Carbon Quantum Dots: The Role of Surface Functional Groups and Proposed Mechanisms for Metal Ion Sensing
Abstract
1. Introduction
2. The Metal Ion Sensing Mechanism
- (1)
- Fluorescence Quenching:
- (2)
- Metal-Enhanced Fluorescence (MEF):
- (1)
- One of the quencher absorption spectra overlaps with the excitation spectrum of CDs, so the quencher absorbs light, and quenching take place [49].
- (2)
- Reabsorption: photons are emitted by one specie and absorbed by others in the solution, and this is due to the weakening of the absorption or excitation radiation by unused quencher and CDs in the solution, so it is not a quenching process by definition [50].
3. Role of Functional Groups in the Metal Ion Sensing Mechanism
3.1. Origin of Optical Properties
3.2. Mercury Detection
3.3. Lead Detection
3.4. Silver Detection
3.5. Chromium Detection
3.6. Iron(III) and Iron(II) Detection
3.7. Copper(II) Detection
4. Summary and Conclusions
- (1)
- Quantum confinement’s effect is most prominent if the CDs do not have a large number of functional groups.
- (2)
- Functional groups of hydroxyl and carboxyl are produced via oxidation, while other functional groups such as amine groups are formed due to precursors and solvents from which nitrogen atoms are taken.
- (3)
- The same precursor and synthesis parameters should be used to produce CDs with consistent properties. Based on the review, the obtained CDs’ chemical and physical properties are not simply and logically correlated with the synthesis conditions. The relationships are probably not linear. Therefore, slight changes in the synthesis method cause significant differences in the properties, while doping with ions or metals can influence the properties of CDs.
- (4)
- Functional group properties should be investigated separately to determine the effect of CD size on properties. This requires a large amount of work related to the blocking of functional groups, their reduction, or other reactions specific to selected types of functional groups, enabling the understanding of the influence of these groups on the optical and chemical properties of CDs.
- (5)
- Metal ions are detectable with some CDs with which they make complexes and bonds, while not being detectable with other types of CDs. This means that producing different quantum dots specifically sensitive to one metal (or compound) is technically possible. Due to the lack of complete knowledge of CDs’ surface chemistry, further research is required.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chu, K.-W.; Lee, S.L.; Chang, C.-J.; Liu, L. Recent progress of carbon dot precursors and photocatalysis applications. Polymers 2019, 11, 689. [Google Scholar] [CrossRef]
- Xie, Z.; Wang, F.; Liu, C.-Y. Organic–inorganic hybrid functional carbon dot gel glasses. Adv. Mater. 2012, 24, 1716–1721. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.T.; Ananthanarayanan, A.; Luo, K.Q.; Chen, P. Glowing graphene quantum dots and carbon dots: Properties, syntheses, and biological applications. Small 2015, 11, 1620–1636. [Google Scholar] [CrossRef]
