Simultaneously Monitoring and Reducing Nε-Carboxymethyl-Lysine and 5-Hydroxymethylfurfural Contents During Soy Sauce Production and Consumption
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
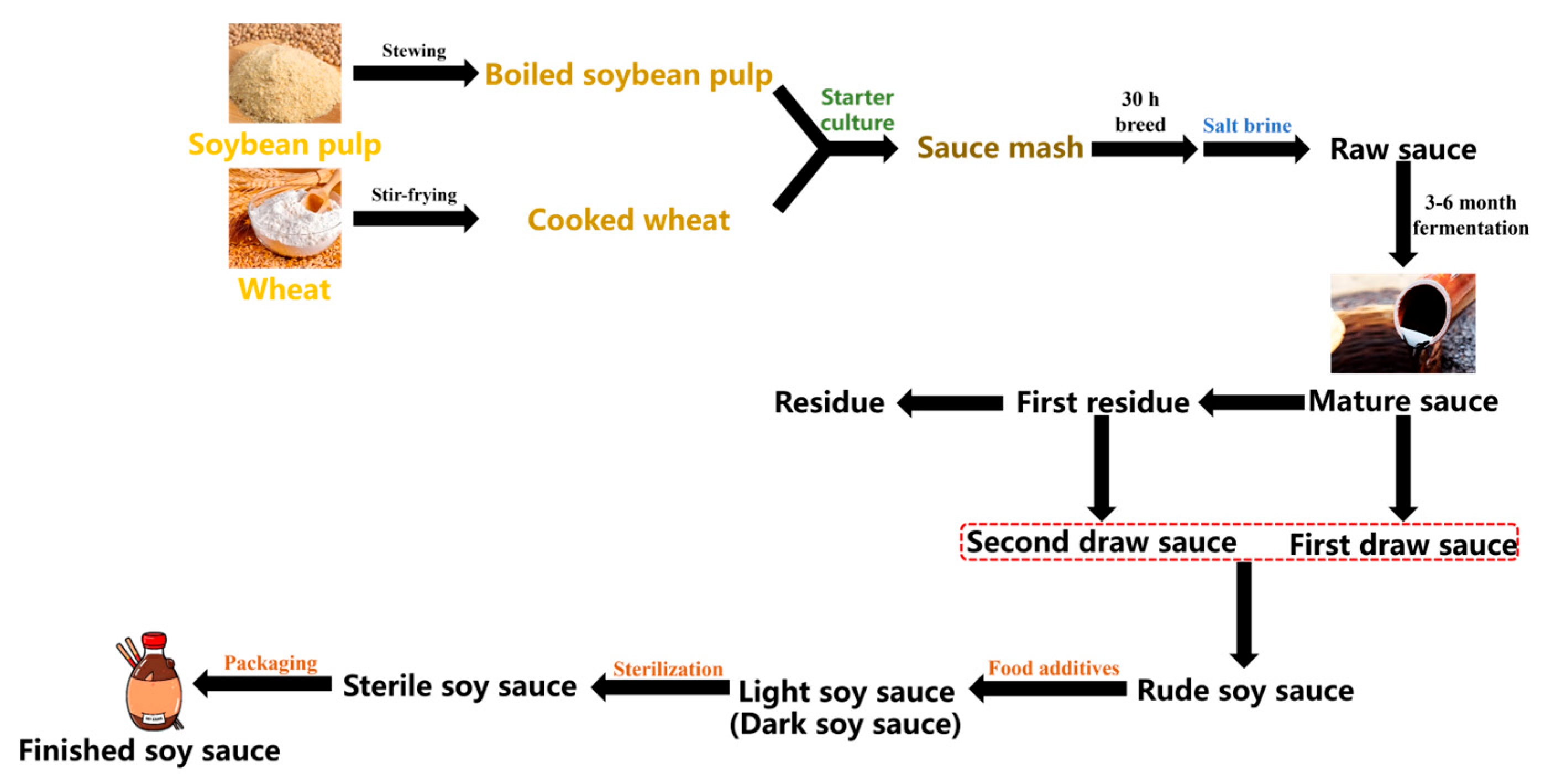
2.2. Sample Preparation
2.3. Detection of Physicochemical Indexes
2.4. Thermal Treatment
2.5. Simultaneous Detection of Free CML and 5-HMF
2.6. Statistical Analysis
3. Results and Discussions
3.1. The CML and 5-HMF Contents During SS Production
3.2. The Contents of CML and 5-HMF in Commercial SS
3.3. The Effect of Thermal Treatment on CML and 5-HMF Contents During SS Production Process
3.4. The Effect of EC, EGEG, and VC on CML and 5-HMF Contents in SS Consumption
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SS | Soy sauce |
| CML | Nε-carboxymethyl-lysine |
| 5-HMF | 5-hydroxymethylfurfural |
| EC | (-)-epicatechin (EC) |
| EGCG | (-)-epigallocatechin gallate (EGCG) |
| VC | Ascorbic acid |
| LSS | Light soy sauce |
| DSS | Dark soy sauce |
| FAAs | Free amino acids |
Appendix A

References
- Liu, Y.; Sun, G.; Li, J.; Cheng, P.; Song, Q.; Lv, W.; Wang, C. Starter molds and multi-enzyme catalysis in koji fermentation of soy sauce brewing: A review. Food Res. Int. 2024, 184, 114273. [Google Scholar] [CrossRef]
- Global Soy Sauce Market Size, Manufacturers, Supply Chain, Sales Channel and Clients, 2025–2031; 4675529; QYResearch: Industry, CA, USA, 2025; p. 151.
- Diez-Simon, C.; Eichelsheim, C.; Mumm, R.; Hall, R.D. Chemical and Sensory Characteristics of Soy Sauce: A Review. J. Agric. Food Chem. 2020, 68, 11612–11630. [Google Scholar] [CrossRef]
- Tang, J.; Tang, X.; Tang, M.; Zhang, X.; Xu, X.; Yi, Y. Analysis of the Bacterial Communities in Two Liquors of Soy Sauce Aroma as Revealed by High-Throughput Sequencing of the 16S rRNA V4 Hypervariable Region. Biomed. Res. Int. 2017, 2017, 6271358. [Google Scholar] [CrossRef]
- Yuan, X.; Nie, C.; Liu, H.; Ma, Q.; Peng, B.; Zhang, M.; Chen, Z.; Li, J. Comparison of metabolic fate, target organs, and microbiota interactions of free and bound dietary advanced glycation end products. Crit. Rev. Food Sci. Nutr. 2021, 63, 3612–3633. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhao, X.; Hu, F.; Fu, J.; Zhang, Z.; Liu, Z.; Wang, B.; He, R.; Ma, H.; Ho, C.T. The latest advances on soy sauce research in the past decade: Emphasis on the advances in China. Food Res. Int. 2023, 173, 113407. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, L.; Li, B.; Han, L.; Li, X.; Xu, Z.; Bian, H. Optimization of Pretreatment for Free and Bound Nε-(carboxymethyl)lysine Analysis in Soy Sauce. Food Anal. Methods 2014, 8, 195–202. [Google Scholar] [CrossRef]
