Google Scholar] [CrossRef]
- Rodrigues, F.T.; Koike, A.C.R.; da Silva, P.G.; Negrão, B.G.; de Alencar, S.M.; Mancini Filho, J.; Villavicencio, A.L.C. Effects of Electron Beam Irradiation on the Bioactive Components of Goji-Berry. Radiat. Phys. Chem. 2021, 179, 109144. [Google Scholar] [CrossRef]
- Kang, S.W.; Hwang, J.H.; Jung, A.H.; Park, E.; Park, S.; Yoon, Y.; Park, S.H. Effect of Non-Thermal Pasteurization on Minced Chicken Meat Based Pet Food and Its Quality Attributes Through Gamma Ray and Electron Beam Irradiation. Food Eng. Prog. 2021, 25, 139–146. [Google Scholar] [CrossRef]
- Wahyono, T.; Ujilestari, T.; Sholikin, M.M.; Muhlisin, M.; Cahyadi, M.; Volkandari, S.D.; Triyannanto, E. Quality of Pork After Electron-Beam Irradiation: A Meta-Analysis Study. Vet. World 2024, 17, 59–71. [Google Scholar] [CrossRef]
- Kwon, J.H.; Kwon, Y.; Nam, K.C.; Lee, E.J.; Ahn, D.U. Effect of Electron-Beam Irradiation Before and After Cooking on The Chemical Properties of Beef, Pork, and Chicken. Meat Sci. 2008, 80, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Sani, M.A.; Velayati, N.; Yazdi, N.B.; Khezerlou, A.; Jafari, S.M. Electron Beam Irradiation: A Non-Thermal Technology for Food Safety and Quality Control. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70205. [Google Scholar] [CrossRef] [PubMed]
- Won, S.; Park, S. Effects of High Temperature Short Time and High Hydrostatic Pressure Treatment on the Microbial and Physicochemical Characteristics of Red Beet Juice. J. Korean Soc. Food Sci. Nutr. 2024, 53, 392–399. [Google Scholar] [CrossRef]
- Kang, K.M.; Lee, S.H.; Kim, H.Y. Changes in Physico-Chemical and Storage Properties of Dry-Aged Beef Loin Using Electric Field Refrigeration System. Foods 2022, 11, 1539. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, H.Y. Analysis of Physicochemical Properties of Dry-Cured Beef Made from Hanwoo and Holstein Meat Distributed in South Korea. Heliyon 2023, 9, e17091. [Google Scholar] [CrossRef]
- Association of Official Agricultural Chemists. Official Methods of Analysis International, 21st ed.; Association of Official Agricultural Chemists: Gaithersburg, MD, USA, 2019. [Google Scholar]
- Lung, H.M.; Cheng, Y.C.; Chang, Y.H.; Huang, H.W.; Yang, B.B.; Wang, C.Y. Microbial Decontamination of Food by Electron Beam Irradiation. Trends Food Sci. Technol. 2015, 44, 66–78. [Google Scholar] [CrossRef]
- Kogut, M.H.; McReynolds, J.L.; He, H.; Genovese, K.J.; Jesudhasan, P.R.; Davidson, M.A.; Cepeda, M.A.; Pillai, S.D. Electron-Beam Irradiation Inactivation of Salmonella: Effects on Innate Immunity and Induction of Protection Against Salmonella enterica serovar Typhimurium Challenge of Chickens. Procedia Vaccinol. 2012, 6, 47–63. [Google Scholar] [CrossRef]
- Hing, J.N.; Jong, B.C.; Liew, P.W.Y.; Ellyna, R.E.; Shamsudin, S. Gamma Radiation Dose-Response of Gram-Positive and Gram-Negative Bacteria. Malays. Appl. Biol. 2022, 51, 107–112. [Google Scholar] [CrossRef]