- Li, X.; Zhao, S.; Li, B.; Yang, K.; Lan, M.; Zeng, L. Advances and perspectives in carbon dot-based fluorescent probes: Mechanism, and application. Coord. Chem. Rev. 2021, 431, 213686. [Google Scholar] [CrossRef]
- Yue, J.; Zhang, K.; Yu, H.; Yu, L.; Hou, T.; Chen, X.; Ge, H.; Hayat, T.; Alsaedi, A.; Wang, S. Mechanism insights into tunable photoluminescence of carbon dots by hydroxyl radicals. J. Mater. Sci. 2019, 54, 6140–6150. [Google Scholar] [CrossRef]
- Liu, W.; Li, C.; Ren, Y.; Sun, X.; Pan, W.; Li, Y.; Wang, J.; Wang, W. Carbon dots: Surface engineering and applications. J. Mater. Chem. B 2016, 4, 5772–5788. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Zhang, W.; Chang, Q.; Yang, J.; Lin, K. A chemical method for identifying the photocatalytic active sites on carbon dots. Carbon 2016, 103, 391–393. [Google Scholar] [CrossRef]
- Dhenadhayalan, N.; Lin, K.-C.; Suresh, R.; Ramamurthy, P. Unravelling the multiple emissive states in citric-acid-derived carbon dots. J. Phys. Chem. C 2016, 120, 1252–1261. [Google Scholar] [CrossRef]
- Wang, H.; Sun, P.; Cong, S.; Wu, J.; Gao, L.; Wang, Y.; Dai, X.; Yi, Q.; Zou, G. Nitrogen-doped carbon dots for “green” quantum dot solar cells. Nanoscale Res. Lett. 2016, 11, 27. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef]
- Papaioannou, N.; Titirici, M.-M.; Sapelkin, A.J.A.o. Investigating the effect of reaction time on carbon dot formation, structure, and optical properties. ACS Omega 2019, 4, 21658–21665. [Google Scholar] [CrossRef]
- Bourlinos, A.B.; Trivizas, G.; Karakassides, M.A.; Baikousi, M.; Kouloumpis, A.; Gournis, D.; Bakandritsos, A.; Hola, K.; Kozak, O.; Zboril, R. Green and simple route toward boron doped carbon dots with significantly enhanced non-linear optical properties. Carbon 2015, 83, 173–179. [Google Scholar] [CrossRef]
- Tepliakov, N.V.; Kundelev, E.V.; Khavlyuk, P.D.; Xiong, Y.; Leonov, M.Y.; Zhu, W.; Baranov, A.V.; Fedorov, A.V.; Rogach, A.L.; Rukhlenko, I.D. sp2–sp3-Hybridized atomic domains determine optical features of carbon dots. ACS Nano 2019, 13, 10737–10744. [Google Scholar] [CrossRef] [PubMed]
- Karami, C.; Taher, M.A.; Shahlaei, M. A simple method for determination of mercury (II) ions by PNBS-doped carbon dots as a fluorescent probe. J. Mater. Sci. Mater. Electron. 2020, 31, 5975–5983. [Google Scholar] [CrossRef]
- Li, W.-K.; Feng, J.-T.; Ma, Z.-Q. Nitrogen, sulfur, boron and flavonoid moiety co-incorporated carbon dots for sensitive fluorescence detection of pesticides. Carbon 2020, 161, 685–693. [Google Scholar] [CrossRef]
- Sagbas, S.; Sahiner, N. Nanocarbon and its Composites; Elsevier: Amsterdam, The Netherlands, 2019; pp. 651–676. [Google Scholar]
- Wang, J.; Liu, G.; Cham-Fai Leung, K.; Loffroy, R.; Lu, P.-X.; Wang, X.J. Opportunities and challenges of fluorescent carbon dots in translational optical imaging. Curr. Pharm. Des. 2015, 21, 5401–5416. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Han, Q.; Wu, J.; Ji, C.; Zhou, Y.; Li, S.; Gao, L.; Leblanc, R.M.; Peng, Z. Synthesis Mechanisms, Structural Models, and Photothermal Therapy Applications of Top-Down Carbon Dots from Carbon Powder, Graphite, Graphene, and Carbon Nanotubes. Int. J. Mol. Sci. 2022, 23, 1456. [Google Scholar] [CrossRef] [PubMed]