- Liu, D.; He, Y.; Xiao, J.; Zhou, Q.; Wang, M. The occurrence and stability of Maillard reaction products in various traditional Chinese sauces. Food Chem. 2021, 342, 128319. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Luo, Y.; Sun, G.; Wang, P.; Hu, X.; Che, F. The simultaneous inhibition of histidine on 5-hydroxymethylfurfural and acrylamide in model systems and cookies. Food Chem. 2022, 370, 131271. [Google Scholar] [CrossRef]
- Wu, Y.; Zong, M.; Wu, H.; He, D.; Li, L.; Zhang, X.; Zhao, D.; Li, B. Dietary Advanced Glycation End-Products Affects the Progression of Early Diabetes by Intervening in Carbohydrate and Lipid Metabolism. Mol. Nutr. Food Res. 2022, 66, e2200046. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Zheng, L.; Wang, P.; Liu, Y.; Wu, Y.; Gong, Z. The neurotoxicity of Nepsilon-(carboxymethyl)lysine in food processing by a study based on animal and organotypic cell culture. Ecotoxicol. Environ. Saf. 2020, 190, 110077. [Google Scholar] [CrossRef]
- Wu, Y.; Zong, M.; Zhang, Z.; Wu, Y.; Li, L.; Zhang, X.; Wu, H.; Li, B. Selective transportation and energy homeostasis regulation of dietary advanced glycation end-products in human intestinal Caco-2 cells. Food Chem. 2022, 391, 133284. [Google Scholar] [CrossRef]
- Mossad, O.; Batut, B.; Yilmaz, B.; Dokalis, N.; Mezö, C.; Nent, E.; Nabavi, L.S.; Mayer, M.; Maron, F.J.M.; Buescher, J.M.; et al. Gut microbiota drives age-related oxidative stress and mitochondrial damage in microglia via the metabolite N6-carboxymethyllysine. Nat. Neurosci. 2022, 25, 295–305. [Google Scholar] [CrossRef]
- Delgado-Andrade, C.; Fogliano, V. Dietary Advanced Glycosylation End-Products (dAGEs) and Melanoidins Formed through the Maillard Reaction: Physiological Consequences of their Intake. Annu. Rev. Food Sci. Technol. 2018, 9, 271–291. [Google Scholar] [CrossRef] [PubMed]
- ALjahdali, N.; Carbonero, F. Impact of Maillard reaction products on nutrition and health: Current knowledge and need to understand their fate in the human digestive system. Crit. Rev. Food Sci. Nutr. 2017, 59, 474–487. [Google Scholar] [CrossRef]
- Delgado-Andrade, C.; Tessier, F.J.; Niquet-Leridon, C.; Seiquer, I.; Pilar Navarro, M. Study of the urinary and faecal excretion of Nepsilon-carboxymethyllysine in young human volunteers. Amino Acids 2012, 43, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Surh, Y.J.; Liem, A.; Miller, J.A.; Tannenbaum, S.R. 5-Sulfooxymethylfurfural as a possible ultimate mutagenic and carcinogenic metabolite of the Maillard reaction product, 5-hydroxymethylfurfural. Carcinogenesis 1994, 15, 2375–2377. [Google Scholar] [CrossRef] [PubMed]
