Abstract
Mycotoxin research is facing unprecedented challenges, starting from the urgent need to cope with the consequences of climate change, the global shortage of grain due to unstable political scenarios, and the major transformation of the supply chains after the COVID-19 pandemic. In this scenario, the mycotoxin contamination of human and animal foods is still unavoidable, thus representing a major challenge to global food security. Next to this, the shift to sustainable and circular food production might be accompanied by an increase in food safety issues involving mycotoxins, e.g., when new technologies are applied to reuse side streams from the food industry, it is not known if and how mycotoxins accumulate in these by-products. MycoTWIN is an EU-funded Horizon 2020 project which fosters knowledge transfer and scientific cooperation within the Mediterranean area, involving worldwide experts, decision makers, and stakeholders in the field of mycotoxigenic fungi and mycotoxins. The MycoTWIN project hosted working group meetings, whose aim was to propose operational plans and/or scientific strategic plans to shape the future research directions to better cope with these challenges. In the working group cycle “Future proof approaches for the management of toxigenic fungi and associated mycotoxins along the food chain”, a multi-actor group was guided in co-creation exercises to elaborate on future research directions and propose relevant actions to be implemented for the present to long-term time periods. The discussion focused on three main topics relevant to the assessment and management of risks associated with mycotoxins and toxigenic fungi: (i) needs for the harmonization of molecular and chemical methods and data analysis, (ii) from lab research to marketable solutions: how to fill the gap, and (iii) gaps in data quality for risk assessment.
1. Introduction
In recent years, the food system has been facing concurrent, emergent, and unexpected challenges, such as climate change, geopolitical tensions, the global shortage of grains, societal changes, and a major transformation of the supply chains after the COVID-19 pandemic. The capability of producing enough, safe, and nutritious food and feed for the growing global population is seriously affected, especially in countries already facing food safety and security issues.
A recent survey covering the first half of 2024 detected high mycotoxin prevalence and associated risks across North and Latin America, Europe, Sub-Saharan Africa, and Asia, while moderate risks were noted in Northern Europe and Oceania [1]. Besides regulated mycotoxins, emerging ones (such as fusaric acid, enniatins, culmorin, apicidin, butenolide, fusaproliferin, Alternaria toxins, auofusarin, emodin, beauvericin (BEA), diacetoxyscirpenol (DAS), moniliformin (MON), and sterigmatocystin) are increasingly detected worldwide in staple cereal commodities. The toxicity of these compounds, as well as their potential to interact with each other and with other xenobiotics, poses an even greater risk to human and animal health [2].
Indeed, despite the efforts of the scientific community and stakeholders, mycotoxins still represent the third most notified hazard category in Europe by the Rapid Alarm Safety System for Food and Feed (RASFF), with f.i. a 10.5% increase in 2022 compared to 2021 [3].
Constantly active research is in progress to develop advanced multi-mycotoxin detection methods for complex samples, as well as prevention strategies (including predictive modeling) and mitigation strategies through physical and biological approaches; however, due to the evolving scenario, several challenges persist. Building capacity for mycotoxin management, especially in resource-limited regions, and harmonizing efforts for effective risk assessment and mitigation is crucial. Achieving transitions towards a healthier, safer, sustainable, and environmentally friendly food system as set out in the Farm2Fork EU strategy [4], and counteracting the global threat of mycotoxin contamination, requires a bold and holistic response by the research community. One solid approach to creating future European Food Safety strategies is to identify the priorities of common interest and share the results, information, and knowledge for institutions and policy makers through cooperation initiatives.
Horizon 2020 was designed to promote scientific excellence, foster innovation, and address major societal challenges. The program prioritized fields such as climate action, sustainability, health, and digitalization, with a strong emphasis on involving business operators, fostering partnerships, and encouraging international cooperation to solve complex global issues. As part of the H2020 scope, during the EU H2020-funded project MycoTWIN [5], working group meetings were organized with the aim of proposing operational plan and/or scientific strategic plans to shape the future research directions in the field of mycotoxigenic fungi and mycotoxins. During the working group cycle “Future proof approaches for the management of toxigenic fungi and associated mycotoxins along the food chain” organized by CNR-ISPA, three trending topics were identified and discussed by the MycoTWIN working with a multidisciplinary, holistic, and collaborative approach provided by the hosting EU Food Safety Platform [6] (see Section 2 for full details).
