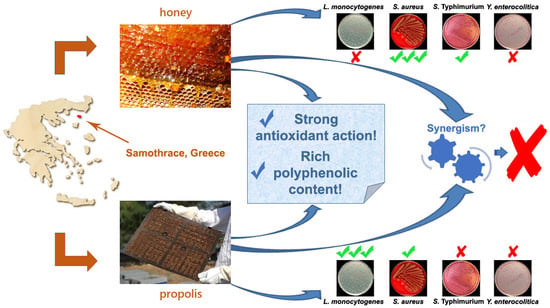
Investigating Possible Synergism in the Antioxidant and Antibacterial Actions of Honey and Propolis from the Greek Island of Samothrace through Their Combined Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Honey and Propolis Samples
2.2. Bacterial Strains and Preparation of Their Working Suspensions
2.3. Chemicals
2.4. Determination of the Antioxidant Activity (AA)
2.5. Determination of the Total Phenolic Content (TPC)
2.6. Determination of Antibacterial Action
2.7. Statistical Analyses
3. Results and Discussion
3.1. AA and TPC of Propolis and Honey Samples
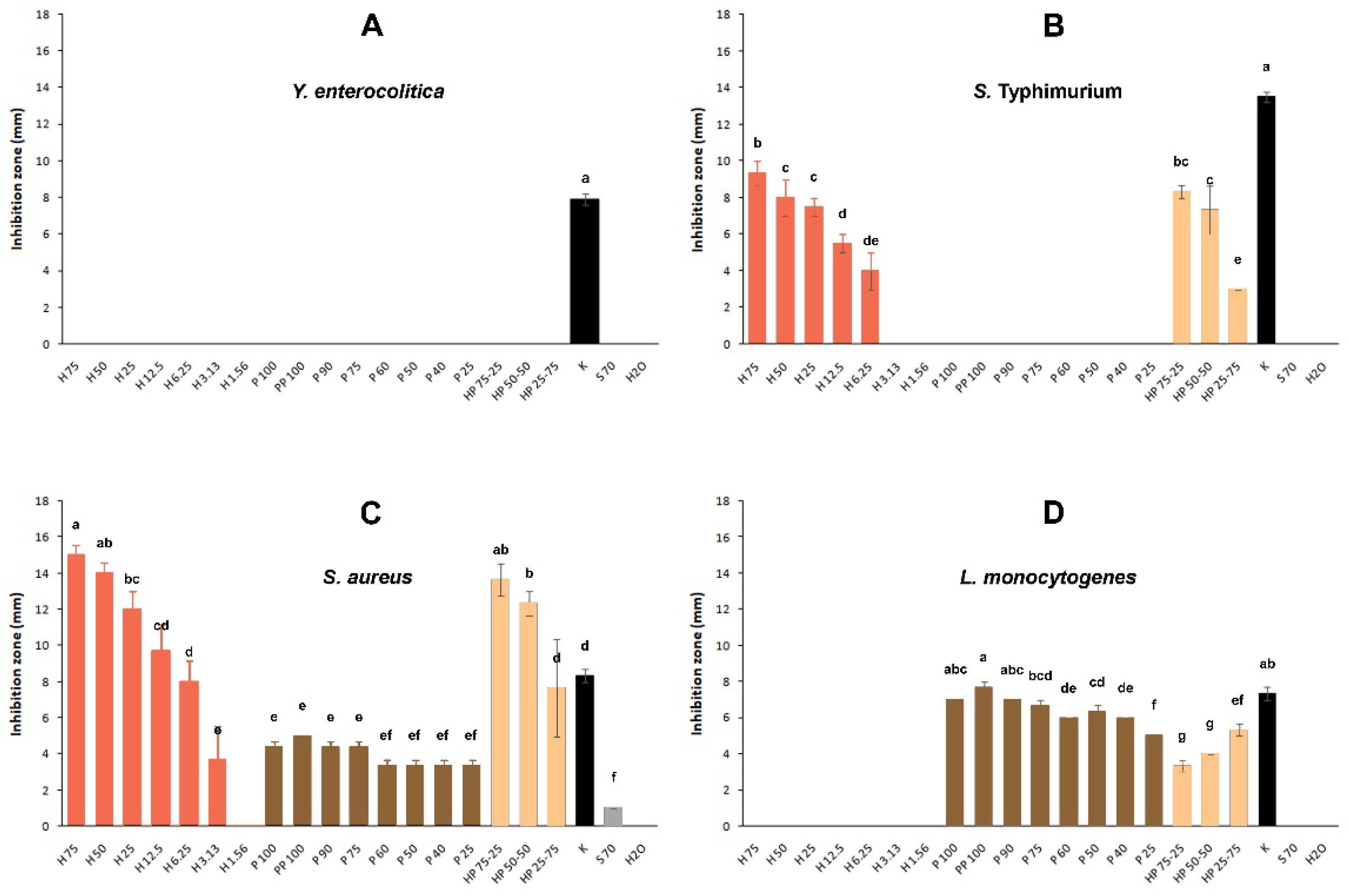
3.2. Antibacterial Action of Honey and Propolis Samples
3.3. Combined Application of Honey and Propolis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pires, S.M.; Desta, B.N.; Mughini-Gras, L.; Mmbaga, B.T.; Fayemi, O.E.; Salvador, E.M.; Gobena, T.; Majowicz, S.E.; Hald, T.; Hoejskov, P.S.; et al. Burden of foodborne diseases: Think global, act local. Curr. Opin. Food Sci. 2021, 39, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Fung, F.; Wang, H.S.; Menon, S. Food safety in the 21st century. Biomed. J. 2018, 41, 88–95. [Google Scholar] [CrossRef]
- Fhogartaigh, C.N.; Edgeworth, J.D. Bacterial gastroenteritis. Medicine 2009, 37, 586–593. [Google Scholar] [CrossRef]
- Majowicz, S.E.; Hall, G.; Scallan, E.; Adak, G.K.; Gauci, C.; Jones, T.F.; O’Brien, S.; Henao, O.; Sockett, P. A common, symptom-based case definition for gastroenteritis. Epidemiol. Infect. 2008, 136, 886–894. [Google Scholar] [CrossRef]
- Desselberger, U. Viral gastroenteritis. Medicine 2017, 45, 690–694. [Google Scholar] [CrossRef] [PubMed]
- Fleckenstein, J.M.; Matthew Kuhlmann, F.; Sheikh, A. Acute bacterial gastroenteritis. Gastroenterol. Clin. N. Am. 2021, 50, 283–304. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.; Fhogartaigh, C.N. Bacterial gastroenteritis. Medicine 2017, 45, 683–689. [Google Scholar] [CrossRef]
- Chlebicz, A.; Śliżewska, K. Campylobacteriosis, salmonellosis, yersiniosis, and listeriosis as zoonotic foodborne diseases: A review. Int. J. Environ. Res. Public Health 2018, 15, 863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control). The European Union one health 2020 zoonoses report. EFSA J. 2021, 19, e06971. [Google Scholar]
- Cheung, G.Y.C.; Bae, J.S.; Otto, M. Pathogenicity and virulence of Staphylococcus aureus. Virulence 2021, 12, 547–569. [Google Scholar] [CrossRef] [PubMed]
- Hennekinne, J.A.; De Buyser, M.L.; Dragacci, S. Staphylococcus aureus and its food poisoning toxins: Characterization and outbreak investigation. FEMS Microbiol. Rev. 2012, 36, 815–836. [Google Scholar] [CrossRef] [Green Version]