- Quan, W.; Lin, Y.; Xue, C.; Cheng, Y.; Luo, J.; Lou, A.; Zeng, M.; He, Z.; Shen, Q.; Chen, J. Metabolic perturbations and health impact from exposure to a combination of multiple harmful Maillard reaction products on Sprague-Dawley rats. Food Funct. 2022, 13, 5515–5527. [Google Scholar] [CrossRef]
- Tian, Z.; Chen, S.; Shi, Y.; Wang, P.; Wu, Y.; Li, G. Dietary advanced glycation end products (dAGEs): An insight between modern diet and health. Food Chem. 2023, 415, 135735. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Y.; Deng, P.; He, Z.; Qin, F.; Chen, Q.; Wang, Z.; Chen, J.; Zeng, M.; Pan, H. Isotope dilution-HPLC-MS/MS to investigate the production patterns and possible pathways of free and protein-bound AGEs and 4-MI in cookies. Food Res. Int. 2023, 173, 113477. [Google Scholar] [CrossRef]
- Nguyen, H.T.; van der Fels-Klerx, H.J.; van Boekel, M.A. Kinetics of N(epsilon)-(carboxymethyl)lysine formation in aqueous model systems of sugars and casein. Food Chem. 2016, 192, 125–133. [Google Scholar] [CrossRef]
- Zeng, R.; Zhang, G.; Zheng, J.; Zhou, H.; Wang, Y.; Huang, C.; Hu, W.; Ou, S. Formation and Identification of Two Hydroxmethylfurfural-Glycine Adducts and Their Cytotoxicity and Absorption in Caco-2 Cells. J. Agric. Food Chem. 2020, 68, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, F.J.; Lavado-Tena, C.M.; Zamora, R. Conversion of 5-Hydroxymethylfurfural into 6-(Hydroxymethyl)pyridin-3-ol: A Pathway for the Formation of Pyridin-3-ols in Honey and Model Systems. J. Agric. Food Chem. 2020, 68, 5448–5454. [Google Scholar] [CrossRef]
- Yeh, W.J.; Hsia, S.M.; Lee, W.H.; Wu, C.H. Polyphenols with antiglycation activity and mechanisms of action: A review of recent findings. J. Food Drug Anal. 2017, 25, 84–92. [Google Scholar] [CrossRef]
- Zhu, H.; Poojary, M.M.; Andersen, M.L.; Lund, M.N. Trapping of Carbonyl Compounds by Epicatechin: Reaction Kinetics and Identification of Epicatechin Adducts in Stored UHT Milk. J. Agric. Food Chem. 2020, 68, 7718–7726. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Zhang, H.; Zhang, H.; Wu, G.; Wang, L.; Qian, H.; Qi, X. Epicatechin Adducting with 5-Hydroxymethylfurfural as an Inhibitory Mechanism against Acrylamide Formation in Maillard Reactions. J. Agric. Food Chem. 2018, 66, 12536–12543. [Google Scholar] [CrossRef]
- Lee, C.-H.; Chen, Y.-T.; Hsieh, H.-J.; Chen, K.-T.; Chen, Y.-A.; Wu, J.-T.; Tsai, M.-S.; Lin, J.-A.; Hsieh, C.-W. Exploring epigallocatechin gallate impregnation to inhibit 5-hydroxymethylfurfural formation and the effect on antioxidant ability of black garlic. Lwt 2020, 117, 108628. [Google Scholar] [CrossRef]
