The Emulsion Properties of Chicken Liver Protein Recovered through Isoelectric Solubilization/Precipitation Processes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. CL Protein Extraction
2.2.2. Recovery Yield
2.2.3. Particle Size and Distribution
2.2.4. Preparation of Emulsions
2.2.5. Emulsion Activity
2.2.6. Emulsion Stability
2.2.7. Optical Microscopy Observation
2.2.8. Statistical Analysis
3. Results and Discussion
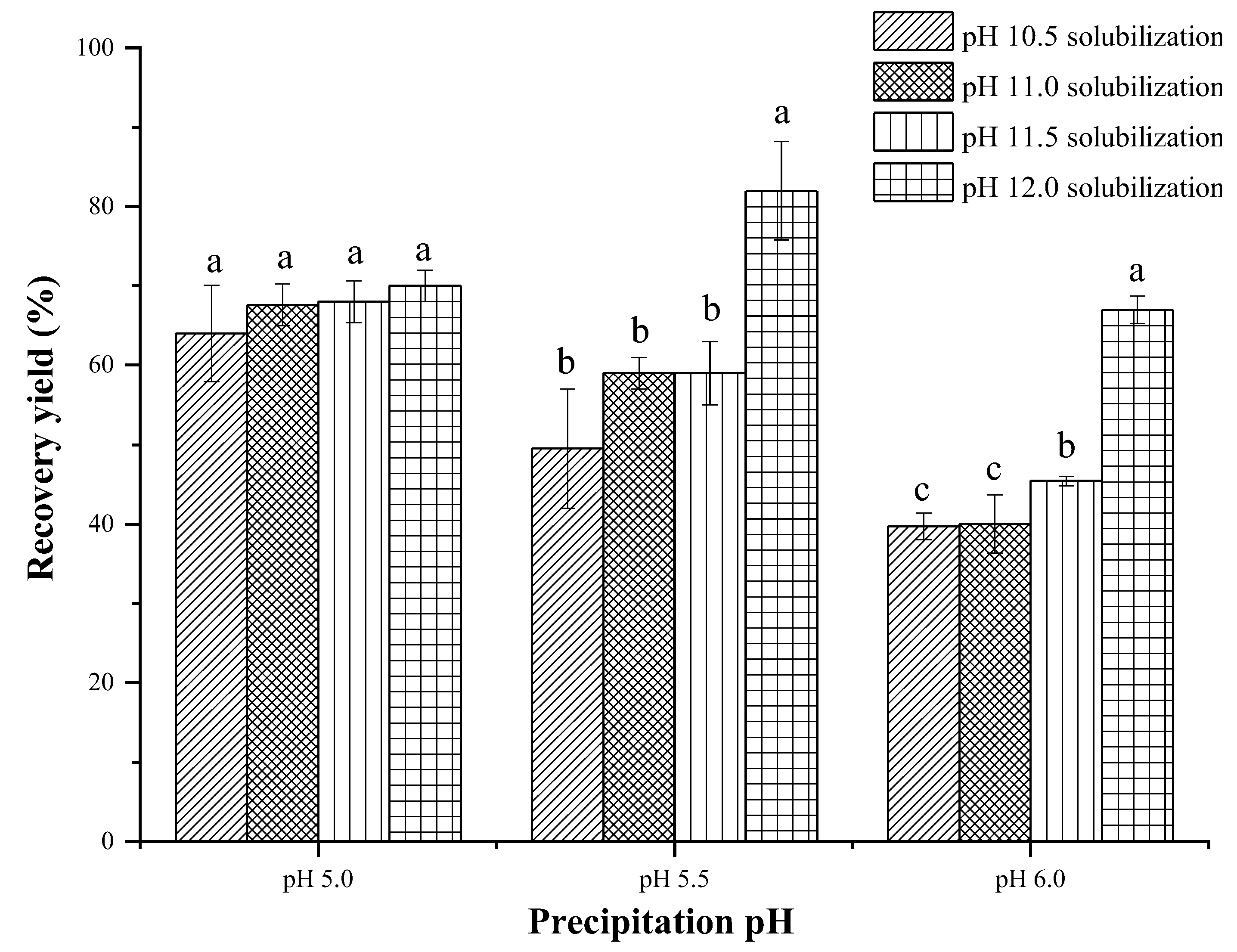
3.1. Recovery Yield
3.2. Particle Size and Distribution
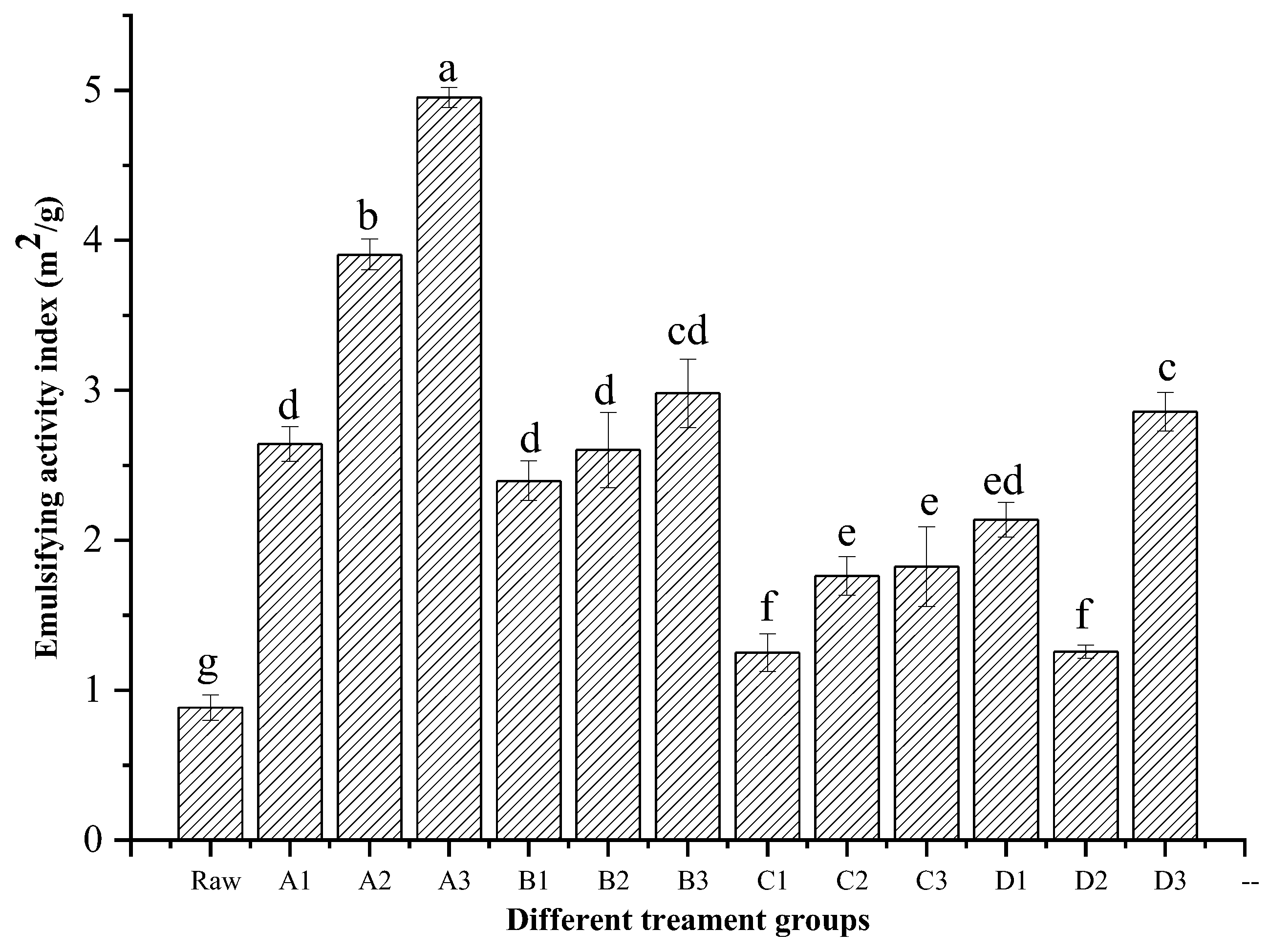
3.3. Emulsion Activity Index (EAI)
3.4. Emulsion Stability Index (ESI)
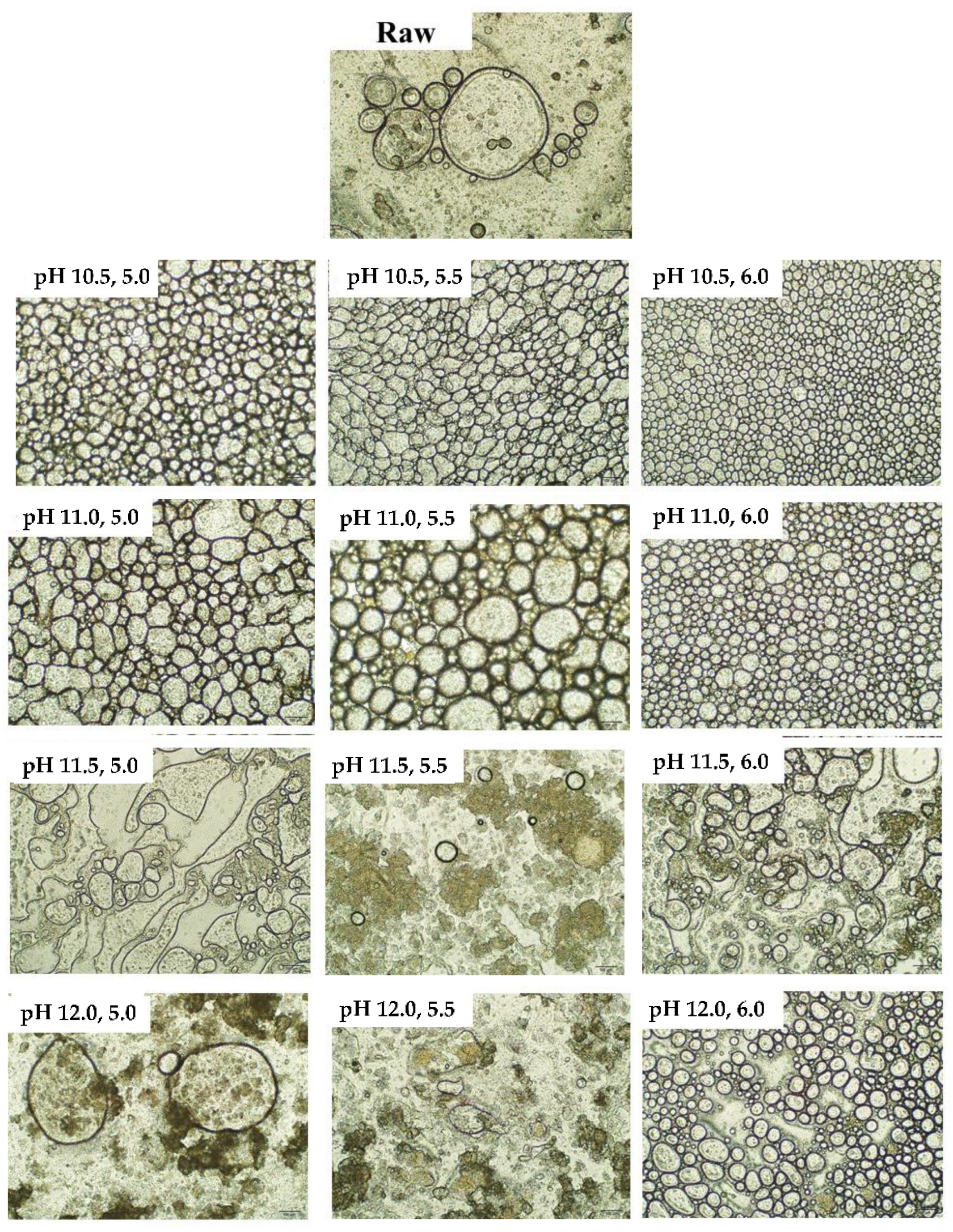
3.5. Microscopic Observation of Emulsion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campos, V.J.; Morais, T.B. Mothers ‘don’t like it; never tried it’: Blind Sensory Test of a Homemade Chicken Liver Baby Food, a Source of Iron, by Infants and their Mothers. J. Trop. Pediatrics 2015, 61, 279–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papazoglou, S.; Tsiraki, M.; Savvaidis, I.N. Effect of thyme oil on the preservation of vacuum-packaged chicken liver. J. Food Sci. 2012, 77, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.; Gao, X.; Zheng, H.; Li, X.; Xu, X.; Zhou, G. Comparison on the physico-chemical and nutritional qualities of normal and abnormal colored fresh chicken liver. Anim. Sci. J. 2017, 88, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.; Jiang, X.; Xie, F.; Fan, Y.; Xu, X.; Zhang, M.; Qi, J.; Wang, S.; Zhou, X. Effect of high-pressure homogenization on structural changes and emulsifying properties of chicken liver proteins isolated by isoelectric solubilization/precipitation. LWT-Food Sci. Technol. 2021, 151, 112092. [Google Scholar] [CrossRef]