When selecting the topics of the working groups, the choice was driven by an extensive review of the existing literature, engaging with scientific networks/research consortia (H2020-funded FoodSafety4EU and EFSA-funded MYCOBOOST projects), monitoring white papers and reports of government agencies (FAO and EFSA), and by the strategic insights gained from the round table discussions held during the first MycoKey International conference, titled “Global Mycotoxin Reduction in the Food and Feed Chain” [6,7,8]. The topics’ selection was conducted to ensure access to up-to-date information on the challenges, research priorities, pressing industry and regulatory issues, and emerging trends shaping the field of mycotoxin research.
Future research needs in the food safety domain are elaborated in recently issued research agendas setting the updated research and innovation framework (f.i. the Food 2030 agenda, and its pathway action “Food Safety Systems of the Future”) [9], or the FoodSafety4EU Strategic Research and Innovation Agenda [10]. In this framework, the generation of and access to Findable, Accessible, Interoperable, and Reusable (FAIR) data are acknowledged as the fundamentals for improving food safety management and risk assessment. Thinking about the rapid advances in toxicology and in the assessment of chemical safety in humans (e.g., mycotoxin mixtures) as well as the spreading of big data (large amount, complex, structured and unstructured data) in this field, the availability of improved and harmonized approaches to generate, analyze, interpret, reuse, and integrate data about mycotoxin contamination at national/EU level is of utmost importance.
The same consideration applies to the design of predictive and modeling tools needed, for instance, to cope with climate change’s impact on mycotoxin/toxigenic fungi distribution patterns.
Next to risk assessment, extensive and systematic mycotoxin/fungi monitoring either in the field or along the chain can contribute to the development and implementation of mitigation measures supporting risk management decisions. In this respect, space and funding for the translation of laboratory research into practical solutions, enabling food business operators to control and share data about contamination, can be found in the Horizon Europe program [11]. The established and emerging technologies for real-time mycotoxin/fungi monitoring, complemented by validated approaches to generate reliable data, are expected to support preventive measures and the reduction in food waste due to product non-compliance.
Based on all the above considerations, the following topics were selected to be elaborated by the working group: (i) Needs for harmonization of molecular and chemical methods and data analysis, (ii) From lab research to marketable solutions: how to fill the gap, and (iii) Gaps in data quality for risk assessment.
2. Methodology for Collaborative Exercise
2.1. Participants
Working group participants were selected among the MycoTWIN consortium, international experts, and the private sector according to diverse research expertise and capabilities, geographic origin, affiliation, and interests in the food safety domain. They represented: research institutions (National Research Council of Italy—Institute of Sciences of Food Production—CNR-ISPA—Bari, Italy; TUBITAK MRC Life Sciences—Gebze/Kocaeli, Turkey; and International Institute of Tropical Agriculture, The Institut National De Recherche Pour L’agriculture, L’alimentation Et L’environnement—INRAE—Bordeaux, France), universities (Valencia University—Valencia, Spain; Piacenza University—Piacenza, Italy; Parma University—Parma, Italy; Liège University—Liège, Belgium; Ghent University—Ghent, Belgium; and Cranfield University—Cranfield, UK), global food authorities (the European Commission, Joint Research Centre—JRC), the EU Food Safety Platform, and national and international companies (Bonifiche Ferraresi, Eurofins Tecna, and Syngenta).
The collaborative exercise was organized as a series of 2 working groups as described in Section 2.2 and Section 2.3; 37 and 11 participants were involved in phase 1 and phase 2, respectively.
2.2. Working Group 1 (1st Phase)
In the preparatory work executed before the working group, based on an extensive review of the available strategic documents (see Section 1), three relevant topics in the mycotoxin field were selected by the authors, and a draft list of issues for the working group participants was prepared.
The first working group was held as a live event on 22 May 2022. For each proposed topic and its related draft list of issues, the discussion was structured as follows:
- Silent reflection: Each participant considered the proposed draft list of issues and annotated amendments or additions of new issues to be included in the subsequent discussion.
- Plenary discussion: The discussion was led by a facilitator, who moderated the discussion, and a reporter, who took notes of the amendments and/or new issues generated by the participants.
- Voting and ranking: Once the participants agreed on the list of issues generated from the plenary discussion, they were asked to vote on them according to the priority of action, with a scale of 1 (low priority) to 10 (high priority). To calculate the weighted mean, the total scores for each issue were divided by the number of participants who ranked that issue. The first six issues, prioritized according to the weighted mean, were subjected to another round of voting according to uncertainty using the same scale (1 to 10).
