Structural and Electronic Properties of Polyoxovanadoborates Containing the [V12B18O60] Core in Different Mixed Valence States
Abstract
:1. Introduction
2. Polyoxovanadoborates: Vanadate and Borate Fragments
2.1. Vanadate Fragments
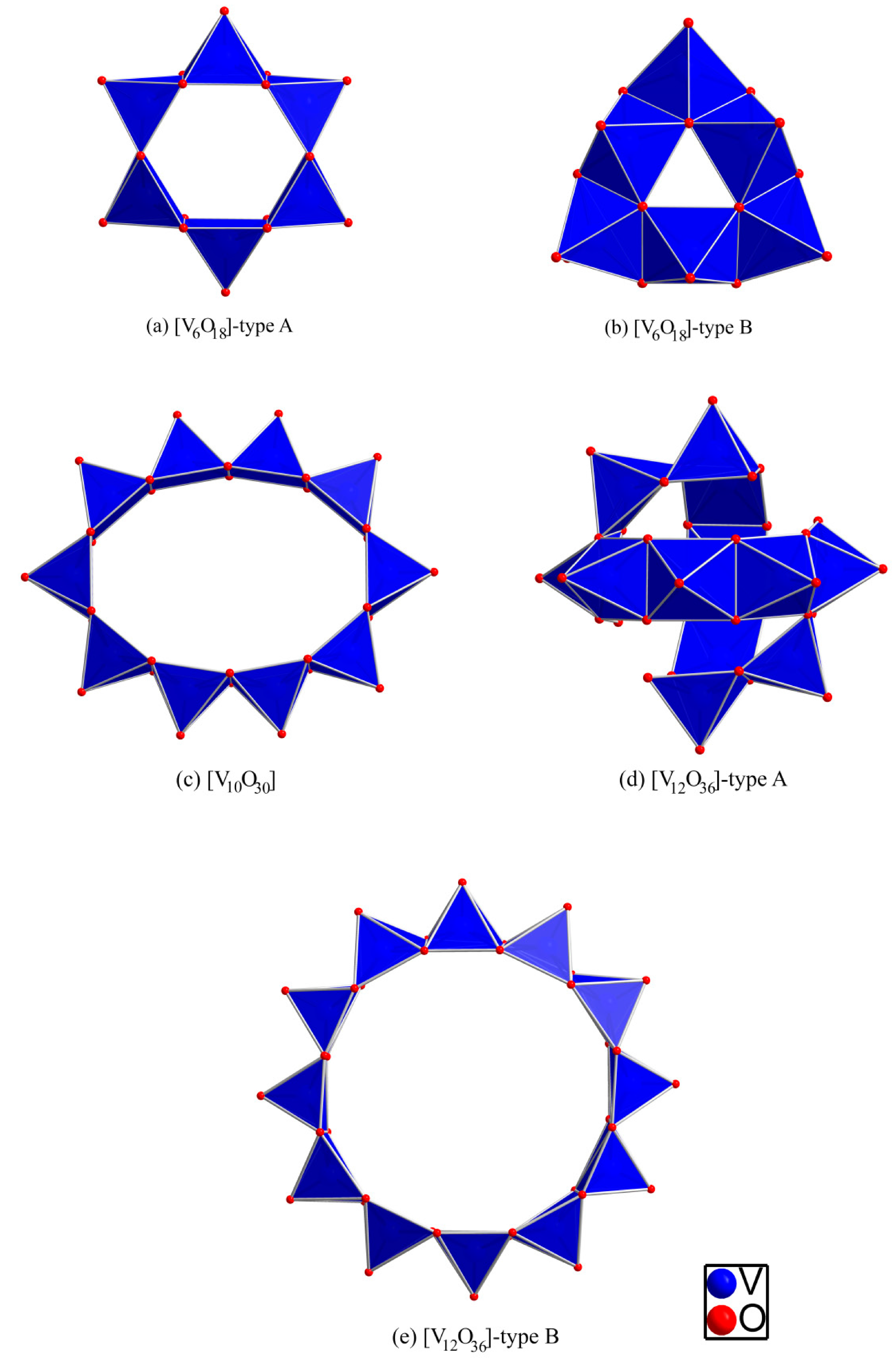
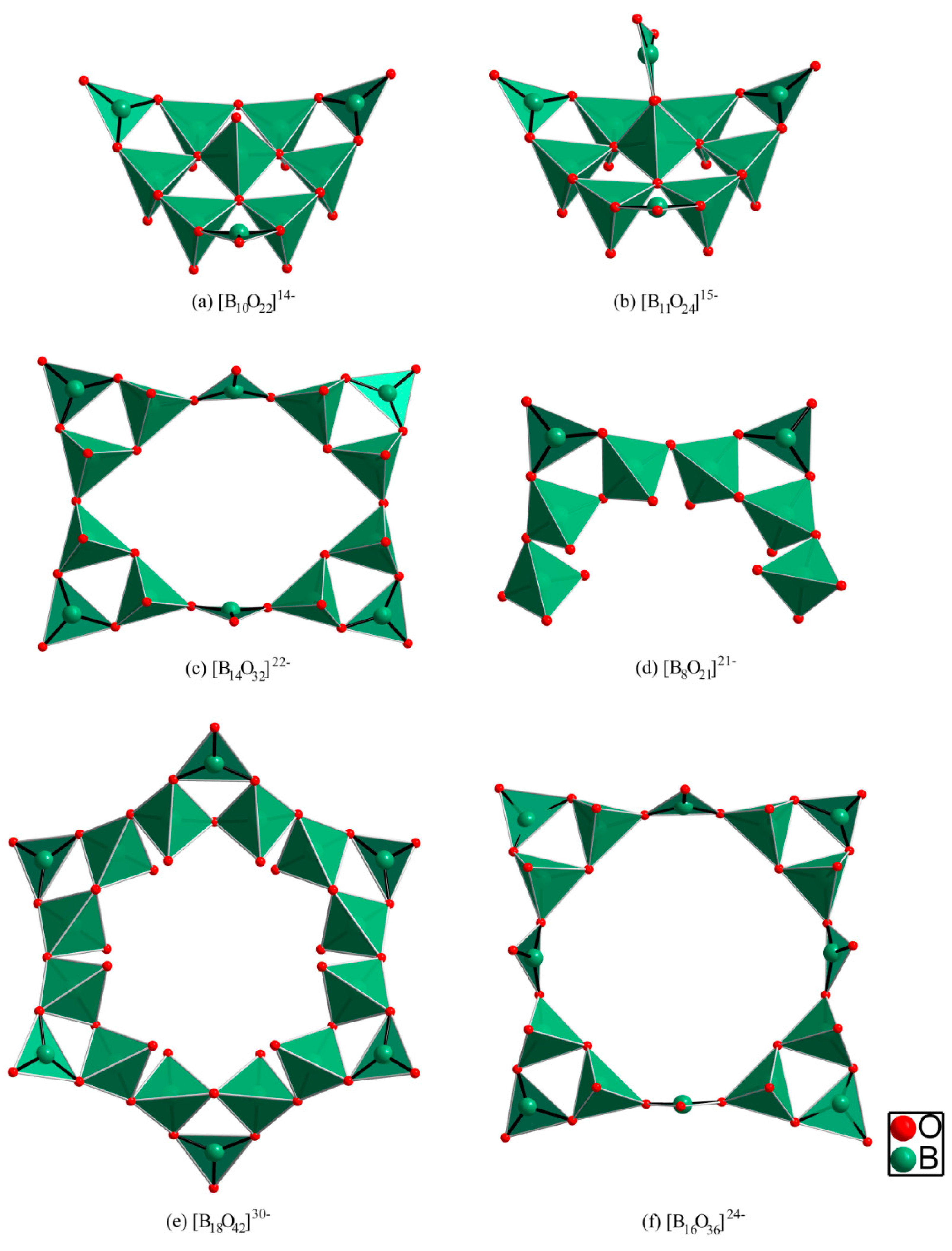
2.2. Borate Fragments
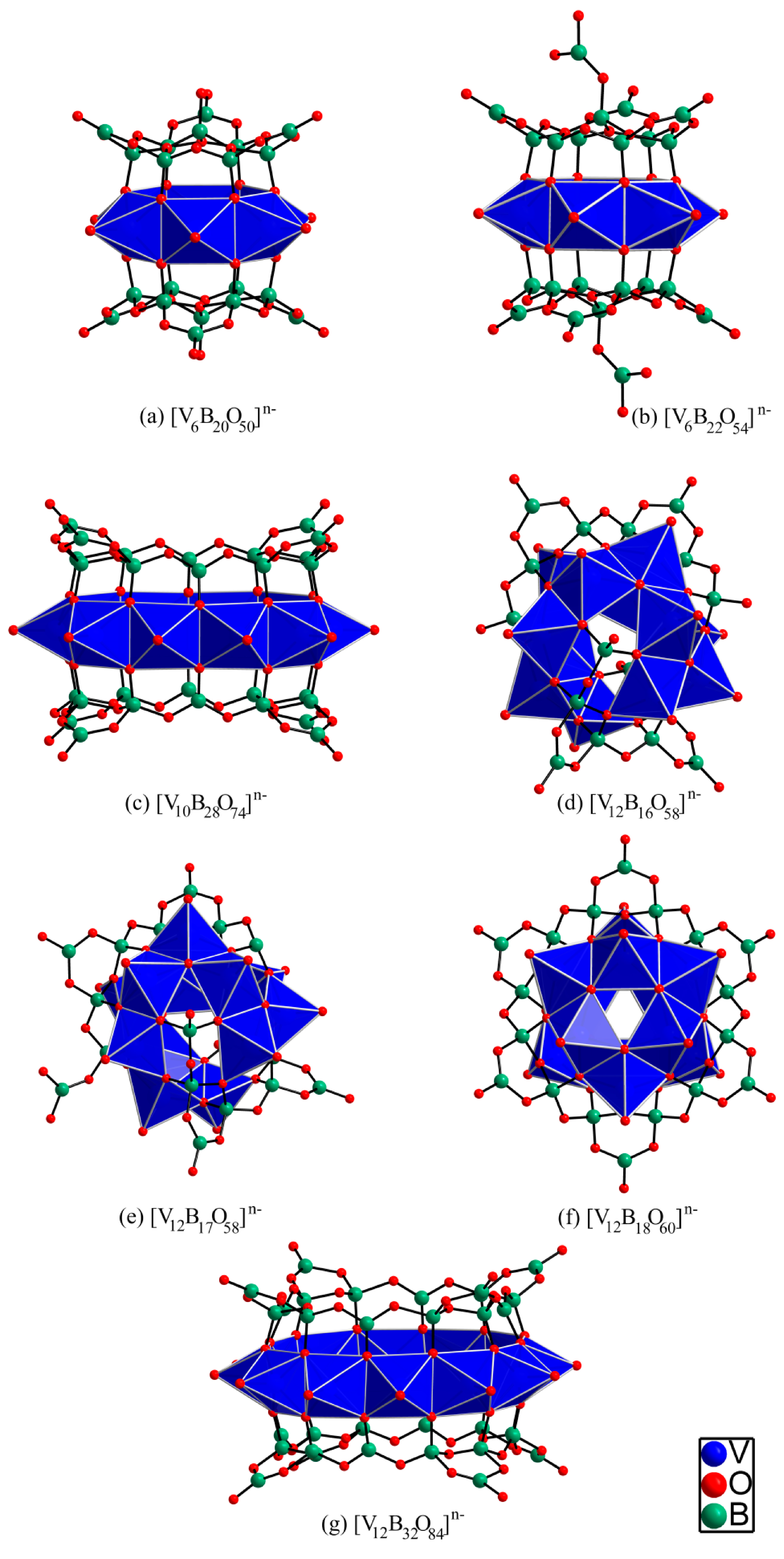
3. Structural Description of the [V12B18O60] Core
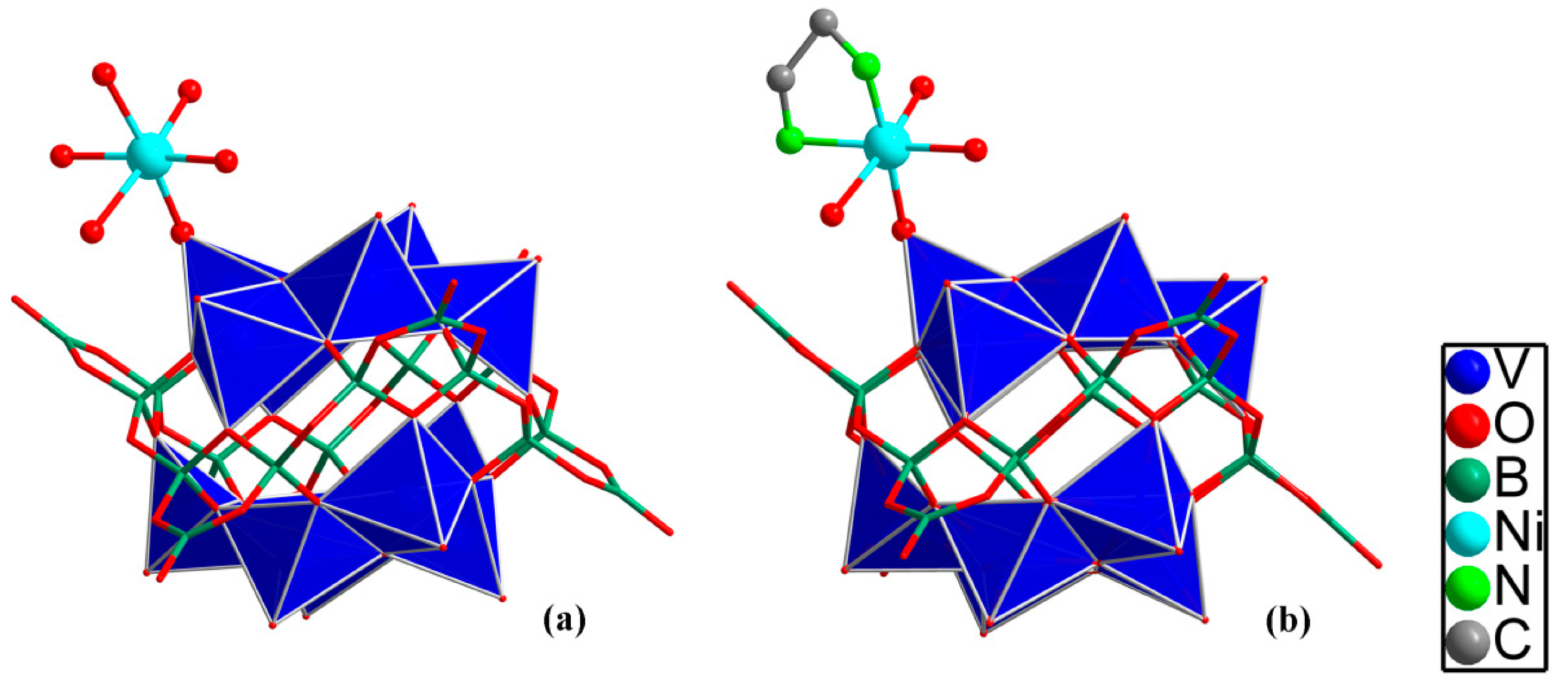
4. [V12B18O60] Cores with Protonated Amines as Counterbalancing Ions
| Compound | Formula | VIV/VV Ratio | Ref. |
|---|---|---|---|
| 1 | (enH2)5{(VO)12O6[B3O6(OH)]6} H2O | 10/2 | [42] |
| 2 | (1,3-diapH2)5{(VO)12O6[B3O6(OH)]6} 6H2O | 10/2 | [42] |
| 3 | (H2dap)2H6{(VO)12O6[B3O6(OH)]6(H2O)} 13H2O | 10/2 | [49] |
| 4 | [H3teta]3[V12B18O54(OH)6(H2O)] (H3O) 5H2O | 10/2 | [34] |
| 5 | (NH4)8(1,3-diapH2)[V12B18O60H6] 5H2O | 10/2 | [57] |
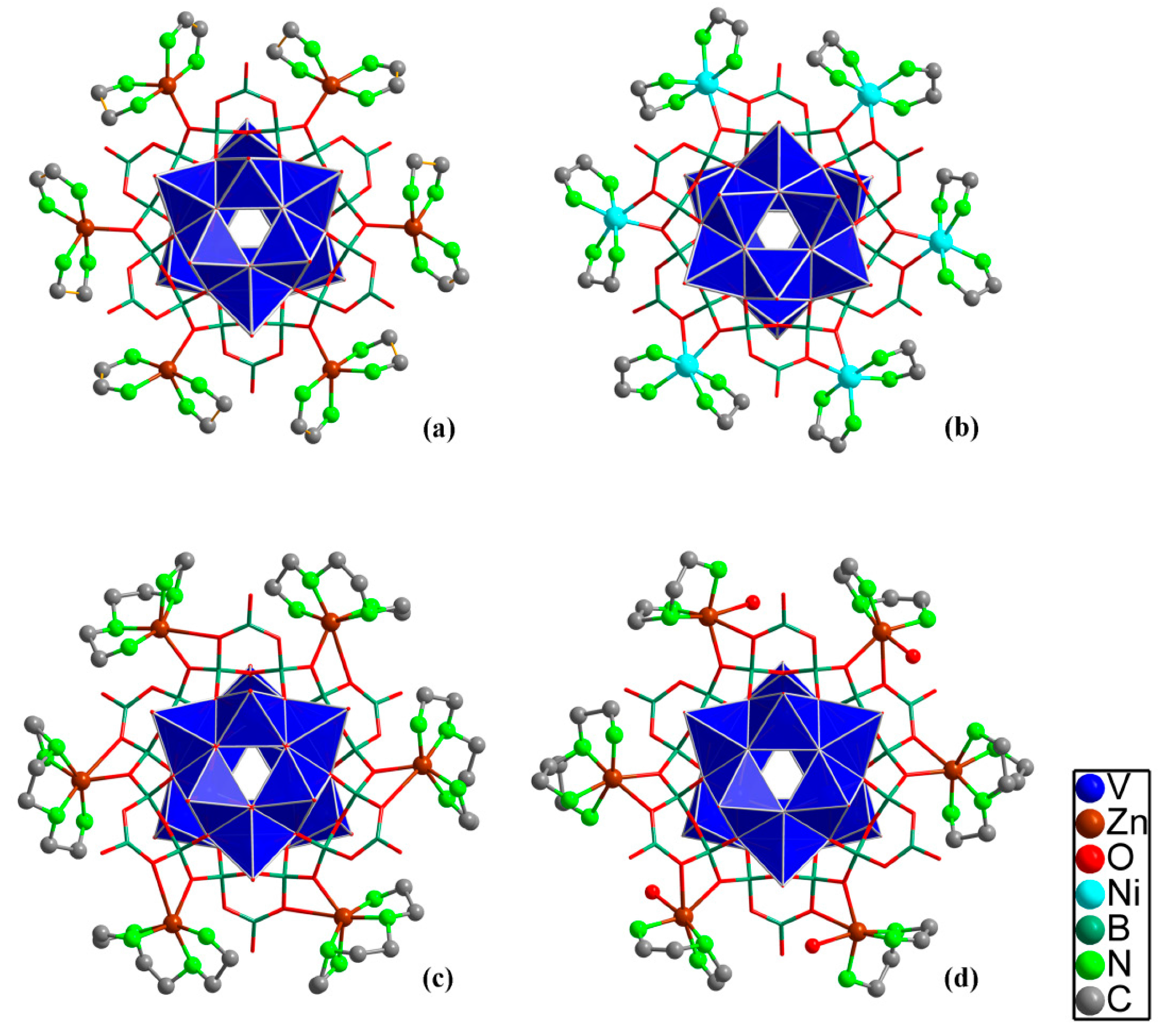
5. [V12B18O60] Cores with Transition Metal Ions and Coordination Compounds as Counterbalancing Cations
| Compound | Formula | VIV/VV Ratio | Ref. |
|---|---|---|---|
| 6 | [Zn(en)2]6[(VO)12O6B18O39(OH)3] 13H2O | 9/3 | [43] |
| 7 | H3{[Cu(en)2]5[(VO)12O6B18O42]}[B(OH)3]2 16H2O | 7/5 † | [44] |
| 8 | [Ni(en)2]6H2[(VO)12O6B18O42] 15H2O | 8/4 | [45] |
| 9 | [Zn(teta)]6[(VO)12O6B18O36(OH)6](H2O) 8H2O | 12/0 | [48] |
| 10 | {[Zn(dien)]2[Zn(dien)(H2O)]4(VO)12O6[B3O6(OH)]6(H2O)}2 15H2O | 12/0 | [49] |
| 11 | {[Cu(dien)(H2O)]3V12B18O54(OH)6(H2O)} 4H3O 5.5H2O | 10/2 | [55] |
| 12 | {[Cd(H2O)2]3V12B18O54(OH)6(H2O)} 4H3O 9.5H2O | 10/2 | [55] |
| 13 | [Zn(H2teta)2V12B18O54(OH)6] 4H3O | 10/2 | [63] |
6. [V12B18O60] Cores with Alkaline Ions as Counterbalancing Cations
| Compound | Formula | VIV/VV Ratio | Ref. |
|---|---|---|---|
| 14 | (Na)10[(H2O)V12B18O60H6] 18H2O | 10/2 | [51] |
| 15 | {Na2B18V12O54(OH)6(H2O)[Na8(H2O)16]} 2H2O | 10/2 | [54] |
| 16 | {K2V12B18O54(OH)6(H2O)[K8(H2O)16]} 3H2O | 10/2 | [67] |
| 17 | {K10V12B18O54(OH)6(H2O)} 14H2O | 10/2 | [67] |
| 18 | K8(NH4)2[V12B18O60H6] 18H2O | 10/2 | [57] |
| 19 | K10[V12B18O60H6] 10H2O | 10/2 | [57] |
| 20 | K8Cs2[V12B18O60H6] 10H2O | 10/2 | [57] |
| 21 | Li8(NH4)2[V12B18O60H6] 8.02H2O | 10/2 | [58] |
7. [V12B18O60] Cores with Organic Ammonium, Alkaline, and/or Transition Metal Ions as Counterbalancing Cations: The Mixed Family
| Compound | Formula | VIV/VV Ratio | Ref. |
|---|---|---|---|
| 22 | (enH2)4Na4H3[(V12O6B18O42] 8H2O | 9/3 | [46] |
| 23 | K3Na5(H2NCH2CH2NH3)2{(VO)12O6[B3O6(OH)]6}(H2O) 12H2O | 10/2 | [47] |
| 24 | Na8[Cu(en)2]2[V12B18O60H6](NO3)2 14.7H2O | 8/4 | [51] |
| 25 | Na7[Cu(en)2]2[V12B18O60H6](NO3) 15.5H2O | 8/4 | [51] |
| 26 | [Na(H2O)]2[Na(H2O)2]2[Cu(en)2][V12B18O54(OH)6] (H3O)2 (H2O)18 | 8/4 | [50] |
| 27 | {[Na(H2O)4]3[V12B18O54(OH)6(H2O)]2}(H4tren)4 (H3O) 41H2O | 10/2 | [53] |
| 28 | Na8(H3O){[Ni(H2O)5][V12B18O60H6]} 12.5H2O | 11/1 | [52] |
| 29 | Na5(H3O)4{[Ni(H2O)3(en)][V12B18O60H6]} 9H2O | 11/1 | [52] |