- Messina, M.M.; Barrionuevo, S.D.; Coustet, M.E.; Kreuzer, M.P.; Saccone, F.D.; dos Santos Claro, P.C.; Ibanez, F.J. Graphene and Carbon Dots for Photoanodes with Enhanced Performance. ACS Appl. Nano Mater. 2021, 4, 7309–7318. [Google Scholar] [CrossRef]
- Choi, Y.; Choi, Y.; Kwon, O.H.; Kim, B.S. Carbon dots: Bottom-up syntheses, properties, and light-harvesting applications. Chem. –Asian J. 2018, 13, 586–598. [Google Scholar] [CrossRef]
- Qu, D.; Sun, Z. The formation mechanism and fluorophores of carbon dots synthesized via a bottom-up route. Mater. Chem. Front. 2020, 4, 400–420. [Google Scholar] [CrossRef]
- Ye, H.-G.; Lu, X.; Cheng, R.; Guo, J.; Li, H.; Wang, C.-F.; Chen, S. Mild bottom-up synthesis of carbon dots with temperature-dependent fluorescence. J. Lumin. 2021, 238, 118311. [Google Scholar] [CrossRef]
- Nguyen, T.N.; Le, P.A.; Phung, V.B. Facile green synthesis of carbon quantum dots and biomass-derived activated carbon from banana peels: Synthesis and investigation. Biomass Convers. Biorefinery 2020, 12, 2407–2416. [Google Scholar] [CrossRef]
- Yousef, S.; Eimontas, J.; Striūgas, N.; Abdelnaby, M.A. Pyrolysis and gasification kinetic behavior of mango seed shells using TG-FTIR-GC–MS system under N2 and CO2 atmospheres. Renew. Energy 2021, 173, 733–749. [Google Scholar] [CrossRef]
- Li, X.; Xing, X.; Zhao, S.; Zhu, S.; Wang, B.; Lan, M.; Song, X. Carbon dot-based fluorescent and colorimetric sensor for sensitive and selective visual detection of benzoyl peroxide. Chin. Chem. Lett. 2022, 33, 1632–1636. [Google Scholar] [CrossRef]
- Cailotto, S.; Amadio, E.; Facchin, M.; Selva, M.; Pontoglio, E.; Rizzolio, F.; Riello, P.; Toffoli, G.; Benedetti, A.; Perosa, A.J.A.m.c.l. Carbon dots from sugars and ascorbic acid: Role of the precursors on morphology, properties, toxicity, and drug uptake. ACS Med. Chem. Lett. 2018, 9, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.; Reckmeier, C.J.; Xiong, Y.; von Seckendorff, M.; Susha, A.S.; Kasák, P.; Rogach, A. Molecular fluorescence in citric acid-based carbon dots. J. Phys. Chem. C 2017, 121, 2014–2022. [Google Scholar] [CrossRef]
- Kalytchuk, S.; Poláková, K.i.; Wang, Y.; Froning, J.P.; Cepe, K.; Rogach, A.L.; Zbořil, R. Carbon dot nanothermometry: Intracellular photoluminescence lifetime thermal sensing. Acs Nano 2017, 11, 1432–1442. [Google Scholar] [CrossRef]
- Yang, K.; Wang, C.; Liu, C.; Ding, S.; Tian, F.; Li, F. Bioluminescence-initiated photodynamic therapy bridged on high-luminescent carbon dots-conjugated protoporphyrin IX. J. Mater. Sci. 2019, 54, 3383–3391. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Wang, Y.; Kalytchuk, S.; Kershaw, S.V.; Wang, Y.; Wang, P.; Zhang, T.; Zhao, Y.; Zhang, H. Color-switchable electroluminescence of carbon dot light-emitting diodes. ACS Nano 2013, 7, 11234–11241. [Google Scholar] [CrossRef]
- Tawfik, W.Z.; Kumar, C.M.; Park, J.; Shim, S.K.; Lee, H.; Lee, J.; Han, J.H.; Ryu, S.-W.; Lee, N.; Lee, J.K. Cathodoluminescence of a 2 inch ultraviolet-light-source tube based on the integration of AlGaN materials and carbon nanotube field emitters. J. Mater. Chem. C 2019, 7, 11540–11548. [Google Scholar] [CrossRef]