2.3. Working Group 2 (2nd Phase)
The second working group discussion was held as a hybrid event among MycoTWIN experts and external guests on 15 April 2024. It was structured as follows:
- Topic discussion: The issues prioritized in the previous working group were briefly discussed, also reviewing the priority and uncertainty scores.
- Target action proposition: For each prioritized issue, the participants were asked to propose a target action or strategic plan by specifying the related hazard. The participants’ contributions were first collected on a digital board and then reviewed and rephrased until consensus was achieved.
- Interlinks: After carrying out step 2 for each of the three topics, the participants were asked to discuss and indicate interlinks between the proposed actions and subtopics.
Common actions or actions with multiple impacts selected with the interlink exercise were discussed considering urgency and uncertainty and classified into the following:
- (i)
- Present (one-year needs): Urgent issues;
- (ii)
- Near future (three-year needs): Emerging issues;
- (iii)
- Normal term (five-year needs): Mid-term issues;
- (iv)
- Long term (eight-year needs): Far future issues.
This was performed with the aim to provide input for future research in the field of mycotoxins and toxigenic fungi.
Clustering issues within specified timeframes: Scores for the prioritized issues (Section 2.2, item 3), based on votes for both the priority of action and level of uncertainty, were normalized on a 0–5 scale, with the highest voted issue across all three topics serving as the benchmark (score 5). The issues were clustered within the four timeframes (present—one-year needs, near future—three-year needs, normal term—five year-needs, and Long term—eight-year needs) based on a qualitative evaluation that, however, took into account not only the normalized scores, but also the experts’ evaluations of issues, i.e., the urgency (priority) of the identified needs, the uncertainty of the available solutions/methodologies to cope with them, and the possible interlinks among the issues.
2.4. Statistical Analyses
The priority of action and uncertainty rank scores (item 3, Section 2.2) were subjected to normality testing distribution using the Shapiro–Wilk test. Following the assessment of non-normal data distribution, the non-parametric Friedman test was applied, along with the post hoc Wilcoxon signed-rank test, to assess statistically significant differences among the voted issues. The Bonferroni-adjusted p-value was applied for pairwise comparisons. All the statistical analyses were performed using the IBM SPSS software (IBM SPSS Statistics for Windows, Version 29.0 (Armonk, NY, USA: IBM Corp, 2022).
2.5. Glossary
For the purpose of the working group meetings, the following glossary was adopted:
- (i)
- High level of priority of action: Something given or meriting attention before competing alternatives since it represents a pre-requisite, or a key requisite, to undertake the other issues in the list;
- (ii)
- High level of uncertainty: not fully investigated and researched; not based on well-established knowledge; values are unknown; possible outcomes are incomplete and ambiguous; unstable; hard to predict; difficult to estimate; unobvious impact.
3. Results
In working group 1 (first phase), for each selected topic, the current constraints, enablers, or requirements (from now on defined as “issues”) were identified by the experts in co-creation exercises and ranked according to the priority of action and uncertainty, according to the methodology described in Section 2.2. Working group 1 resulted in a list of six prioritized issues for each of the three discussed topics. In working group 2 (second phase), the prioritized issues were further explored, identifying the main related hazards and proposing actions to cope with them. Finally, the issues and relevant proposed actions were clustered in four timeframes according to the priority of action, uncertainty, and interlinks identified between the issues.
3.1. Topic 1: Needs for Harmonization of Molecular and Chemical Methods and Data Analysis
Harmonized, quality data are essential to ensure the consistency, comparability, and reliability of results. They represent a mandatory requirement to perform large-scale monitoring, gain insight into mycotoxin and fungal contamination, support the establishment or revision of mycotoxin regulatory limits in food and feed, and manage recommendations and guidelines at various levels. Nonetheless, given the current increase in data generation driven by high-throughput and AI-based approaches, harmonizing molecular and chemical methods and data analysis has become a critical issue that requires immediate attention from the research community.