| 30 | Na9(H3O){Zn0.5[V12B18O60H6]} 11H2O | 11/1 | [52] |
| 31 | [Hen][H2en]{[Zn(en)2]3[V12B18O60H6]} 3H2O | 9/3 | [52] |
| 32 | {[Na(H2O)3]4Na2V12B18O56(OH)4(H2O)}(H3dien)2 | 10/2 | [55] |
| 33 | {V12B18O54(OH)6(H2O)[K6(H2O)12]} 2(H2dien) 3H2O | 10/2 | [67] |
| 34 | K6(CH3NH3)4[V12B18O54(OH)6(H2O)] 2en 12H2O | 10/2 | [68] |
| 35 | K(H3O)(enH2)4[V12B18O60H6] 9.60H2O | 10/2 | [58] |
| 36 | K5(H3O)2(1,3-diapH2)2[V12B18O60H6] 10.8H2O | 11/1 | [59] |
| 37 | K2(H3O)7(enH2)[V12B18O60H6] 9.0H2O | 11/1 | [59] |
| 38 | [Zn(H3tepa)V12B18O54(OH)6][H2en]2 H3O 3H2O | 10/2 | [63] |
| 39 | [V12B18Zn3O63H12] 3(C4N3H16) 10NH4 5H2O | - | [56] |
| 40 | [V12B18Mn3O63H12] 3(C4N3H16) 10NH4 5H2O | - | [56] |
| 41 | [V12B18Ni3O63H12] 3(C4N3H16) 10NH4 5H2O | - | [56] |
8. Coordination Geometry Analysis of the Counterbalancing Alkaline and Secondary Transition Metal Ions
| Cation | Geometry | Geometry Symbol |
|---|---|---|
| Li | Square Pyramid | (SPY-5) |
| Na | Vacant Octahedron | (vOC-5) |
| Trigonal Bipyramid | (TBPY-5) | |
| Square Pyramid | (SPY-5) | |
| Pentagonal Pyramid | (PPY-6) | |
| Octahedron | (OC-6) | |
| Trigonal Prism | (TPR-6) | |
| K | Pentagonal Pyramid | (PPY-6) |
| Octahedron | (OC-6) | |
| Trigonal Prism | (TPR-6) | |
| Capped Octahedron | (COC-7) | |
| Capped Trigonal Prism | (CTPR-7) | |
| Square Antiprism | (SAPR-8) | |
| Triangular Dodecahedron | (TDD-8) | |
| Tricaped Trigonal Prism | (TCTPR-9) | |
| Cs | Hexagonal Bipyramid | (HBPY-8) |
| Mn | Trigonal Prism | (TPR-6) |
| Ni | Octahedron | (OC-6) |
| Cu | Square | (SP-4) |
| Octahedron | (OC-6) | |
| Zn | Tetrahedron | (T-4) |
| Square Pyramid | (SPY-5) | |
| Vacant Octahedron | (vOC-5) | |
| Octahedron | (OC-6) |
9. Spectroscopic Properties
10. Magnetic Properties
| Compound | Auxiliary cations | VIV/VV Ratio | χT (emu K mol−1) (300 K) | χT (emu K mol−1) (2 K) | Ref. |
|---|---|---|---|---|---|
| 5 | NH4+, (1,3-diapH2)2+ | 10/2 | 3.34 | 0.33 | [57] |
| 11 | [Cu(dien)(H2O)]2+ | 10/2 | 4.81 (3.68) * | 0.56 | [55] |
| 12 | Cd(H2O)22+ | 10/2 | 3.60 | 0.10 | [55] |
| 15 | Na+ | 10/2 | 3.53 | 0.23 | [54] |
| 18 | K+, NH4+ | 10/2 | 3.57 | 0.40 | [57] |
| 19 | K+ | 10/2 | 3.58 | 0.38 | [57] |
| 27 | [Na(H2O)4]+, (H4tren)4+ | 10/2 | 3.83 | 0.15 | [53] |
| 38 | Zn(H3tepa)2+, (enH2)2+ | 10/2 | 1.54 | 0.11 | [63] |

11. Final Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pope, M.T.; Müller, A. Polyoxometalate Chemistry From Topology via Self-Assembly to Applications; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001. [Google Scholar]
- Yamase, T.; Pope, M.T. Polyoxometalate Chemistry for Nano-Composite Design; Kluwer Academic: Dordrecht, The Netherlands; Plenum Publishers: New York, NY, USA, 2002. [Google Scholar]
- Borrás-Almenar, J.J.; Coronado, E.; Müller, A.; Pope, M.T. Polyoxometalate Molecular Science; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003. [Google Scholar]