- Skowron, K.; Grudlewska, K.; Gryń, G.; Skowron, K.J.; Świeca, A.; Paluszak, Z.; Zimek, A.; Rafalski, E.; Gospodarek-Komkowska, E. Effect of Electron Beam and Gamma Radiation on Drug-Susceptible and Drug-Resistant Listeria monocytogenes Strains in Salmon Under Different Temperature. J. Appl. Microbiol. 2018, 125, 828–842. [Google Scholar] [CrossRef] [PubMed]
- Setlow, P. Spores of Bacillus subtilis: Their Resistance to and Killing by Radiation, Heat and Chemicals. J. Appl. Microbiol. 2006, 101, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Venugopal, A.P.; Sutar, P.P.; Xiao, H.; Zhang, Q. Mechanism of Microbial Spore Inactivation Through Electromagnetic Radiations: A Review. J. Future Foods 2024, 4, 324–334. [Google Scholar] [CrossRef]
- Xu, J.; Xu, Y.; Guan, X.; Yang, G.; Wang, S. Effects of Sequential Treatments Using Radio Frequency Energy and Ultraviolet Light on Inactivation of Bacillus cereus Spores and Quality Attributes of Buckwheat. Int. J. Food Microbiol. 2023, 385, 109997. [Google Scholar] [CrossRef]
- Arshad, M.S.; Amjad, Z.; Yasin, M.; Saeed, F.; Imran, A.; Sohaib, M.; Anjum, F.M.; Hussain, S. Quality and Stability Evaluation of Chicken Meat Treated with Gamma Irradiation and Turmeric Powder. Int. J. Food Prop. 2019, 22, 154–172. [Google Scholar] [CrossRef]
- Wong, P.Y.; Kitts, D.D. Factors Influencing Ultraviolet and Electron Beam Irradiation-Induced Free Radical Damage of Ascorbic Acid. Food Chem. 2001, 74, 75–84. [Google Scholar] [CrossRef]
- Abeyrathne, E.D.N.S.; Nam, K.; Ahn, D.U. Analytical Methods for Lipid Oxidation and Antioxidant Capacity in Food Systems. Antioxidants 2021, 10, 1587. [Google Scholar] [CrossRef]
- Ameer, A.; Seleshe, S.; Kang, S.N. Effect of Various Doses of Electron Beam Irradiation Treatment on the Quality Characteristics of Vacuum-Packed Dry Fermented Sausage during Refrigerated Storage. Prev. Nutr. Food Sci. 2022, 27, 323. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, F.; Ghoorchi, T.; Shawrang, P.; Mansouri, H.; Torbati-Nejad, N.M. Comparison of Electron Beam and Gamma Ray Irradiations Effects on Ruminal Crude Protein and Amino Acid Degradation Kinetics, and in vitro Digestibility of Cottonseed Meal. Radiat. Phys. Chem. 2012, 81, 672–678. [Google Scholar] [CrossRef]
- Shawrang, P.; Sadeghi, A.A.; Behgar, M.; Zareshahi, H.; Shahhoseini, G. Study of Chemical Compositions, Anti-Nutritional Contents and Digestibility of Electron Beam Irradiated Sorghum Grains. Food Chem. 2011, 125, 376–379. [Google Scholar] [CrossRef]
- Tolentino, M.; Diano, G.; Abrera, G.; Montefalcon, D.R.; Cobar, M.L.; Deocaris, C.; Baule, A.; Asaad, C. Electron Beam Irradiation of Raw Ground Beef Patties in the Philippines: Microbial Quality, Sensory Characteristics, and Cost-Analysis. Radiat. Phys. Chem. 2021, 186, 109536. [Google Scholar] [CrossRef]
- Ham, Y.K.; Kim, H.W.; Hwang, K.E.; Song, D.H.; Kim, Y.J.; Choi, Y.S.; Song, B.S.; Park, J.H.; Kim, C.J. Effects of Irradiation Source and Dose Level on Quality Characteristics of Processed Meat Products. Radiat. Phys. Chem. 2017, 130, 259–264. [Google Scholar] [CrossRef]
- Schmidt, K.H.; Ander, S.M. Formation and Recombination of the Hydronium Ion (H3O+) and Hydroxide in Irradiated Water. J. Phys. Chem. 1969, 73, 2846–2852. [Google Scholar] [CrossRef]