3.1.1. Topic 1 Issue Definition and Prioritization
During the first working group meeting, eleven issues related to constraints and enablers for method harmonization were identified (Table 1). The experts identified as major issues the need for standardization protocols/procedures (issues n. 1, 2, 7, 8, 9, and 10), need for reference material (issue n. 3), data generation and sharing (issues n. 4, 6, and 7), and method validation (issues n. 8 and 11). The ranked issues are shown in Table 1 (priority of action) and Table 2 (top six, uncertainty). Looking at the uncertainty ranking, the group’s view revealed that there are still gaps in traditional issues that need to evolve in the new scenario. These include reference material—with emphasis on multi-mycotoxin or emerging/modified mycotoxin reference materials—sampling and the validation of new analytical approaches.

Table 1.
Prioritized issues (priority of action) for Topic 1. Priority scores range from 1 (low priority) to 10 (high priority).

Table 2.
Prioritized issues (priority of action and uncertainty) for Topic 1. Uncertainty scores from 1 (low uncertainty) to 10 (high uncertainty).
3.1.2. Definition of Hazards and Relevant Actions for Topic 1
The development of collaborative, data-driven platforms, infrastructures, and online repositories were identified as crucial actions to undertake. These platforms enable data sharing and use across different research fields and link molecular/predictive data to phenotype and bioanalysis, as also highlighted during the plenary discussion. In addition, simplified and harmonized data formats and management, also supported by artificial intelligence, were classified as emerging issues (Table 3).

Table 3.
Hazards and relevant actions proposed by the MycoTWIN working group participants for each prioritized issue of Topic 1—needs for the harmonization of molecular and chemical methods and data analysis.
Interestingly, while it is clear that new hazards have been identified for emerging mycotoxins and mycotoxins in biofluids and new commodities, such as fermented foods, novel foods, and side streams, the situation for toxigenic fungi is less clear as they are not regulated. A notable normal-term issue that emerged from the discussion regarded fungal taxonomy, for which there is the need for reference strains to define fungal chemotypes based on the mycotoxigenic potential rather than core features. Hence, fungal taxonomy should be revised according to the toxigenic potential, especially in light of climate change and the emergence of new commodities of interest.
3.2. Topic 2: From Lab Research to Marketable Solutions: How to Fill the Gap
Filling the gap between laboratory research and marketable solutions is a crucial task to ensure that scientific advancements are translated into tangible benefits for stakeholders and society. This process involves a multi-actor, multidisciplinary intervention to address the multifaceted challenges, different backgrounds, and perspectives. Indeed, often the information or efforts required to bring innovation into the market rely also on other stakeholders, such as large-scale and/or smallholder farmers, and observations made by extension agents, policy makers, and private industry, rather than on solely scientists [12].
3.2.1. Topic 2 Issue Definition and Prioritization
Twelve issues related to constraints and enablers for bridging the gap between laboratory research and marketable solutions were identified (Table 4). The experts highlighted not only the need for improvements in technologies (issues n. 1, 3, 4, 6, 8, 9, 10, and 11), but also the need to bring awareness and a structured, improved communication (issue n. 2), and to engage with the regulatory bodies (issues n. 5 and 7) and companies (issues n. 2, 9, and 12). These latter were also listed among the most uncertain issues to tackle (Table 5), also due to the lack of well-established and trusted channels of communication between these actors of the food safety system, especially when thinking about local authorities and small enterprises/farmers. However, issues like the verification of the analytical process and full traceability were put on the basis of acceptance by regulatory bodies and trustful communication.

Table 4.
Prioritized issues (priority of action) for Topic 2.

Table 5.
Prioritized issues (priority of action and uncertainty) for Topic 2.
3.2.2. Definition of Hazards and Actions for Topic 2
Among the actions to be undertaken in the present–near future, the experts highlighted the need for fitness-for-purpose approaches in method validation, performance, and verification protocols. They also emphasized the importance of promoting automated procedures and providing on-site training to improve reliability, validity, and quality control. Investments and cooperation were considered urgent actions as well.
As for normal-term improvements (over the next 5 years), the experts only identified technical improvements, also based on AI, to improve the ease of use and method reliability.
Concerning hazard identification, regulated mycotoxins and mycotoxin adducts/metabolites in the blood and urine emerged as important hazards, for which the gap from lab research to marketable solutions is still high.
3.3. Topic 3: Gaps in Data Quality for Risk Assessment
Continuous risk assessment is crucial to comprehensively understand and mitigate the potential adverse effects of mycotoxin contamination, thereby ensuring safe food and feed. High-quality data represent the foundation for each step of risk assessment, i.e., hazard identification and characterization, exposure assessment, and risk characterization.