- Müller, A.; Peters, F.; Pope, M.T.; Gatteschi, D. Polyoxometalates: Very large clusters-nanoscale magnets. Chem. Rev. 1998, 98, 239–271. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Juan, J.M.; Coronado, E.; Gaita-Ariño, A. Magnetic polyoxometalates: from molecular magnetism to molecular spintronics and quantum computing. Chem. Soc. Rev. 2012, 41, 7464–7478. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Sato, R.; Mizuno, N. Reversible switching of single-molecule magnet behaviors by transformation of dinuclear dysprosium cores in polyoxometalates. Chem. Sci. 2013, 4, 596–600. [Google Scholar] [CrossRef]
- Mizuno, N.; Misono, M. Heterogeneous catalysis. Chem. Rev. 1998, 98, 199–217. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Geletii, Y.V.; Zhao, C.; Vickers, J.W.; Zhu, G.; Luo, Z.; Song, J.; Lian, T.; Musaev, D.G.; Hill, C.L. Polyoxometalate water oxidation catalysts and the production of green fuel. Chem. Soc. Rev. 2012, 41, 7572–7589. [Google Scholar] [CrossRef] [PubMed]
- Rhule, J.T.; Hill, C.L.; Judd, D.A.; Schinazi, R.F. Polyoxometalates in medicine. Chem. Rev. 1998, 98, 327–357. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Weinstock, I.A. Polyoxometalate-decorated nanoparticles. Chem. Soc. Rev. 2012, 41, 7479–7496. [Google Scholar] [CrossRef] [PubMed]
- Miras, H.N.; Yan, J.; Long, D.-L.; Cronin, L. Engineering polyoxometalates with emergent properties. Chem. Soc. Rev. 2012, 41, 7403–7430. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.-F.; Tsunashima, R. Recent advances on polyoxometalate-based molecular and composite materials. Chem. Soc. Rev. 2012, 41, 7384–7402. [Google Scholar] [CrossRef] [PubMed]
- Long, D.-L.; Tsunashima, R.; Cronin, L. Polyoxometalates: Building blocks for functional nanoscale systems. Angew. Chem. Int. Ed. 2010, 49, 1736–1758. [Google Scholar] [CrossRef] [PubMed]
- Streb, C.; Long, D.-L.; Cronin, L. Influence of organic amines on the self-assembly of hybrid polyoxo-molybdenum(V) phosphate frameworks. CrystEngComm 2006, 8, 629–634. [Google Scholar] [CrossRef]
- Yi, Z.; Yu, X.; Xia, W.; Zhao, L.; Yang, C.; Chen, Q.; Wang, X.; Xu, X.; Zhang, X. Influence of the steric hindrance of organic amines on the supramolecular network based on polyoxovanadates. CrystEngComm. 2010, 12, 242–249. [Google Scholar] [CrossRef]
- Long, D.-L.; Kögerler, P.; Farrugia, L.J.; Cronin, L. Restraining symmetry in the formation of small polyoxomolybdates: Building blocks of unprecedented topology resulting from “shrink-wrapping” [H2Mo16O52]10−-type clusters. Angew. Chem. Int. Ed. 2003, 42, 4180–4183. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.; Pickering, A.L.; Long, D.-L.; Kögerler, P.; Cronin, L. Controllable growth of chains and grids from polyoxomolybdate building blocks linked by Silver(I) dimers. Chem. Eur. J. 2005, 11, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Streb, G.; McGlone, T.; Brücher, O.; Long, D.-L.; Cronin, L. Hybrid host-guest complexes: Directing the supramolecular structure through secondary host-guest interactions. Chem. Eur. J. 2008, 14, 8861–8868. [Google Scholar] [CrossRef] [PubMed]