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Kritikos, A.; Zanella, M.C.; Huttner, B.; Boillat-Blanco, N. Side effects of selected antibiotics, not to be missed. Rev. Med. Suisse 2020, 16, 719–723. [Google Scholar] [PubMed]
- Ramirez, J.; Guarner, F.; Bustos Fernandez, L.; Maruy, A.; Sdepanian, V.L.; Cohen, H. Antibiotics as major disruptors of gut microbiota. Front. Cell. Infect. Microbiol. 2020, 10, 572912. [Google Scholar] [CrossRef] [PubMed]
- Morehead, M.S.; Scarbrough, C. Emergence of global antibiotic resistance. Prim. Care. 2018, 45, 467–484. [Google Scholar] [CrossRef]
- Huemer, M.; Mairpady Shambat, S.; Brugger, S.D.; Zinkernagel, A.S. Antibiotic resistance and persistence-implications for human health and treatment perspectives. EMBO Rep. 2020, 21, e51034. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Pop, A.; Georgescu, C.; Turcuş, V.; Olah, N.K.; Mathe, E. An overview of natural antimicrobials role in food. Eur. J. Med. Chem. 2018, 143, 922–935. [Google Scholar] [CrossRef] [PubMed]
- Gkoutzouvelidou, M.; Panos, G.; Xanthou, M.N.; Papachristoforou, A.; Giaouris, E. Comparing the antimicrobial actions of Greek honeys from the island of Lemnos and manuka honey from New Zealand against clinically important bacteria. Foods 2021, 10, 1402. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.M.; Molan, P.C.; Cursons, R.T. The in vitro susceptibility of Campylobacter spp. to the antibacterial effect of manuka honey. Eur. J. Clin. Microbiol. Infect. Dis. 2009, 28, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Abdulrhman, M.A.; Mekawy, M.A.; Awadalla, M.M.; Mohamed, A.H. Bee honey added to the oral rehydration solution in treatment of gastroenteritis in infants and children. J. Med. Food 2010, 13, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.; Lamas, L.B.; Flórez, S.M.; Toyos, P.A.; et al. Phenolic compounds in honey and their associated health benefits: A review. Molecules 2018, 23, 2322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nolan, V.C.; Harrison, J.; Cox, J.A.G. Dissecting the antimicrobial composition of honey. Antibiotics 2019, 8, 251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, L.M.; Fonseca, M.S.; Sokolonski, A.R.; Deegan, K.R.; Araújo, R.P.; Umsza-Guez, M.A.; Barbosa, J.D.; Portela, R.D.; Machado, B.A. Propolis: Types, composition, biological activities, and veterinary product patent prospecting. J. Sci. Food Agric. 2020, 100, 1369–1382. [Google Scholar] [CrossRef] [PubMed]
- Pobiega, K.; Kraśniewska, K.; Gniewosz, M. Application of propolis in antimicrobial and antioxidative protection of food quality —A review. Trends Food Sci. Technol. 2019, 83, 53–62. [Google Scholar] [CrossRef]