- Song, Y.; Zhu, S.; Xiang, S.; Zhao, X.; Zhang, J.; Zhang, H.; Fu, Y.; Yang, B. Investigation into the fluorescence quenching behaviors and applications of carbon dots. Nanoscale 2014, 6, 4676–4682. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Sahu, S.; Cao, L.; Bunker, C.E.; Peng, G.; Liu, Y.; Fernando, K.S.; Wang, P.; Guliants, E.A.; Meziani, M.J. Efficient fluorescence quenching in carbon dots by surface-doped metals-disruption of excited state redox processes and mechanistic implications. Langmuir 2012, 28, 16141–16147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Goncalves, H.; da Silva, J.C.E.; Geddes, C.D. Metal-enhanced photoluminescence from carbon nanodots. Chem. Commun. 2011, 47, 5313–5315. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhu, Y.; Zhang, X.; Yang, X.; Li, C. Metal-enhanced fluorescence of carbon dots adsorbed Ag@ SiO2 core-shell nanoparticles. RSC Adv. 2012, 2, 1765–1768. [Google Scholar] [CrossRef]
- Dubois, F.; Mahler, B.; Dubertret, B.; Doris, E.; Mioskowski, C. A versatile strategy for quantum dot ligand exchange. J. Am. Chem. Soc. 2007, 129, 482–483. [Google Scholar] [CrossRef]
- Tian, T.; He, Y.; Ge, Y.; Song, G. One-pot synthesis of boron and nitrogen co-doped carbon dots as the fluorescence probe for dopamine based on the redox reaction between Cr (VI) and dopamine. Sens. Actuators B Chem. 2017, 240, 1265–1271. [Google Scholar] [CrossRef]
- Zu, F.; Yan, F.; Bai, Z.; Xu, J.; Wang, Y.; Huang, Y.; Zhou, X. The quenching of the fluorescence of carbon dots: A review on mechanisms and applications. Microchim. Acta 2017, 184, 1899–1914. [Google Scholar] [CrossRef]
- Gao, X.; Du, C.; Zhuang, Z.; Chen, W. Carbon quantum dot-based nanoprobes for metal ion detection. J. Mater. Chem. C 2016, 4, 6927–6945. [Google Scholar] [CrossRef]
- Batool, M.; Junaid, H.M.; Tabassum, S.; Kanwal, F.; Abid, K.; Fatima, Z.; Shah, A.T. Metal ion detection by carbon dots—A review. Crit. Rev. Anal. Chem. 2022, 52, 756–767. [Google Scholar] [CrossRef]
- Jiang, W.; Zhao, Y.; Zhu, X.; Liu, H.; Sun, B. Carbon dot-based biosensors. Crit. Rev. Anal. Chem. 2021, 1, 2000042. [Google Scholar] [CrossRef]
- Clapp, A.R.; Medintz, I.L.; Mauro, J.M.; Fisher, B.R.; Bawendi, M.G.; Mattoussi, H. Fluorescence resonance energy transfer between quantum dot donors and dye-labeled protein acceptors. J. Am. Chem. Soc. 2004, 126, 301–310. [Google Scholar] [CrossRef]
- Clapp, A.R.; Medintz, I.L.; Fisher, B.R.; Anderson, G.P.; Mattoussi, H. Can luminescent quantum dots be efficient energy acceptors with organic dye donors? J. Am. Chem. Soc. 2005, 127, 1242–1250. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Uhlikova, N.; Xu, Z.; Zhu, Y.; Huang, Y.; Egap, E.; Lian, T. Competition of Dexter, Förster, and charge transfer pathways for quantum dot sensitized triplet generation. J. Chem. Phys. 2020, 152, 214702. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhao, N.; Cheng, Z.; Liu, H. Amino-functionalized green fluorescent carbon dots as surface energy transfer biosensors for hyaluronidase. Nanoscale 2015, 7, 6836–6842. [Google Scholar] [CrossRef] [PubMed]