- Mcglone, T.; Streb, C.; Long, D.-L.; Cronin, L. Guest-directed supramolecular architectures of {W36} polyoxometalate crowns. Chem. Asian J. 2009, 4, 1612–1618. [Google Scholar] [CrossRef] [PubMed]
- Day, V.W.; Klemperer, W.G.; Yaghi, O.M. A new structure type in polyoxoanion chemistry: synthesis and structure of the V5O143− anion. J. Am. Chem. Soc. 1989, 111, 4518–4519. [Google Scholar] [CrossRef]
- Evans, H.T. The molecular structure of the isopoly complex ion, decavanadate (V10O2816−). Inorg. Chem. 1966, 5, 967–977. [Google Scholar] [CrossRef]
- Day, V.W.; Klemperer, W.G.; Yaghi, O.M. Synthesis and characterization of a soluble oxide inclusion complex, [CH3CN⊂(V12O124−)]. J. Am. Chem. Soc. 1989, 111, 5959–5962. [Google Scholar] [CrossRef]
- Hou, D.; Hagen, K.S.; Hill, C.L. Tridecavanadate, [V13O34]3−, a new high-potential isopolyvanadate. J. Am. Chem. Soc. 1992, 114, 5864–5866. [Google Scholar] [CrossRef]
- Hou, D.; Hagen, K.S.; Hill, C.L. Pentadecavanadate, V15O429−, a new highly condensed fully oxidized isopolyvanadate with kinetic stability in water. J. Chem. Soc. Chem. Commun. 1993, 20, 426–428. [Google Scholar] [CrossRef]
- Müller, A.; Krickemeyer, E.; Penk, M.; Walberg, H.-J.; Bögge, H. Spherical mixed-valence [V15O36]5−, an example from an unusual cluster family. Angew. Chem. Int. Ed. Engl. 1987, 26, 1045–1046. [Google Scholar] [CrossRef]
- Müller, A.; Sessoli, R.; Krickemeyer, E.; Bögge, H.; Meyer, J.; Gatteschi, D.; Pardi, L.; Westphal, J.; Hovemeier, K.; Rohlfing, R.; Döring, J.; Hellweg, F.; Beugholt, C.; Schmidtmann, M. Polyoxovanadates: High-nuclearity spin clusters with interesting host−guest systems and different electron populations. synthesis, spin organization, magnetochemistry, and spectroscopic studies. Inorg. Chem. 1997, 36, 5239–5250. [Google Scholar] [CrossRef]
- Suber, L.; Bonamico, M.; Fares, V. Synthesis, magnetism, and X-ray molecular structure of the mixed-valence vanadium(IV/V)−oxygen cluster [VO4⊂(V18O45)]9−. Inorg. Chem. 1997, 36, 2030–2033. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.; Krickemeyer, E.; Penk, M.; Rohlfing, R.; Armatage, A.; Bögge, H. Template-controlled formation of cluster shells or a type of molecular recognition: Synthesis of [HV22O54(ClO4)]6− and [H2V18O44(N3)]5−. Angew. Chem. Int. Ed. 1991, 30, 1674–1677. [Google Scholar] [CrossRef]
- Müller, A.; Rohlfing, R.; Döring, J.; Penk, M. Formation of a cluster sheath around a central cluster by a“self-organization process”: the mixed valence polyoxovanadate[V34O82]10−. Angew. Chem. Int. Ed. 1991, 30, 588–590. [Google Scholar] [CrossRef]