- Przybyłek, I.; Karpiński, T.M. Antibacterial properties of propolis. Molecules 2019, 24, 2047. [Google Scholar] [CrossRef] [Green Version]
- Kocot, J.; Kiełczykowska, M.; Luchowska-Kocot, D.; Kurzepa, J.; Musik, I. Antioxidant potential of propolis, bee pollen, and royal jelly: Possible medical application. Oxid. Med. Cell. Longev. 2018, 2018, 7074209. [Google Scholar] [CrossRef] [PubMed]
- Sforcin, J.M. Biological properties and therapeutic applications of propolis. Phytother. Res. 2016, 30, 894–905. [Google Scholar] [CrossRef]
- Cornara, L.; Biagi, M.; Xiao, J.; Burlando, B. Therapeutic properties of bioactive compounds from different honeybee products. Front. Pharmacol. 2017, 8, 412. [Google Scholar] [CrossRef]
- Al-Waili, N.; Al-Ghamdi, A.; Ansari, M.J.; Al-Attal, Y.; Salom, K. Synergistic effects of honey and propolis toward drug multi-resistant Staphylococcus aureus, Escherichia coli and Candida albicans isolates in single and polymicrobial cultures. Int. J. Med. Sci. 2012, 9, 793–800. [Google Scholar] [CrossRef] [Green Version]
- Freitas, A.S.; Cunha, A.; Oliveira, R.; Almeida-Aguiar, C. Propolis antibacterial and antioxidant synergisms with gentamicin and honey. J. Appl. Microbiol. 2022, 132, 2733–2745. [Google Scholar] [CrossRef]
- Osés, S.M.; Pascual-Maté, A.; Fernández-Muiño, M.A.; López-Díaz, T.M.; Sancho, M.T. Bioactive properties of honey with propolis. Food Chem. 2016, 196, 1215–1223. [Google Scholar] [CrossRef]
- Biel, B.; Tan, K. Flora of Samothraki; The Goulandris Natural History Museum: Kifissia, Greece, 2014; ISBN 9789604645855. [Google Scholar]
- Abidi, E.; Habib, J.; Yassine, A.; Chahine, N.; Mahjoub, T.; Elkak, A. Effects of methanol extracts from roots, leaves, and fruits of the Lebanese strawberry tree (Arbutus andrachne) on cardiac function together with their antioxidant activity. Pharm. Biol. 2016, 54, 1035–1041. [Google Scholar] [CrossRef]
- Louveaux, J.; Maurizio, A.; Vorwohl, G. Methods of melissopalynology. Bee World 1978, 59, 139–157. [Google Scholar] [CrossRef]
- Karousou, D. Enrichment of Honey from Samothrace with Phytochemicals of Arbutus andrachne Plant. Master’s Thesis, Aristotle University of Thessaloniki, Thessaloniki, Greece, 2019. [Google Scholar]
- Papachristoforou, A.; Koutouvela, E.; Menexes, G.; Gardikis, K.; Mourtzinos, I. Photometric analysis of propolis from the island of Samothraki, Greece. The discovery of red propolis. Chem. Biodivers. 2019, 16, e1900146. [Google Scholar] [CrossRef] [PubMed]
- Bankova, V.; Trusheva, B.; Popova, M. Propolis extraction methods: A review. J. Apic. Res. 2021, 60, 734–743. [Google Scholar] [CrossRef]