- Xiong, G.; Chen, X.; Gao, X.; Yin, C.; Xu, X.; Qi, J. Comparison on the emulsion properties of normal colour and discolouration fresh chicken liver. Ital. J. Anim. Sci. 2020, 19, 551–559. [Google Scholar] [CrossRef]
- Kamal, H.; Le, C.F.; Salter, A.M.; Ali, A. Extraction of protein from food waste: An overview of current status and opportunities. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2455–2475. [Google Scholar] [CrossRef]
- Xiong, G.; Gao, X.; Wang, P.; Xu, X.; Zhou, G. Comparative study of extraction efficiency and composition of protein recovered from chicken liver by acid–alkaline treatment. Process Biochem. 2016, 51, 1629–1635. [Google Scholar] [CrossRef]
- Nolsøe, H.; Undeland, I. The Acid and Alkaline Solubilization Process for the Isolation of Muscle Proteins: State of the Art. Food Bioprocess Technol. 2009, 2, 1–27. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, W.; Zhao, X.; Xu, X. Comparison of the interfacial properties of native and refolded myofibrillar proteins subjected to pH-shifting. Food Chem. 2022, 380, 131734. [Google Scholar] [CrossRef]
- Cha, J.W.; Yoon, I.S.; Lee, G.-W.; Kang, S.I.; Park, S.Y.; Kim, J.-S.; Heu, M.S. Food functionalities and bioactivities of protein isolates recovered from skipjack tuna roe by isoelectric solubilization and precipitation. Food Sci. Nutr. 2020, 8, 1874–1887. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Bai, Y.; Xing, T.; Xu, X.