- Tvrdy, K.; Frantsuzov, P.A.; Kamat, P.V. Photoinduced electron transfer from semiconductor quantum dots to metal oxide nanoparticles. Proc. Natl. Acad. Sci. USA 2011, 108, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cao, L.; Lu, F.; Meziani, M.J.; Li, H.; Qi, G.; Zhou, B.; Harruff, B.A.; Kermarrec, F.; Sun, Y.-P. Photoinduced electron transfers with carbon dots. Chem. Commun. 2009, 25, 3774–3776. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Xie, Z.; Qu, D.; Li, D.; Du, P.; Jing, X.; Sun, Z. On–off–on fluorescent carbon dot nanosensor for recognition of chromium (VI) and ascorbic acid based on the inner filter effect. ACS Appl. Mater. Interfaces 2013, 5, 13242–13247. [Google Scholar] [CrossRef]
- Panigrahi, S.K.; Mishra, A.K. Inner filter effect in fluorescence spectroscopy: As a problem and as a solution. J. Photochem. Photobiol. C 2019, 41, 100318. [Google Scholar] [CrossRef]
- Wang, T.; Zeng, L.-H.; Li, D.-L. A review on the methods for correcting the fluorescence inner-filter effect of fluorescence spectrum. Appl. Spectrosc. Rev. 2017, 52, 883–908. [Google Scholar] [CrossRef]
- Yan, F.; Sun, Z.; Zhang, H.; Sun, X.; Jiang, Y.; Bai, Z. The fluorescence mechanism of carbon dots, and methods for tuning their emission color: A review. Microchim. Acta 2019, 186, 583. [Google Scholar] [CrossRef]
- Li, D.; Kou, E.; Li, W.; Zhang, H.; Zhang, X.; Zhuang, J.; Liu, Y.; Hu, C.; Zheng, Y.; Yang, Q. Oxidation-induced quenching mechanism of ultrabright red carbon dots and application in antioxidant RCDs/PVA film. Chem. Eng. J. 2021, 425, 131653. [Google Scholar] [CrossRef]
- Yang, X.; Sui, L.; Wang, B.; Zhang, Y.; Tang, Z.; Yang, B.; Lu, S. Red-emitting, self-oxidizing carbon dots for the preparation of white LEDs with super-high color rendering index. Sci. China Chem. 2021, 64, 1547–1553. [Google Scholar] [CrossRef]
- Yoo, H.J.; Kwak, B.E. Competition of the roles of π-conjugated domain between emission center and quenching origin in the photoluminescence of carbon dots depending on the interparticle separation. Carbon 2021, 183, 560–570. [Google Scholar] [CrossRef]
- Zhai, L.; Ren, X.-M.; Xu, Q. Carbogenic π-conjugated domains as the origin of afterglow emissions in carbon dot-based organic composite films. Mater. Chem. Front. 2021, 5, 4272–4279. [Google Scholar] [CrossRef]
- Tian, Z.; Zhang, X.; Li, D.; Zhou, D.; Jing, P.; Shen, D.; Qu, S.; Zboril, R.; Rogach, A.L. Full-color inorganic carbon dot phosphors for white-light-emitting diodes. Adv. Opt. Mater. 2017, 5, 1700416. [Google Scholar] [CrossRef]
- Miao, X.; Qu, D.; Yang, D.; Nie, B.; Zhao, Y.; Fan, H.; Sun, Z. Synthesis of carbon dots with multiple color emission by controlled graphitization and surface functionalization. Adv. Mater. 2018, 30, 1704740. [Google Scholar] [CrossRef]
- Yuan, T.; Meng, T.; He, P.; Shi, Y.; Li, Y.; Li, X.; Fan, L.; Yang, S. Carbon quantum dots: An emerging material for optoelectronic applications. J. Mater. Chem. C 2019, 7, 6820–6835. [Google Scholar] [CrossRef]
- Xiong, Y.; Schneider, J.; Ushakova, E.V.; Rogach, A.L. Influence of molecular fluorophores on the research field of chemically synthesized carbon dots. Nano Today 2018, 23, 124–139. [Google Scholar] [CrossRef]