- Tu, A.-T.; Lin, J.-A.; Lee, C.-H.; Chen, Y.-A.; Wu, J.-T.; Tsai, M.-S.; Cheng, K.-C.; Hsieh, C.-W. Reduction of 3-Deoxyglucosone by Epigallocatechin Gallate Results Partially from an Addition Reaction: The Possible Mechanism of Decreased 5-Hydroxymethylfurfural in Epigallocatechin Gallate-Treated Black Garlic. Molecules 2021, 26, 4746. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, Y.; Shu, L.; He, Y. Study on metabolites of Bacillus producing soy sauce-like aroma in Jiang-flavor Chinese spirits. Food Sci. Nutr. 2020, 8, 97–103. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Feng, Y.Z.; Cui, C.; Zhao, H.F.; Zhao, M.M. Effects of koji-making with mixed strains on physicochemical and sensory properties of Chinese-type soy sauce. J. Sci. Food Agric. 2015, 95, 2145–2154. [Google Scholar] [CrossRef]
- Chou, C.-C.; Ling, M.-Y. Biochemical changes in soy sauce prepared with extruded and traditional raw materials. Food Res. Int. 1998, 31, 487–492. [Google Scholar] [CrossRef]
- Hu, B.; Li, B.; Chen, S.; Zhang, X.; Li, Y.; Wen, Y.; Liu, Z.; Li, L. Simultaneous detection of free Nε-(carboxymethyl)lysine and 5-hydroxymethylfurfural in soy sauce production. Food Sci. Technol. 2019, 44, 293–299. [Google Scholar] [CrossRef]
- Chen, G. Dietary N-epsilon-carboxymethyllysine as for a major glycotoxin in foods: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4931–4949. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Barringer, S.A. Kinetics of furan formation during pasteurization of soy sauce. LWT-Food Sci. Technol. 2016, 67, 200–205. [Google Scholar] [CrossRef]
- Hellwig, M.; Henle, T. Baking, ageing, diabetes: A short history of the Maillard reaction. Angew. Chem. Int. Ed. Engl. 2014, 53, 10316–10329. [Google Scholar] [CrossRef]
- Nikolov, P.Y.; Yaylayan, V.A. Reversible and covalent binding of 5-(hydroxymethyl)-2-furaldehyde (HMF) with lysine and selected amino acids. J. Agric. Food Chem. 2011, 59, 6099–6107. [Google Scholar] [CrossRef] [PubMed]
- da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.; Fett, R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef]
- Ramonaitytė, D.T.; Keršienė, M.; Adams, A.; Tehrani, K.A.; Kimpe, N.D. The interaction of metal ions with Maillard reaction products in a lactose–glycine model system. Food Res. Int. 2009, 42, 331–336. [Google Scholar] [CrossRef]