-L.; Zhou, G. Use of an isoelectric solubilization/precipitation process to modify the functional properties of PSE (pale, soft, exudative)-like chicken meat protein: A mechanistic approach. Food Chem. 2018, 248, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Yu, X.; Li, X.; Zhao, X.; Han, M.; Xu, X.; Zhou, G. Structural changes and emulsion properties of goose liver proteins obtained by isoelectric solubilisation/precipitation processes. LWT-Food Sci. Technol. 2019, 102, 190–196. [Google Scholar] [CrossRef]
- Zouari, N.; Fakhfakh, N.; Amara-Dali, W.B.; Sellami, M.; Msaddak, L.; Ayadi, M.A. Turkey liver: Physicochemical characteristics and functional properties of protein fractions. Food Bioprod. Process. 2011, 89, 142–148. [Google Scholar] [CrossRef]
- Hrynets, Y.; Omana, D.A.; Xu, Y.; Betti, M. Effect of Acid- and Alkaline-Aided Extractions on Functional and Rheological Properties of Proteins Recovered from Mechanically Separated Turkey Meat (MSTM). J. Food Sci. 2010, 75, E477–E486. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wu, T.; Xing, T.; Xu, X.-L.; Zhou, G. Rheological and physical properties of O/W protein emulsions stabilized by isoelectric solubilization/precipitation isolated protein: The underlying effects of varying protein concentrations. Food Hydrocoll. 2019, 95, 580–589. [Google Scholar] [CrossRef]
- Kristinsson, H.G.; Ingadottir, B. Recovery and Properties of Muscle Proteins Extracted from Tilapia (Oreochromis niloticus) Light Muscle by pH Shift Processing. J. Food Sci. 2006, 71, E132–E141. [Google Scholar] [CrossRef]
- Abdollahi, M.; Undeland, I. Physicochemical and gel-forming properties of protein isolated from salmon, cod and herring by-products using the pH-shift method. LWT-Food Sci. Technol. 2019, 101, 678–684. [Google Scholar] [CrossRef]