- Yu, J.; Liu, C.; Yuan, K.; Lu, Z.; Cheng, Y.; Li, L.; Zhang, X.; Jin, P.; Meng, F.; Liu, H. Luminescence mechanism of carbon dots by tailoring functional groups for sensing Fe3+ ions. Nanomaterials 2018, 8, 233. [Google Scholar] [CrossRef]
- Ding, H.; Li, X.-H.; Chen, X.-B.; Wei, J.-S.; Li, X.-B.; Xiong, H.-M. Surface states of carbon dots and their influences on luminescence. J. Appl. Phys. 2020, 127, 231101. [Google Scholar] [CrossRef]
- Ding, H.; Yu, S.-B.; Wei, J.-S.; Xiong, H.-M. Full-color light-emitting carbon dots with a surface-state-controlled luminescence mechanism. ACS Nano 2016, 10, 484–491. [Google Scholar] [CrossRef]
- Yuan, B.; Guan, S.; Sun, X.; Li, X.; Zeng, H.; Xie, Z.; Chen, P.; Zhou, S. Highly efficient carbon dots with reversibly switchable green–red emissions for trichromatic white light-emitting diodes. ACS Appl. Mater. Interfaces 2018, 10, 16005–16014. [Google Scholar] [CrossRef]
- Gude, V.; Das, A.; Chatterjee, T.; Mandal, P.K. Molecular origin of photoluminescence of carbon dots: Aggregation-induced orange-red emission. Phys. Chem. Chem. Phys. 2016, 18, 28274–28280. [Google Scholar] [CrossRef]
- Liu, M.L.; Yang, L.; Li, R.S.; Chen, B.B.; Liu, H.; Huang, C.Z. Large-scale simultaneous synthesis of highly photoluminescent green amorphous carbon nanodots and yellow crystalline graphene quantum dots at room temperature. Green Chem. 2017, 19, 3611–3617. [Google Scholar] [CrossRef]
- Kundelev, E.V.; Tepliakov, N.V.; Leonov, M.Y.; Maslov, V.G.; Baranov, A.V.; Fedorov, A.V.; Rukhlenko, I.D.; Rogach, A.L. Amino functionalization of carbon dots leads to red emission enhancement. J. Phys. Chem. Lett. 2019, 10, 5111–5116. [Google Scholar] [CrossRef]
- Li, Y.; Lin, H.; Luo, C.; Wang, Y.; Jiang, C.; Qi, R.; Huang, R.; Travas-sejdic, J.; Peng, H. Aggregation induced red shift emission of phosphorus doped carbon dots. Rsc Adv. 2017, 7, 32225–32228. [Google Scholar] [CrossRef]
- Arul, V.; Sethuraman, M.G. Facile green synthesis of fluorescent N-doped carbon dots from Actinidia deliciosa and their catalytic activity and cytotoxicity applications. Opt. Mater. 2018, 78, 181–190. [Google Scholar] [CrossRef]
- Lu, S.; Sui, L.; Wu, M.; Zhu, S.; Yong, X.; Yang, B. Graphitic nitrogen and high-crystalline triggered strong photoluminescence and room-temperature ferromagnetism in carbonized polymer dots. Adv. Sci. 2019, 6, 1801192. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yuan, F.; Li, X.; Li, Y.; Zhong, H.; Fan, L.; Yang, S. 53% efficient red emissive carbon quantum dots for high color rendering and stable warm white-light-emitting diodes. Adv. Mater. 2017, 29, 1702910. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Liu, C.; Zhang, Z.L.; Pang, D.W. Photoluminescence-tunable carbon nanodots: Surface-state energy-gap tuning. Adv. Mater. 2015, 27, 1663–1667. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhu, M.; Huang, H.; Liu, Y.; Kang, Z. Advances, challenges and promises of carbon dots. Inorg. Chem. Front. 2017, 4, 1963–1986. [Google Scholar] [CrossRef]
- Liu, K.K.; Song, S.Y.; Sui, L.Z.; Wu, S.X.; Jing, P.T.; Wang, R.Q.; Li, Q.Y.; Wu, G.R.; Zhang, Z.Z.; Yuan, K.J. Efficient red/near-infrared-emissive carbon nanodots with multiphoton excited upconversion fluorescence. Adv. Sci. 2019, 6, 1900766. [Google Scholar] [CrossRef]