- Warren, C.J.; Rijssenbeek, J.T.; Rose, D.J.; Haushalter, R.C.; Zubieta, J. Hydrothermal synthesis and characterization of an unusual polyoxovanadium borate cluster: Structure of Rb4[(VO)6{{B10O16(OH)6}}2] 0.5H2O. Polyhedron 1998, 17, 2599–2605. [Google Scholar] [CrossRef]
- Williams, I.D.; Wu, M.; Sung, H.H.-Y.; Zhang, X.X.; Yu, J. An organotemplated vanadium(IV) borate polymer from boric acid “flux” synthesis, [H2en]4[Hen]2[V6B22O53H8] 5H2O. Chem. Commun. 1998, 2463–2464. [Google Scholar] [CrossRef]
- Cao, Y.-N.; Zhang, H.-H.; Huang, C.-C.; Sun, Y.-X.; Chen, Y.-P.; Guo, W.-J.; Zhang, F.-L. Synthesis and structural characterization of a new polyoxovanadium borate: (H3NCH2CH2NH3)4[(VO)6(B10O22)2](H3O)7. Chin. J. Struct. Chem. 2005, 24, 525–530. [Google Scholar]
- Cai, Q.; Lu, B.; Zhang, J.; Shan, Y. Synthesis, structure and properties of (H2NCH2CH2NH3)3 {(VO)6[B10O16(OH)6]2} 11H2O. J. Chem. Crystallogr. 2008, 38, 321–325. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, J.; An, L.; Chen, R.; Hu, F.; Tang, Q. Hydrothermal syntheses, crystal structures and characterization of new vanadoborates: The novel decorated cage cluster [V6B22O44(OH)10]. J. Solid State Chem. 2013, 201, 79–84. [Google Scholar] [CrossRef]
- Wu, M.; Law, T.S.-C.; Sung, H.H.-Y.; Cai, J.; Williams, I.D. Synthesis of elliptical vanadoborates housing bimetallic centers [Zn4(B2O4H2)(V10B28O74]8− and [Mn4(C2O4)(V10B28O74H8)]10−. Chem. Commun. 2005, 4, 1827–1829. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhang, H.; Huang, C.; Yang, Q.; Chen, Y.; Sun, R.; Zhang, F.; Guo, W. Synthesis, crystal structure and two-dimensional infrared correlation spectroscopy of a layer-like transition metal (TM)-oxalate templated polyoxovanadium borate. J. Solid State Chem. 2005, 178, 3563–3570. [Google Scholar] [CrossRef]
- Chen, H.; Yu, Z.-B.; Bacsik, Z.; Zhao, H.; Yao, Q.; Sun, J. Construction of mesoporous frameworks with vanadoborate clusters. Angew. Chem. Int. Ed. 2014, 53, 3608–3611. [Google Scholar] [CrossRef] [PubMed]
- Warren, C.J.; Haushalter, R.C.; Rose, D.J.; Zubieta, J. A bimetallic main group oxide cluster of the oxovanadium borate system: (H3NCH2CH2NH3)4[(VO)12O4{B8O17(OH)4}2{Mn(H2O)2}2] H2O. Inorg. Chim. Acta 1998, 282, 123–129. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, H.; Huang, C.; Chen, Y.; Sun, R.; Guo, W. Hydrothermal synthesis and crystal structure of a novel 1D polyoxovanadium borate: (H3NCH2CH2NH3)3[(VO)12O4{B8O17(OH)4}2{Na(H2O)}2 (H3O)2(H2O)6.5. J. Mol. Struct. 2005, 733, 211–216. [Google Scholar] [CrossRef]
- Sun, Y.-Q.; Li, G.-M.; Chen, Y.-P. A novel polyoxovanadium borate incorporating an organic amine ligand: synthesis and structure of [V12B16O50(OH)7(en)]7−. Dalton Trans. 2012, 41, 5774–5777. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhou, J.; Xiao, H.-P.; Kong, C.; Zou, H.; Tang, Q.; Li, J. Two new 3-D boratopolyoxovanadate architectures based on the [V12B16O50(OH)8]12− cluster with different metal linkers. New J. Chem. 2013, 37, 4077–4082. [Google Scholar] [CrossRef]