- Yan, L.; Greenwood, A.A.; Hossain, A.; Yang, B. A comprehensive mechanistic kinetic model for dilute acid hydrolysis of switchgrass cellulose to glucose, 5-HMF and levulinic acid. RSC Adv. 2014, 4, 23492–23504. [Google Scholar] [CrossRef]
- Zhang, L.L.; Kong, Y.; Yang, X.; Zhang, Y.Y.; Sun, B.G.; Chen, H.T.; Sun, Y. Kinetics of 5-hydroxymethylfurfural formation in the sugar-amino acid model of Maillard reaction. J. Sci. Food Agric. 2019, 99, 2340–2347. [Google Scholar] [CrossRef]
- Nowotny, K.; Schroter, D.; Schreiner, M.; Grune, T. Dietary advanced glycation end products and their relevance for human health. Ageing Res. Rev. 2018, 47, 55–66. [Google Scholar] [CrossRef]
- Chen, X.M.; Dai, Y.; Kitts, D.D. Detection of Maillard Reaction Product [5-(5,6-Dihydro-4H-pyridin-3-ylidenemethyl)furan-2-yl]methanol (F3-A) in Breads and Demonstration of Bioavailability in Caco-2 Intestinal Cells. J. Agric. Food Chem. 2016, 64, 9072–9077. [Google Scholar] [CrossRef]
- Zhang, L.-L.; Sun, Y.; Zhang, Y.-Y.; Sun, B.-G.; Chen, H.-T. Determination and Quantification of 5-Hydroxymethylfurfural in Vinegars and Soy Sauces. J. Food Qual. 2017, 2017, 1–8. [Google Scholar] [CrossRef]
- Nomi, Y.; Annaka, H.; Sato, S.; Ueta, E.; Ohkura, T.; Yamamoto, K.; Homma, S.; Suzuki, E.; Otsuka, Y. Simultaneous Quantitation of Advanced Glycation End Products in Soy Sauce and Beer by Liquid Chromatography-Tandem Mass Spectrometry without Ion-Pair Reagents and Derivatization. J. Agric. Food Chem. 2016, 64, 8397–8405. [Google Scholar] [CrossRef]
- Jia, W.; Guo, A.; Zhang, R.; Shi, L. Mechanism of natural antioxidants regulating advanced glycosylation end products of Maillard reaction. Food Chem. 2023, 404, 134541. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, Q.; Yu, X.; Liu, Y.; Li, L.; Li, B.; Zhang, X.; Chen, S.; Liu, Z.; Zhao, X.; et al. Study of reactions of Nepsilon-(carboxymethyl) lysine with o-benzoquinones by cyclic voltammetry. Food Chem. 2020, 307, 125554. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, L.; Lund, M.N.; Li, B.; Hu, Y.; Zhang, X. Reduction of Nepsilon-(carboxymethyl) lysine by (-)-epicatechin and (-)-epigallocatechin gallate: The involvement of a possible trapping mechanism by catechin quinones. Food Chem. 2018, 266, 427–434. [Google Scholar] [CrossRef]
- Craft, B.D.; Kerrihard, A.L.; Amarowicz, R.; Pegg, R.B. Phenol-Based Antioxidants and the In Vitro Methods Used for Their Assessment. Compr. Rev. Food Sci. Food Saf. 2012, 11, 148–173. [Google Scholar] [CrossRef]
- Totlani, V.M.; Peterson, D.G. Epicatechin Carbonyl-Trapping Reactions in Aqueous Maillard Systems_ Identification and Structural Elucidation. J. Agric. Food Chem. 2006, 54, 7311–7318. [Google Scholar] [CrossRef]