- Li, D.; Liang, C.; Ushakova, E.V.; Sun, M.; Huang, X.; Zhang, X.; Jing, P.; Yoo, S.J.; Kim, J.G.; Liu, E. Thermally activated upconversion near-infrared photoluminescence from carbon dots synthesized via microwave assisted exfoliation. Small 2019, 15, 1905050. [Google Scholar] [CrossRef]
- Su, R.; Guan, Q.; Cai, W.; Yang, W.; Xu, Q.; Guo, Y.; Zhang, L.; Fei, L.; Xu, M. Multi-color carbon dots for white light-emitting diodes. Rsc Adv. 2019, 9, 9700–9708. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Zhan, L.; Liu, Y.; Huang, C. One-step synthesis of fluorescent hydroxyls-coated carbon dots with hydrothermal reaction and its application to optical sensing of metal ions. Sci. China Chem. 2011, 54, 1342. [Google Scholar] [CrossRef]
- Tsubokawa, N.; Hosoya, M. Reactive carbon black having acyl imidazole or acid anhydride groups: Preparation and reaction with functional polymers having hydroxyl or amino groups. React. Polym. 1991, 14, 33–40. [Google Scholar] [CrossRef]
- Ogi, T.; Aishima, K.; Permatasari, F.A.; Iskandar, F.; Tanabe, E.; Okuyama, K. Kinetics of nitrogen-doped carbon dot formation via hydrothermal synthesis. New J. Chem. 2016, 40, 5555–5561. [Google Scholar] [CrossRef]
- Gong, X.; Zhang, Q.; Gao, Y.; Shuang, S.; Choi, M.M.; Dong, C. Phosphorus and nitrogen dual-doped hollow carbon dot as a nanocarrier for doxorubicin delivery and biological imaging. ACS Appl. Mater. Interfaces 2016, 8, 11288–11297. [Google Scholar] [CrossRef]
- Wang, S.; Yang, D.-S.; Yang, F. Nitrogen-induced shift of photoluminescence from green to blue emission for xylose-derived carbon dots. Nano Express 2020, 1, 020018. [Google Scholar] [CrossRef]
- Li, F.; Yang, D.; Xu, H. Non-metal-heteroatom-doped carbon dots: Synthesis and properties. Chem. –A Eur. J. 2019, 25, 1165–1176. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, J. Bioinspired thiol functionalized carbon dots for rapid detection of lead (II) ions in human serum. Opt. Mater. 2020, 99, 109514. [Google Scholar] [CrossRef]
- Zhou, J.; Shan, X.; Ma, J.; Gu, Y.; Qian, Z.; Chen, J.; Feng, H. Facile synthesis of P-doped carbon quantum dots with highly efficient photoluminescence. Rsc Adv. 2014, 4, 5465–5468. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, S.J.; Wang, H.Y.; Qu, S.N.; Zhang, Y.L.; Zhang, J.H.; Chen, Q.D.; Xu, H.L.; Han, W.; Yang, B.; et al. Common Origin of Green Luminescence in Carbon Nanodots and Graphene Quantum Dots. Acs Nano 2014, 8, 2541–2547. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Tong, C. Nitrogen-and sulfur-codoped carbon dots for highly selective and sensitive fluorescent detection of Hg2+ ions and sulfide in environmental water samples. J. Agric. Food Chem. 2019, 67, 2794–2800. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Zhang, Y.; Li, G.; Li, B. Fluorescent determination of mercury (II) by green carbon quantum dots synthesized from eggshell membrane. Anal. Lett. 2020, 53, 2841–2853. [Google Scholar] [CrossRef]