- Leite, T.S.; Augusto, P.E.D.; Cristianini, M. The use of high pressure homogenization (HPH) to reduce consistency of concentrated orange juice (COJ). Innov. Food Sci. Emerg. Technol. 2014, 26, 124–133. [Google Scholar] [CrossRef]
- Kristinsson, H.G. Conformational and Functional Changes of Hemoglobin and Myosin Induced by pH: Functional Role in Fish Quality. Ph.D. Thesis, University of Massachusetts Amherst, Amherst, MA, USA, 2002. [Google Scholar]
- Hu, H.; Cheung, I.W.Y.; Pan, S.; Li-Chan, E.C.Y. Effect of high intensity ultrasound on physicochemical and functional properties of aggregated soybean β-conglycinin and glycinin. Food Hydrocoll. 2015, 45, 102–110. [Google Scholar] [CrossRef]
- Yanjun, S.; Jianhang, C.; Shuwen, Z.; Hongjuan, L.; Jing, L.; Lu, L.; Uluko, H.; Yanling, S.; Wenming, C.; Wupeng, G.; et al. Effect of power ultrasound pre-treatment on the physical and functional properties of reconstituted milk protein concentrate. J. Food Eng. 2014, 124, 11–18. [Google Scholar] [CrossRef]
- Abdollahi, M.; Undeland, I. Structural, functional, and sensorial properties of protein isolate produced from salmon, cod, and herring by-products. Food Bioprocess Technol. 2018, 11, 1733–1749. [Google Scholar] [CrossRef] [Green Version]
- Panpipat, W.; Chaijan, M. Functional properties of pH-shifted protein isolates from bigeye snapper (Priacanthus tayenus) head by-product. Int. J. Food Prop. 2017, 20, 596–610. [Google Scholar] [CrossRef] [Green Version]
- Kristinsson, H.G.; Hultin, H.O. Effect of low and high pH treatment on the functional properties of cod muscle proteins. J. Agric. Food Chem. 2003, 51, 5103–5110. [Google Scholar] [CrossRef] [PubMed]