- Kostoglou, D.; Tsaklidou, P.; Iliadis, I.; Garoufallidou, N.; Skarmoutsou, G.; Koulouris, I.; Giaouris, E. Advanced killing potential of thymol against a time and temperature optimized attached Listeria monocytogenes population in lettuce broth. Biomolecules 2021, 11, 397. [Google Scholar] [CrossRef] [PubMed]
- Kostaki, M.; Chorianopoulos, N.; Braxou, E.; Nychas, G.J.; Giaouris, E. Differential biofilm formation and chemical disinfection resistance of sessile cells of Listeria monocytogenes strains under monospecies and dual-species (with Salmonella enterica) conditions. Appl. Environ. Microbiol. 2012, 78, 2586–2595. [Google Scholar] [CrossRef] [Green Version]
- Schleifstein, J.I.; Coleman, M.B. An unidentified microorganism resembling B. ligniere and Past. pseudotuberculosis, and pathogenic for man. N. Y. State J. Med. 1939, 39, 1749–1753. [Google Scholar]
- Graikini, D.; Papachristoforou, A.; Mourtzinos, I. Comparison of qualitative characteristics of propolis extracts using different purification methods. J. Apic. Res. 2019, 58, 792–799. [Google Scholar] [CrossRef]
- Kalogeropoulos, N.; Konteles, S.J.; Troullidou, E.; Mourtzinos, I.; Karathanos, V.T. Chemical composition, antioxidant activity and antimicrobial properties of propolis extracts from Greece and Cyprus. Food Chem. 2009, 116, 452–461. [Google Scholar] [CrossRef]
- Kasiotis, K.M.; Anastasiadou, P.; Papadopoulos, A.; Machera, K. Revisiting Greek propolis: Chromatographic analysis and antioxidant activity study. PLoS ONE 2017, 12, e0170077. [Google Scholar] [CrossRef] [Green Version]
- Al-Naggar, Y.; Sun, J.; Robertson, A.; Giesy, J.P.; Wiseman, S. Chemical characterization and antioxidant properties of Canadian propolis. J. Apic. Res. 2016, 55, 305–314. [Google Scholar] [CrossRef]
- Hamasaka, T.; Kumazawa, S.; Fujimoto, T.; Nakayama, T. Antioxidant activity and constituents of propolis collected in various areas of Japan. Food Sci. Technol. Res. 2004, 10, 86–92. [Google Scholar] [CrossRef] [Green Version]
- Kumazawa, S.; Hamasaka, T.; Nakayama, T. Antioxidant activity of propolis of various geographic origins. Food Chem. 2004, 84, 329–339. [Google Scholar] [CrossRef]
- Righi, A.A.; Alves, T.R.; Negri, G.; Marques, L.M.; Breyer, H.; Salatino, A. Brazilian red propolis: Unreported substances, antioxidant and antimicrobial activities. J. Sci. Food Agric. 2011, 91, 2363–2370. [Google Scholar] [CrossRef] [PubMed]
- Stagos, D.; Soulitsiotis, N.; Tsadila, C.; Papaeconomou, S.; Arvanitis, C.; Ntontos, A.; Mossialos, D. Antibacterial and antioxidant activity of different types of honey derived from Mount Olympus in Greece. Int. J. Mol. Med. 2018, 42, 726–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez-Suarez, J.M.; Gasparrini, M.; Forbes-Hernández, T.Y.; Mazzoni, L.; Giampieri, F. The composition and biological activity of honey: A focus on manuka honey. Foods 2014, 3, 420–432. [Google Scholar] [CrossRef] [Green Version]