- Song, Z.; Jing, Y.; Wei, X.; Li, H.; Xie, J.; Shen, M. Mechanistic insights into advanced glycation end products production in glucose-lysine model system involved with ascorbic acid. Food Chem. 2025, 474, 143178. [Google Scholar] [CrossRef]
- Zhu, Q.Y.; Zhang, A.; Tsang, D.; Huang, Y.; Chen, Z.-Y. Stability of Green Tea Catechins. J. Agric. Food Chem. 1997, 45, 4624–4628. [Google Scholar] [CrossRef]
- Akyildiz, A.; Mertoglu, T.S.; Agcam, E. Kinetic study for ascorbic acid degradation, hydroxymethylfurfural and furfural formations in Orange juice. J. Food Compos. Anal. 2021, 102, 103996. [Google Scholar] [CrossRef]
- Smuda, M.; Glomb, M.A. Maillard degradation pathways of vitamin C. Angew. Chem. Int. Ed. Engl. 2013, 52, 4887–4891. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.A.; Ahmed, M.U.; Murtiashaw, M.H.; Richardson, J.M.; Walla, M.D.; Thorpe, S.R.; Baynes, J.W. Reaction of ascorbate with lysine and protein under autoxidizing conditions: Formation of N.epsilon.-(carboxymethyl)lysine by reaction between lysine and products of autoxidation of ascorbate. Biochemistry 1990, 29, 10964–10970. [Google Scholar] [CrossRef] [PubMed]




| Reducing Sugars | Soluble Salt-Free Solids | Protein | Total Nitrogen | FAAs | |
|---|---|---|---|---|---|
| CML | −0.015 | 0.576 * | 0.015 | 0.026 | 0.003 |
| 5-HMF | −0.600 ** | 0.0482 | −0.800 ** | −0.765 ** | −0.841 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Hu, B.; Wen, Y.; Xiao, Z.; Li, L.; Zhang, X.; Zhang, Z.; Li, B. Simultaneously Monitoring and Reducing Nε-Carboxymethyl-Lysine and 5-Hydroxymethylfurfural Contents During Soy Sauce Production and Consumption. Foods 2025, 14, 2437. https://doi.org/10.3390/foods14142437
Wu Y, Hu B, Wen Y, Xiao Z, Li L, Zhang X, Zhang Z, Li B. Simultaneously Monitoring and Reducing Nε-Carboxymethyl-Lysine and 5-Hydroxymethylfurfural Contents During Soy Sauce Production and Consumption. Foods. 2025; 14(14):2437. https://doi.org/10.3390/foods14142437
Chicago/Turabian StyleWu, Yongtai, Bei Hu, Yuxin Wen, Zuowei Xiao, Lin Li, Xia Zhang, Zhenhui Zhang, and Bing Li. 2025. "Simultaneously Monitoring and Reducing Nε-Carboxymethyl-Lysine and 5-Hydroxymethylfurfural Contents During Soy Sauce Production and Consumption" Foods 14, no. 14: 2437. https://doi.org/10.3390/foods14142437
APA StyleWu, Y., Hu, B., Wen, Y., Xiao, Z., Li, L., Zhang, X., Zhang, Z., & Li, B. (2025). Simultaneously Monitoring and Reducing Nε-Carboxymethyl-Lysine and 5-Hydroxymethylfurfural Contents During Soy Sauce Production and Consumption. Foods, 14(14), 2437. https://doi.org/10.3390/foods14142437