- An, K.A.; Arshad, M.S.; Jo, Y.; Chung, N.; Kwon, J.H. E-beam Irradiation for Improving the Microbiological Quality of Smoked Duck Meat with Minimum Effects on Physicochemical Properties During Storage. J. Food Sci. 2017, 82, 865–872. [Google Scholar] [CrossRef]
- Arshad, M.S.; Kwon, J.H.; Ahmad, R.S.; Ameer, K.; Ahmad, S.; Jo, Y. Influence of E-Beam Irradiation on Microbiological and Physicochemical Properties and Fatty Acid Profile of Frozen Duck Meat. Food Sci. Nutr. 2020, 8, 1020–1029. [Google Scholar] [CrossRef]
- Orynbekov, D.; Amirkhanov, K.; Kalibekkyzy, Z.; Muslimova, N.; Nurymkhan, G.; Nurgazezova, A.; Kassymov, S.; Kassenov, A.; Maizhanova, A.; Kulushtayeva, B.; et al. Effect of Electron-Beam Irradiation on Microbiological Safety, Nutritional Quality, and Structural Characteristics of Meat. Foods 2025, 14, 1460. [Google Scholar] [CrossRef]
- Garcia-Marquez, I.; Cambero, M.I.; Ordonez, J.A.; Cabeza, M.C. Shelf-Life Extension and Sanitation of Fresh Pork Loin by E-Beam Treatment. J. Food Prot. 2012, 75, 2179–2189. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Yu, J.; Teng, Y.; Xiang, X.; Zhang, D.; Kang, L.; Niu, Y.; Feng, X.; Chen, L. Effect of Electron Beam Irradiation on the Quality of Chicken During Post-Mortem Ageing. Food Chem. 2025, 480, 143869. [Google Scholar] [CrossRef]
- Bai, R.; Han, J.; Ye, X.; Yu, J.; Jiang, S.; Li, Z.; Zhang, L.; Yang, C.; Chen, Y.; Ding, W. Improvement on Gel Properties of Chicken Myofibrillar Protein with Electron Beam Irradiation: Based on Protein Structure, Gel Quality, Water State. Int. J. Biol. Macromol. 2024, 280, 135806. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.; Da Silva, P.F.; Castell-Perez, M.E.; Moreira, R.G. Quality and Microbial Population of Cornish Game Hen Carcasses as Affected by Electron Beam Irradiation. J. Food Sci. 2006, 71, E327–E336. [Google Scholar] [CrossRef]
- Zhang, M.; He, L.; Li, C.; Yang, F.; Zhao, S.; Liang, Y.; Jin, G. Effects of Gamma Ray Irradiation-Induced Protein Hydrolysis and Oxidation on Tenderness Change of Fresh Pork During Storage. Meat Sci. 2020, 163, 108058. [Google Scholar] [CrossRef]
- Wang, H.; Suo, R.; Wang, Y.; Sun, J.; Liu, Y.; Wang, W.; Wang, J. Effects of Electron Beam Irradiation on Protein Oxidation and Textural Properties of Shrimp (Litopenaeus vannamei) During Refrigerated Storage. Food Chem. X 2023, 20, 101009. [Google Scholar] [CrossRef]
- Lewis, S.J.; Velasquez, A.; Cuppett, S.L. Effect of Electron Beam Irradiation on Poultry Meat Safety and Quality. Poult. Sci. 2002, 81, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Cha, J.Y.; Kim, T.K.; Lee, J.H.; Jung, S.; Choi, Y.S. The Effect of Irradiation on Meat Products. Food Sci. Anim. Resour. 2024, 44, 779. [Google Scholar] [CrossRef] [PubMed]
- King, D.A.; Hunt, M.C.; Barbut, S.; Claus, J.R.; Cornforth, D.P.; Joseph, P.; Kim, Y.H.; Lindahl, G.; Mancini, R.A.; Nair, M.N.; et al. American Meat Science Association Guidelines for Meat Color Measurement. Meat Muscle Biol. 2023, 6, 12473. [Google Scholar] [CrossRef]