- Higuera-Barraza, O.A.; Torres-Arreola, W.; Ezquerra-Brauer, J.M.; Cinco-Moroyoqui, F.J.; Rodríguez Figueroa, J.C.; Marquez-Ríos, E. Effect of pulsed ultrasound on the physicochemical characteristics and emulsifying properties of squid (Dosidicus gigas) mantle proteins. Ultrason. Sono 2017, 38, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Shi, H.; Chen, X.; Xu, P.; Jiang, D.; Xu, W.; Wang, D. Modifying the structure, emulsifying and rheological properties of water-soluble protein from chicken liver by low-frequency ultrasound treatment. Int. J. Biol. Macromol. 2019, 139, 810–817. [Google Scholar] [CrossRef]
- Zhao, X.; Zou, Y.-F.; Shao, J.-J.; Chen, X.; Han, M.-Y.; Xu, X.-L. Comparison of the Acidic and Alkaline Treatment on Emulsion Composite Gel Properties of the Proteins Recovered from Chicken Breast by Isoelectric Solubilization/Precipitation Process. J. Food Process. Preserv. 2017, 41, e12884. [Google Scholar] [CrossRef]
- Li-Chan, E.; Nakai, S.; Wood, D.F. Hydrophobicity and Solubility of Meat Proteins and Their Relationship to Emulsifying Properties. J. Food Sci. 1984, 49, 345–350. [Google Scholar] [CrossRef]
- Pearce, K.N.; Kinsella, J.E. Emulsifying properties of proteins: Evaluation of a turbidimetric technique. J. Agric. Food Chem. 1978, 26, 716–723. [Google Scholar] [CrossRef]
- Hu, H.-Y.; Xing, L.-J.; Hu, Y.-Y.; Qiao, C.-L.; Wu, T.; Zhou, G.-H.; Zhang, W.-G. Effects of regenerated cellulose on oil-in-water emulsions stabilized by sodium caseinate. Food Hydrocoll. 2016, 52, 38–46. [Google Scholar] [CrossRef]