- Tsavea, E.; Vardaka, F.-P.; Savvidaki, E.; Kellil, A.; Kanelis, D.; Bucekova, M.; Grigorakis, S.; Godocikova, J.; Gotsiou, P.; Dimou, M.; et al. Physicochemical characterization and biological properties of pine honey produced across Greece. Foods 2022, 11, 943. [Google Scholar] [CrossRef]
- Bartoncelj, J.; Doberšek, U.; Jamnik, M.; Golob, T. Evaluation of the phenolic content, antioxidant activity and colour of Slovenian honey. Food Chem. 2007, 105, 822–828. [Google Scholar] [CrossRef]
- Pontis, J.A.; da Costa, L.A.M.A.; da Silva, S.J.R.; Flach, A. Color, phenolic and flavonoid content, and antioxidant activity of honey from Roraima, Brazil. Food Sci. Technol. 2014, 34, 69–73. [Google Scholar] [CrossRef] [Green Version]
- Kurek-Górecka, A.; Rzepecka-Stojko, A.; Górecki, M.; Stojko, J.; Sosada, M.; Swierczek-Zieba, G. Structure and antioxidant activity of polyphenols derived from propolis. Molecules 2014, 19, 78–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almasaudi, S. The antibacterial activities of honey. Saudi J. Biol. Sci. 2021, 28, 2188–2196. [Google Scholar] [CrossRef] [PubMed]
- Johnston, M.; McBride, M.; Dahiya, D.; Owusu-Apenten, R.; Nigam, P.S. Antibacterial activity of manuka honey and its components: An overview. AIMS Microbiol. 2018, 4, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Brudzynski, K. A current perspective on hydrogen peroxide production in honey. A review. Food Chem. 2020, 332, 127229. [Google Scholar] [CrossRef]
- Machado De-Melo, A.A.; de Almeida-Muradian, L.B.; Sancho, M.T.; Pascual-Maté, A. Composition and properties of Apis mellifera honey: A review. J. Apic. Res. 2017, 57, 5–37. [Google Scholar] [CrossRef]
- Taormina, P.J.; Niemira, B.A.; Beuchat, L.R. Inhibitory activity of honey against foodborne pathogens as influenced by the presence of hydrogen peroxide and level of antioxidant power. Int. J. Food Microbiol. 2001, 69, 217–225. [Google Scholar] [CrossRef]
- Masoura, M.; Passaretti, P.; Overton, T.W.; Lund, P.A.; Gkatzionis, K. Use of a model to understand the synergies underlying the antibacterial mechanism of H2O2-producing honeys. Sci. Rep. 2020, 10, 17692–17696. [Google Scholar] [CrossRef] [PubMed]
- Sforcin, J.M.; Fernandes, A.; Lopes, C.A.M.; Bankova, V.; Funari, S.R.C. Seasonal effect on Brazilian propolis antibacterial activity. J. Ethnopharmacol. 2000, 73, 243–249. [Google Scholar] [CrossRef]
- Karlsson, P.A.; Tano, E.; Jernberg, C.; Hickman, R.A.; Guy, L.; Järhult, J.D.; Wang, H. Molecular characterization of multidrug-resistant Yersinia enterocolitica from foodborne outbreaks in Sweden. Front. Microbiol. 2021, 12, 664665. [Google Scholar] [CrossRef] [PubMed]