Nonetheless, researchers consistently point out the limited availability, inconsistency, and lack of harmonization and integration in data concerning fungal and mycotoxin contamination and the need for robust methodologies and cutting-edge technologies for data collection, analysis, and interpretation [13].
3.3.1. Topic 3 Issue Definition and Prioritization
Nine issues were identified by the experts (Table 6) regarding protocols and guidelines for data and metadata collection and sharing (issues n. 1, 2, 3, 6, and 7), platforms for data storage and management (issues n. 4, 5, and 9), and privacy/reputation (issue 8). The most urgent issues identified regarded harmonizing data and promoting data sharing (Table 7).

Table 6.
Prioritized issues (priority of action) for Topic 3.

Table 7.
Prioritized issues (priority of action and uncertainty) for Topic 3.
3.3.2. Definition of Hazards and Actions for Topic 3
The actions identified by the experts in the near- and normal-term timeframe aimed at supporting data collection and curation, and platforms in which they should be transparently shared. These actions targeted users (involvement and training) and digital infrastructures (user-friendly and accessible software, workflows, and open access tools).
For the first time, long-term actions (8-year needs) were identified and focused on metadata. In the era of omics techniques, metadata are becoming as important as the data themselves, as they include the full description of how the data were obtained, processed, and annotated [14]. Besides actions aimed to establish harmonized protocols and formats, the experts highlighted the need to implement knowledge and awareness on metadata, their acquisition through New Approach Methodologies (in silico, in vitro, omics, predictive, and biomarker-based technologies), and their sharing among researchers, risk assessors, and risk managers.
4. Discussion
In relation to the three discussed topics, a number of strategic actions was proposed to address relevant hazards that included regulated and emerging mycotoxins as well as mycotoxigenic fungi (Table 3, Table 8 and Table 9).

Table 8.
Hazards and relevant actions proposed by the MycoTWIN working group participants for each prioritized issue of Topic 2—from lab research to marketable solutions: how to fill the gap.

Table 9.
Hazards and relevant actions proposed by the MycoTWIN working group participants for each prioritized issue of Topic 3—gaps in data availability and quality for risk assessment.
The issues emerging from Topic 1 were among the ones with a higher impact on the issues of the other topics. Indeed, method harmonization is often a prerequisite to implementing the transferability of research to the market (Topic 2) and to defining data used in risk assessment (topic 3). A consistent methodology, which includes standardized data collection and curation, the availability of reference material, and fit-for-purpose validation programs, enables one to easily use and implement novel methods into the market and verify them against the official ones (Figure 1). Sharing data was also regarded as a tool to create awareness and promote communication among research, private companies, and consumers, which in turn fosters transparency, collaboration, and trust-building (Figure 1). Regulatory bodies support the strategic goals of the European Union, encouraging innovation and scientific advancement. Their role in promoting policies, education, and funding to support metadata and data sharing was acknowledged (Figure 1). The statistical analyses revealed significant differences only among the priority scores of Topics 1 and 2, while no differences were found in their uncertainty scores or among all the Topic 3 scores. Overall, the closely grouped scores—including those in which statistically significant differences were observed—indicated that the working group participants assigned a consistent level of recognition to the issues for all the topics. Their role in promoting policies, education, and funding to support metadata and data sharing was acknowledged (Figure 1). It is important to note that the scores were assigned individually, reflecting the individual participants’ experience and expertise. To reach a consensus, the issues were further elaborated within the working group taking into account the scoring results. By elaborating on (i) the urgency (priority) of the identified needs, (ii) the uncertainty of the available solutions/methodologies to cope with them, and (iii) the interlinks showing which actions were pre-requisite for others, the experts clustered the proposed actions in four timeframes (present, near, medium, and long term) to envisage a possible strategic action plan. The actions to be undertaken in the short–medium terms were identified mainly in Topics 1 and 2. The discussion highlighted that advanced research on regulated mycotoxins (and relevant fungi) and emerging ones is already in progress. The needed actions deal mainly with better exploitation/transfer and sharing of already available research results. Therefore, approaches and guidelines for the harmonization and standardization of data and protocols, which in turn will enable data sharing and joint exploitation, resulted to be of high priority.
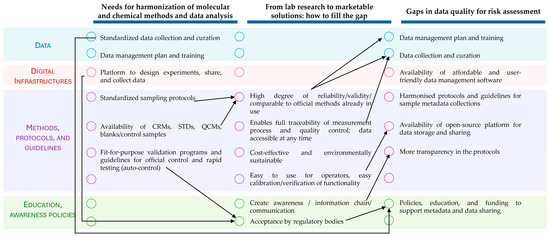
Figure 1.