- Rijssenbeek, J.T.; Rose, D.J.; Haushalter, R.C.; Zubieta, J. Novel clusters of transition metals and main group oxides in the alkylamine/oxovanadium/borate system. Angew. Chem. Int. Ed. 1997, 36, 1008–1010. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, Z.; Yang, G.; Chen, X.; Feng, S. Hydrothermal synthesis and X-ray single crystal structure of [Zn(en)2]6[(VO)12O6B18O39(OH)3] 13H2O. J. Solid State Chem. 1999, 148, 450–454. [Google Scholar] [CrossRef]
- Lin, Z.-H.; Zhang, H.-H.; Huang, C.-C.; Sun, R.-Q.; Chen, Y.-P.; Wu, X.-Y. Hydrothermal synthesis, crystal structure and properties of polyoxovanadium borate H3{[Cu(en)2]5[(VO)12O6B18O42]}[B(OH)3]2 16H2O. Acta Chim. Sin. 2004, 62, 391–398. [Google Scholar]
- Lin, Z.-H.; Zhang, H.-H.; Huang, C.-C.; Sun, R.-Q.; Yang, Q.-Y.; Wu, X.-Y. Hydrothermal synthesis and crystal structure of [Ni(en)2]6H2[(VO)12O6B18O42] 15H2O. Chin. J. Struct. Chem. 2004, 23, 83–86. [Google Scholar]
- Lin, Z.-H.; Yang, Q.-Y.; Zhang, H.-H.; Huang, C.-C.; Sun, R.-Q.; Wu, X.-Y. Hydrothermal synthesis and crystal structure of (enH2)4Na4H3[(VO)12O6B18O42] 8H2O. Chin. J. Struct. Chem. 2004, 23, 590–595. [Google Scholar]
- Lu, B.; Wang, H.; Zhang, L.; Dai, C.-Y.; Cai, Q.-H.; Shan, Y.-K. Hydrothermal synthesis and structure of K3Na5(H2NCH2CH2NH3)2{(VO)12O6[B3O6(OH)]6}(H2O) 12H2O. Chin. J. Chem. 2005, 23, 137–143. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, J. The new vanadoborate-supported hexanuclear zinc complex [Zn(teta)]6[(VO)12O6B18O36(OH)6](H2O) 8H2O. Z. Naturforsch. 2011, 66b, 115–118. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, J.; Zhou, Z.; Zhang, F. Hydrothermal syntheses and crystal structures of two new heteropolyoxovanadoborates containing {(VO)12O6[B3O6(OH)]6(H2O)} cluster. J. Clust. Sci. 2011, 22, 65–72. [Google Scholar] [CrossRef]
- Li, G.-M.; Mei, H.-X.; Chen, X.-Y.; Chen, Y.-P.; Sun, Y.-Q.; Zhang, H.-H.; Chen, X.-P. A porous organic-inorganic hybrid compound constructed from polyoxovanadium borate anions, dinuclear Na sites and metal-organic units. Chin. J. Struct. Chem. 2011, 30, 785–792. [Google Scholar]
- Brown, K.; Car, P.-E.; Vega, A.; Venegas-Yazigi, D.; Paredes-García, V.; Vaz, M.G.F.; Allao, R.A.; Pivan, J.-Y.; Le Fur, E.; Spodine, E. Polyoxometalate cluster [V12B18O60H6] functionalized with the copper(II) bis-ethylenediamine complex. Inorg. Chim. Acta 2011, 367, 21–28. [Google Scholar] [CrossRef]
- Hermosilla-Ibáñez, P.; Car, P.E.; Vega, A.; Costamagna, J.; Caruso, F.; Pivan, J.-Y.; Le Fur, E.; Spodine, E.; Venegas-Yazigi, D. New structures based on the mixed valence polyoxometalate cluster [V12B18O60H6]n−. CrystEngComm 2012, 14, 5604–5612. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, X.; Hu, F.; Zou, H.; Li, X. A new 1-D extended vanadoborate containing triply bridged metal complex units. Inorg. Chem. Commun. 2012, 25, 51–54. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, X.; Hu, F.; Zou, H.; Li, R.; Li, X. One novel 3-D vanadoborate with unusual 3-D Na–O–Na network. RSC Adv. 2012, 2, 10937–10940. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, X.; Chen, R.; Xiao, H.-P.; Hu, F.; Zou, H.; Zhou, Y.; Liu, C.; Zhu, L. New 3-D polyoxovanadoborate architectures based on [V12B18O60]16− clusters. CrystEngComm 2013, 15, 5057–5063. [Google Scholar] [CrossRef]