- Habryka, C.; Socha, R.; Juszczak, L. The Effect of enriching honey with propolis on the antioxidant activity, sensory characteristics, and quality parameters. Molecules 2020, 25, 1176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boukraa, L.; Benbarek, H.; Aissat, S. Synergistic action of starch and honey against Pseudomonas aeruginosa in correlation with diastase number. J. Altern. Complement. Med. 2008, 14, 181–184. [Google Scholar] [CrossRef] [Green Version]
- Botelho, J.; Grosso, F.; Peixe, L. Antibiotic resistance in Pseudomonas aeruginosa—Mechanisms, epidemiology and evolution. Drug Resist. Updat. 2019, 44, 100640. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, S.; Makarewicz, M. Functional properties of honey supplemented with bee bread and propolis. Nat. Prod. Res. 2017, 31, 2680–2683. [Google Scholar] [CrossRef] [PubMed]
- Boukraâ, L.; Niar, A.; Benbarek, H.; Benhanifia, M. Additive action of royal jelly and honey against Staphylococcus aureus. J. Med. Food. 2008, 11, 190–192. [Google Scholar] [CrossRef] [PubMed]

| Bacterial Species | Gram Reaction | Strain Code | Isolation Origin | Other Strain Information | Reference |
|---|---|---|---|---|---|
| Staphylococcus aureus | + | DFSN 1_B37 | Greek traditional cheese | − | unpublished |
| Listeria monocytogenes | + | AAL 2 20107 | Mixed green salad | serovar 1/2b | [38] |
| Salmonellla enterica | − | FMCC 3_B137 | Human salmonellosis outbreak | serovar Typhimurium, phage type DT193 | [39] |
| Yersinia enterocolitica | − | DSM 4 4780 | Glanders-like infection of face | subsp. enterocolitica, type strain | [40] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Postali, E.; Peroukidou, P.; Giaouris, E.; Papachristoforou, A. Investigating Possible Synergism in the Antioxidant and Antibacterial Actions of Honey and Propolis from the Greek Island of Samothrace through Their Combined Application. Foods 2022, 11, 2041. https://doi.org/10.3390/foods11142041
Postali E, Peroukidou P, Giaouris E, Papachristoforou A. Investigating Possible Synergism in the Antioxidant and Antibacterial Actions of Honey and Propolis from the Greek Island of Samothrace through Their Combined Application. Foods. 2022; 11(14):2041. https://doi.org/10.3390/foods11142041
Chicago/Turabian StylePostali, Evdoxia, Panagiota Peroukidou, Efstathios Giaouris, and Alexandros Papachristoforou. 2022. "Investigating Possible Synergism in the Antioxidant and Antibacterial Actions of Honey and Propolis from the Greek Island of Samothrace through Their Combined Application" Foods 11, no. 14: 2041. https://doi.org/10.3390/foods11142041