- Ruedt, C.; Gibis, M.; Weiss, J. Meat Color and Iridescence: Origin, Analysis, and Approaches to Modulation. Compr. Rev. Food Sci. Food Saf. 2023, 22, 3366–3394. [Google Scholar] [CrossRef] [PubMed]


| Trait (%) | Dose (kGy) | ||||
|---|---|---|---|---|---|
| 0 (Control) | 1 | 2 | 3 | ||
| Moisture | Chicken | 75.11 ± 0.71 Y | 75.07 ± 0.71 Y | 74.93 ± 0.01 Y | 74.86 ± 0.01 Y |
| Duck | 76.61 ± 0.35 X | 76.38 ± 0.10 X | 76.78 ± 0.24 X | 76.72 ± 0.03 X | |
| Protein | Chicken | 22.13 ± 0.46 X | 22.20 ± 0.58 X | 22.18 ± 0.38 X | 22.20 ± 0.37 X |
| Duck | 19.56 ± 0.68 Y | 19.51 ± 0.57 Y | 19.55 ± 0.86 Y | 19.48 ± 0.39 Y | |
| Fat | Chicken | 0.40 ± 0.04 Y | 0.41 ± 0.01 Y | 0.41 ± 0.05 Y | 0.42 ± 0.07 Y |
| Duck | 1.20 ± 0.06 X | 1.28 ± 0.17 X | 0.94 ± 0.19 X | 0.93 ± 0.18 X | |
| Ash | Chicken | 1.22 ± 0.02 Y | 1.24 ± 0.03 Y | 1.24 ± 0.02 Y | 1.26 ± 0.01 Y |
| Duck | 1.54 ± 0.07 X | 1.60 ± 0.08 X | 1.66 ± 0.17 X | 1.69 ± 0.08 X | |
| Trait | Dose (kGy) | ||||
|---|---|---|---|---|---|
| 0 (Control) | 1 | 2 | 3 | ||
| pH | Chicken | 5.98 ± 0.01 Xa | 5.98 ± 0.01 Xa | 5.96 ± 0.01 Xb | 5.96 ± 0.01 Xb |
| Duck | 5.84 ± 0.03 Ya | 5.81 ± 0.03 Yab | 5.77 ± 0.02 Yb | 5.77 ± 0.02 Yb | |
| WHC (%) | Chicken | 50.81 ± 0.48 Ya | 45.15 ± 0.33 b | 41.36 ± 0.71 Yc | 34.95 ± 0.71 Yd |
| Duck | 55.71 ± 0.43 Xa | 46.12 ± 0.70 b | 42.82 ± 0.54 Xc | 40.18 ± 0.40 Xd | |
| Shear force (kgf) | Chicken | 1.58 ± 0.18 | 1.47 ± 0.43 | 1.47 ± 0.08 | 1.27 ± 0.17 |
| Duck | 1.64 ± 0.33 | 1.48 ± 0.39 | 1.47 ± 0.07 | 1.34 ± 0.08 | |
| Trait | Dose (kGy) | ||||
|---|---|---|---|---|---|
| 0 (Control) | 1 | 2 | 3 | ||
| CIE L* | Chicken | 50.63 ± 0.51 X | 50.53 ± 0.25 X | 50.02 ± 0.72 X | 49.73 ± 0.49 X |
| Duck | 40.67 ± 0.83 Ya | 39.70 ± 0.20 Yab | 39.63 ± 0.52 Yab | 38.47 ± 0.86 Yb | |
| CIE a* | Chicken | 5.83 ± 0.36 Yd | 8.30 ± 0.30 Yc | 9.50 ± 0.29 Yb | 11.97 ± 0.45 Ya |
| Duck | 15.67 ± 0.67 Xc | 18.60 ± 0.10 Xb | 19.03 ± 0.58 Xb | 28.97 ± 0.64 Xa | |
| CIE b* | Chicken | 8.33 ± 0.05 Xa | 8.03 ± 0.12 Xb | 7.13 ± 0.17 c | 6.13 ± 0.13 Xd |
| Duck | 7.70 ± 0.10 Ya | 7.30 ± 0.17 Yab | 7.03 ± 0.31 b | 5.00 ± 0.10 Yc | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, K.-M.; Kim, H.-Y. Effects of Electron Beam Irradiation on the Storage Stability and Quality Characteristics of Chicken and Duck Meat. Foods 2025, 14, 3867. https://doi.org/10.3390/foods14223867
Kang K-M, Kim H-Y. Effects of Electron Beam Irradiation on the Storage Stability and Quality Characteristics of Chicken and Duck Meat. Foods. 2025; 14(22):3867. https://doi.org/10.3390/foods14223867
Chicago/Turabian StyleKang, Kyu-Min, and Hack-Youn Kim. 2025. "Effects of Electron Beam Irradiation on the Storage Stability and Quality Characteristics of Chicken and Duck Meat" Foods 14, no. 22: 3867. https://doi.org/10.3390/foods14223867
APA StyleKang, K.-M., & Kim, H.-Y. (2025). Effects of Electron Beam Irradiation on the Storage Stability and Quality Characteristics of Chicken and Duck Meat. Foods, 14(22), 3867. https://doi.org/10.3390/foods14223867