- Chen, H.; Deng, Y.; Yu, Z.; Zhao, H.; Yao, Q.; Zou, X.; Bäckvall, J.E.; Sun, J. 3D open-framework vanadoborate as a highly effective heterogeneous pre-catalyst for the oxidation of alkylbenzenes. Chem. Mater. 2013, 25, 5031–5036. [Google Scholar] [CrossRef]
- Hermosilla-Ibáñez, P.; Cañon-Mancisidor, W.; Costamagna, J.; Vega, A.; Paredes-García, V.; Garland, M.T.; Le Fur, E.; Cador, O.; Spodine, E.; Venegas-Yazigi, D. Crystal lattice effect on the quenching of the intracluster magnetic interaction in [V12B18O60H6]10− polyoxometalate. Dalton Trans. 2014, 43, 14132–14141. [Google Scholar] [CrossRef] [PubMed]
- Hermosilla-Ibáñez, P.; Costamagna, J.; Vega, A.; Paredes-García, V.; Le Fur, E.; Spodine, E.; Venegas-Yazigi, D. Coordination interactions in the crystalline lattice of alkaline ions with the polyoxometalate [V12B18O60H6]10− ligand. J. Coord. Chem. 2014, 67, 3940–3952. [Google Scholar] [CrossRef]
- Hermosilla-Ibáñez, P.; Costamagna, J.; Vega, A.; Paredes-García, V.; Garland, M.T.; Le Fur, E.; Spodine, E.; Venegas-Yazigi, D. Protonated diamines as linkers in the supramolecular assemblies based on the [V12B18O60H6] polyoxovanadoborate anion. J. Struct. Chem. 2014, 55, 1453–1465. [Google Scholar] [CrossRef]
- Yamase, T.; Suzuki, M.; Ohtaka, K. Structures of photochemically prepared mixed-valence polyoxovanadate clusters: oblong [V18O44(N3)]14− , superkeggin [V18O42(PO4)]11− and doughnut-shaped [V12B32O84Na4]15− anions. Dalton Trans. 1997, 44, 2463–2472. [Google Scholar] [CrossRef]
- Warren, C.J.; Rose, D.J.; Haushalter, R.C.; Zubieta, J. A new transition metal−main group oxide cluster in the oxovanadium−borate system: hydrothermal synthesis and structure of (H3O)12[(VO)12{B16O32(OH)4}2] 28H2O. Inorg. Chem. 1998, 37, 1140–1141. [Google Scholar] [CrossRef] [PubMed]
- Williams, I.D.; Wu, M.; Sung, H.H.-Y.; Law, T.S.-C.; Zhang, X.X. Contemporary Boron Chemistry: Synthesis and Properties of Vanadoborate Cluster Materials; Davidson, M.G., Hughes, A.K., Marder, T.B., Wade, K., Eds.; Royal Society of Chemistry: Cambridge, UK, 2000. [Google Scholar]
- Liu, X.; Zhao, R.; Zhou, J.; Liu, M. Three new vanadoborates functionalized with zinc complexes. Inorg. Chem. Commun. 2014, 43, 101–104. [Google Scholar] [CrossRef]
- Christ, C.L.; Clark, J.R. A crystal-chemical classification of borate structures with emphasis on hydrated borates. Phys. Chem. Miner. 1977, 2, 59–87. [Google Scholar] [CrossRef]
- Hagrman, P.J.; Finn, R.C.; Zubieta, J. Molecular manipulation of solid state structure: Influences of organic components on vanadium oxide architectures. Solid State Sci. 2001, 3, 745–774. [Google Scholar] [CrossRef]