Simplified scheme of the topic interlinks proposed by the MycoTWIN working group. For each topic (column), the issues were categorized by color: Blue—issues related to data; Orange—issues related to digital infrastructures; Purple—issues related to methods, protocols, and guidelines; Green—issues related to education, awareness, and policies. The arrows indicate the interlinks among the issues of different topics.
A major impact will result from the increasing application of AI-based approaches in mycotoxin risk prediction, characterization, assessment, and management. In this respect, full compliance with the FAIR principles in data generation, collection, and storage will require actions in the medium–long term to cope with big data and issues related to Intellectual Property (IP) and data curation.
Common issues in Topics 1 and 3 highlighted the need for improving data collection, curation, and platforms/software for their management. Initiatives led by the World Health Organization (WHO) jointly with the Food and Agriculture Organization (FAO), and EFSA have made several progresses towards the definition of descriptors for mycotoxin contamination data [15]. Nonetheless, the occurrence of mycotoxigenic fungi in food and feed remains largely unregulated. Although there is a significant body of literature addressing the occurrence of these fungi in specific commodities and in specific geographical areas, the data reported in these studies are not systematically reported nor analyzed, often resulting in fragmented or inconsistent data. In addition, the current taxonomic definition for fungal species is not adequate to describe the toxigenic potential of a fungal strain in a specified matrix in a geographical area under a defined climatic condition. Harmonized approaches to identify mycotoxigenic fungi would facilitate more effective monitoring, risk assessment, and management of fungal contamination along the supply chain.
A cross-cutting need over the three topics resulted to be the availability of platforms and infrastructures for data storage, knowledge sharing, and access. This addresses not only mycotoxins (food safety and risk assessment) but also fungi, since further developments were advocated to establish and maintain international microbial collections as well as new and harmonized approaches for species classifications or re-definition based on their mycotoxigenic potential. Capitalizing and making the already existing platforms interoperable is an action that can be planned in the short term, while the achievement of fully open-source solutions is envisaged in the long term.
Long-term issues were identified within Topic 3 and deal with (i) the harmonization of data, metadata, and protocols for emerging risk assessment and toxicological assessment methodologies (NAMs) and (ii) long-term strategies to shape future policies and orienteering future funding schemes and promoting investments, also through education and training. The latter is expected to contribute to facilitating the relationship between risk assessors and risk managers with a view to the evolving food safety scenarios.
5. Conclusions
Starting from the available knowledge, a co-creation process was implemented, along with a workshop series, to generate a shared vision of the challenges related to the management of food safety risks, arising from mycotoxin and toxigenic fungi contamination along the food/feed chain. The multi-actor composition of the working groups allowed them to capture stakeholders’ needs, fostering a multi-perspective approach in prioritizing and undertaking these challenges.
In line with the main objective of the MycoTWIN project, which was capacity building, this collaborative exercise might facilitate the incorporation of new researchers into the group and foster collaborative networks around topics of common interest, while the outcomes of the discussion are expected to serve the scientific community by guiding authors in developing new research projects.
By identifying common actions or actions with multiple impacts on the prioritized issues, the MycoTWIN working group selected and proposed three emerging research topics in the field of mycotoxigenic fungi and mycotoxins for the European framework.
- (1)
- Further development of in silico models for emerging risk assessment (NAMs), including harmonized models and protocols for computational modeling, high-throughput screening, omics technologies, and mechanistic toxicology;
- (2)
- To develop new and more robust approaches for the taxonomic classification of fungal species, also based on interlaboratory comparisons;
- (3)
- To map the available resources and propose pathways for the integration and interoperability of the existing platforms/infrastructures connecting data and metadata from different sources (i.e., genomic analyses, transcriptomic studies, biological assays, expression profiles, and more).
If complemented by adequate measures for awareness building and training, future research in these domains is expected to provide input for future food safety R&I programs.