- Li, L.; Cai, R.; Wang, P.; Xu, X.; Zhou, G.; Sun, J. Manipulating interfacial behavior and emulsifying properties of myosin through alkali-heat treatment. Food Hydrocoll. 2018, 85, 69–74. [Google Scholar] [CrossRef]


| Solubilization and Precipitation pH | D3,2 (μm) | D4,3 (μm) |
|---|---|---|
| pH 10.5, 5.0 | 13.056 ± 0.20 f | 39.954 ± 0.52 d |
| pH 10.5, 5.5 | 10.635 ± 0.04 h | 23.539 ± 0.07 ef |
| pH 10.5, 6.0 | 9.197 ± 0.50 i | 14.576 ± 0.14 h |
| pH 11.0, 5.0 | 11.008 ± 0.48 gh | 25.425 ± 0.71 e |
| pH 11.0, 5.5 | 11.358 ± 0.02 g | 19.172 ± 0.04 g |
| pH 11.0, 6.0 | 14.525 ± 0.05 e | 22.589 ± 0.07 f |
| pH 11.5, 5.0 | 15.153 ± 0.56 d | 45.534 ± 1.88 c |
| pH 11.5, 5.5 | 18.376 ± 0.12 b | 44.563 ± 0.92 c |
| pH 11.5, 6.0 | 17.794 ± 0.14 c | 39.743 ± 0.99 d |
| pH 12.0, 5.0 | 14.075 ± 0.42 e | 39.858 ± 1.22 d |
| pH 12.0, 5.5 | 19.849 ± 0.12 a | 53.654 ± 0.43 a |
| pH 12.0, 6.0 | 18.499 ± 0.22 b | 49.069 ± 3.45 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pu, S.; Yin, C.; Zhang, X.; Zhang, Y.; Lu, N.; Xiong, G. The Emulsion Properties of Chicken Liver Protein Recovered through Isoelectric Solubilization/Precipitation Processes. Foods 2022, 11, 1644. https://doi.org/10.3390/foods11111644
Pu S, Yin C, Zhang X, Zhang Y, Lu N, Xiong G. The Emulsion Properties of Chicken Liver Protein Recovered through Isoelectric Solubilization/Precipitation Processes. Foods. 2022; 11(11):1644. https://doi.org/10.3390/foods11111644
Chicago/Turabian StylePu, Shunchang, Cong Yin, Xingguo Zhang, Yu Zhang, Ning Lu, and Guoyuan Xiong. 2022. "The Emulsion Properties of Chicken Liver Protein Recovered through Isoelectric Solubilization/Precipitation Processes" Foods 11, no. 11: 1644. https://doi.org/10.3390/foods11111644