- Zhang, Y.-N.; Zhou, B.-B.; Sha, J.-Q.; Su, Z.-H.; Cui, J.-W. Assembly of two layered cobalt–molybdenum phosphates: Changing interlayer distances by tuning the lengths of amine ligands. J. Solid State Chem. 2011, 184, 419–426. [Google Scholar] [CrossRef]
- An, L.; Zhou, J.; Xiao, H.-P.; Liu, X.; Zou, H.; Pan, C.-Y.; Liu, M.; Li, J. A series of new 3-D boratopolyoxovanadates containing five types of [KxOy]n building units. CrystEngComm 2014, 16, 4236–4244. [Google Scholar] [CrossRef]
- Meng, Q.; He, H.; Yang, B.-F.; Zhao, J.-W.; Yang, G.-Y. Synthesis and characterization of a 3-D framework constructed from [V12B18O54(OH)6(H2O)]10− clusters and K+ cations. J. Clust. Sci. 2014, 25, 1273–1282. [Google Scholar] [CrossRef]
- Llunell, M.; Casanova, D.; Cirera, J.; Alemany, P.; Alvarez, S. SHAPE, version 2.1; Universitat de Barcelona: Barcelona, Spain, 2013. [Google Scholar]
- Delgado, F.S.; Ruiz-Pérez, C.; Sanchiz, J.; Lloret, F.; Julve, M. Versatile supramolecular self-assembly. Part I. Network formation and magnetic behaviour of the alkaline salts of the bis(malonate)cuprate(II) anion. CrystEngComm 2006, 8, 507–529. [Google Scholar] [CrossRef]
- Askarinejad, A.; Morsali, A. Potassium(I)thallium(I) heterometallic 3D polymeric mixed-anions compound, succinate-nitrate [K2Tl(MU-C4H4O4)(MU-NO3)]n. Inorg. Chim. Acta 2006, 359, 3379–3383. [Google Scholar] [CrossRef]
- Nagasubramanian, S.; Jayamani, A.; Thamilarasan, V.; Aravindan, G.; Ganesan, V.; Sengottuvelan, N. Hetero-metallic trigonal cage-shaped dimeric Ni3K core complex of L-proline ligand: Synthesis, structural, electrochemical and DNA binding and cleavage activities. J. Chem. Sci. 2014, 126, 771–781. [Google Scholar] [CrossRef]
- Robin, M.B.; Day, P. Mixed Valence Chemistry-A Survey and Classification. Adv. Inorg. Chem. Radiochem. 1967, 10, 247–422. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hermosilla-Ibáñez, P.; Muñoz-Becerra, K.; Paredes-García, V.; Fur, E.L.; Spodine, E.; Venegas-Yazigi, D. Structural and Electronic Properties of Polyoxovanadoborates Containing the [V12B18O60] Core in Different Mixed Valence States. Inorganics 2015, 3, 309-331. https://doi.org/10.3390/inorganics3030309
Hermosilla-Ibáñez P, Muñoz-Becerra K, Paredes-García V, Fur EL, Spodine E, Venegas-Yazigi D. Structural and Electronic Properties of Polyoxovanadoborates Containing the [V12B18O60] Core in Different Mixed Valence States. Inorganics. 2015; 3(3):309-331. https://doi.org/10.3390/inorganics3030309
Chicago/Turabian StyleHermosilla-Ibáñez, Patricio, Karina Muñoz-Becerra, Verónica Paredes-García, Eric Le Fur, Evgenia Spodine, and Diego Venegas-Yazigi. 2015. "Structural and Electronic Properties of Polyoxovanadoborates Containing the [V12B18O60] Core in Different Mixed Valence States" Inorganics 3, no. 3: 309-331. https://doi.org/10.3390/inorganics3030309