Author Contributions
Conceptualization, V.M.T.L. and M.L.; methodology, V.M.T.L. and M.L.; investigation, M.L.; resources V.M.T.L.; writing—original draft preparation, M.L. and V.M.T.L.; writing—review and editing, M.L., A.M., V.L., L.M., P.V.-D., H.Ö., C.P.K., E.Y., İ.D. and V.M.T.L.; visualization, M.L. and V.M.T.L.; supervision, A.M. and H.Ö.; project administration, A.M. and H.Ö.; funding acquisition, A.M. and H.Ö. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by H2020-WIDESPREAD-2020-5 MycoTWIN “Enhancing Research and Innovation Capacity of TÜBITAK MAM Food Institute on Management of Mycotoxigenic Fungi And Mycotoxins” Grant Agreement-952337.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to thank Sarah De Saeger and Riccardo Baroncelli for their valuable suggestions during the second working group.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- DSM Mycotoxin Survey 2024. Available online: https://www.dsm.com/ (accessed on 29 October 2024).
- Kolawole, O.; Siri-Anusornsak, W.; Petchkongkaew, A.; Elliott, C. A systematic review of global occurrence of emerging mycotoxins in crops and animal feeds, and their toxicity in livestock. Emerg. Contam. 2024, 10, 100305. [Google Scholar] [CrossRef]
- RASFF. 2022 Annual Report Alert and Cooperation Network. Available online: https://food.ec.europa.eu/system/files/2023-10/acn_annual-report_2022.pdf (accessed on 14 June 2024).
- European Environmental Agency. Transforming Europe’s Food System—Assessing the EU Policy Mix; European Environmental Agency: Copenhagen, Denmark, 2022; Available online: https://www.eea.europa.eu/publications/transforming-europes-food-system (accessed on 17 June 2024).
- MycoTWIN Project. Available online: https://www.mycotwin.eu/ (accessed on 20 June 2024).
- FoodSafety4EU Project. Available online: https://foodsafety4.eu/ (accessed on 21 June 2024).
- Dall’Asta, C.; De Boevre, M.; Dellafiora, L.; De Saeger, S.; Moretti, A.; Pinson-Gadais, L.; Susca, A. Boosting knowledge and harmonisation in the mycotoxin field through sustainable scientific alliances—MYCOBOOST. EFSA Supp. Publ. 2023, 20, 8420E. [Google Scholar] [CrossRef]
- Leslie, J.F.; Lattanzio, V.; Audenaert, K.; Battilani, P.; Cary, J.; Chulze, S.N.; De Saeger, S.; Gerardino, A.; Karlovsky, P.; Liao, Y.-C.; et al. MycoKey Round Table Discussions of Future Directions in Research on Chemical Detection Methods, Genetics and Biodiversity of Mycotoxins. Toxins 2018, 10, 109. [Google Scholar] [CrossRef] [PubMed]
- Food 2030: Green and Resilient Food System. Available online: https://research-innovation-community.ec.europa.eu/events/5XBL7D4zP2PsfvrXmAJPv1/overview (accessed on 19 July 2024).
- Nastasia, B.; Duta, D.; Cito, N.; Rychlik, M.; Lattanzio, V.M.T. Strategic Research and Innovation Agenda for Food Safety in Europe. EU Food Safety Forum, Brussels. Zenodo 2023. [Google Scholar] [CrossRef]
- Horizon Europe Programme. Available online: https://research-and-innovation.ec.europa.eu/funding/funding-opportunities/funding-programmes-and-open-calls/horizon-europe_en (accessed on 22 June 2024).
- Ortega-Beltran, A.; Bandyopadhyay, R. Aflatoxin Biocontrol in Practice Requires a Multidisciplinary, Long-Term Approach. Front. Sustain. Food Syst. 2023, 7, 1110964. [Google Scholar] [CrossRef]
- Chhaya, R.S.; O’Brien, J.; Cummins, E. Feed to Fork Risk Assessment of Mycotoxins under Climate Change Influences-Recent Developments. Trends Food Sci. Technol. 2022, 126, 126–141. [Google Scholar] [CrossRef]
- Cernava, T.; Rybakova, D.; Buscot, F.; Clavel, T.; McHardy, A.C.; Meyer, F.; Berg, G. Metadata Harmonization–Standards Are the Key for a Better Usage of Omics Data for Integrative Microbiome Analysis. Environ. Microbiome 2022, 17, 33. [Google Scholar] [CrossRef] [PubMed]
- Mesfin, A.; Lachat, C.; Vidal, A.; Croubels, S.; Haesaert, G.; Ndemera, M.; Matumba, L. Essential Descriptors for Mycotoxin Contamination Data in Food and Feed. Food Res. Int. 2022, 152, 110883. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).