- Vandarkuzhali, S.A.A.; Natarajan, S.; Jeyabalan, S.; Sivaraman, G.; Singaravadivel, S.; Muthusubramanian, S.; Viswanathan, B. Pineapple peel-derived carbon dots: Applications as sensor, molecular keypad lock, and memory device. ACS Omega 2018, 3, 12584–12592. [Google Scholar] [CrossRef] [PubMed]
- Atchudan, R.; Edison, T.N.J.I.; Aseer, K.R.; Perumal, S.; Karthik, N.; Lee, Y.R. Highly fluorescent nitrogen-doped carbon dots derived from Phyllanthus acidus utilized as a fluorescent probe for label-free selective detection of Fe3+ ions, live cell imaging and fluorescent ink. Biosens. Bioelectron. 2018, 99, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Yantasee, W.; Lin, Y.; Hongsirikarn, K.; Fryxell, G.E.; Addleman, R.; Timchalk, C. Electrochemical sensors for the detection of lead and other toxic heavy metals: The next generation of personal exposure biomonitors. Environ. Health Perspect. 2007, 115, 1683–1690. [Google Scholar] [CrossRef]
- Bhatia, M.; Gupta, R.; Srivastava, S. Migraine associated with water deprivation and progressive myopia. Cephalalgia 2006, 26, 758–760. [Google Scholar] [CrossRef]
- Papanikolaou, N.C.; Hatzidaki, E.G.; Belivanis, S.; Tzanakakis, G.N.; Tsatsakis, A.M. Lead toxicity update. A brief review. Med. Sci. Monit. 2005, 11, RA329. [Google Scholar]
- Niu, X.; Liu, Y.; Wang, F.; Luo, D. Highly sensitive and selective optical sensor for lead ion detection based on liquid crystal decorated with DNAzyme. Opt. Express 2019, 27, 30421–30428. [Google Scholar] [CrossRef]
- Chauhan, P.; Chaudhary, S.; Kumar, R. Biogenic approach for fabricating biocompatible carbon dots and their application in colorimetric and fluorometric sensing of lead ion. J. Clean. Prod. 2021, 279, 123639. [Google Scholar] [CrossRef]
- Radhakrishnan, K.; Panneerselvam, P.; Marieeswaran, M. A green synthetic route for the surface-passivation of carbon dots as an effective multifunctional fluorescent sensor for the recognition and detection of toxic metal ions from aqueous solution. Anal. Methods 2019, 11, 490–506. [Google Scholar] [CrossRef]
- Ansi, V.; Renuka, N.K. Table sugar derived Carbon dot–a naked eye sensor for toxic Pb2+ ions. Sens. Actuators B Chem. 2018, 264, 67–75. [Google Scholar] [CrossRef]
- Bala, T.; Prasad, B.; Sastry, M.; Kahaly, M.U.; Waghmare, U.V. Interaction of different metal ions with carboxylic acid group: A quantitative study. J. Phys. Chem. A 2007, 111, 6183–6190. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Q.; Li, J.; Lei, M.; Yan, X. Selective and sensitive chemosensor for lead ions using fluorescent carbon dots prepared from chocolate by one-step hydrothermal method. Sens. Actuators B Chem. 2016, 237, 597–604. [Google Scholar] [CrossRef]
- Pudza, M.Y.; Abidin, Z.Z.; Abdul-Rashid, S.; Yasin, F.M.; Noor, A.S.M.; Abdullah, J. Selective and simultaneous detection of cadmium, lead and copper by tapioca-derived carbon dot–modified electrode. Environ. Sci. Pollut. Res. 2020, 27, 13315–13324. [Google Scholar] [CrossRef]
- Paladini, F.; Pollini, M. Antimicrobial silver nanoparticles for wound healing application: Progress and future trends. Materials 2019, 12, 2540. [Google Scholar] [CrossRef]
- Chen, D.; Qiao, X.; Qiu, X.; Chen, J. Synthesis and electrical properties of uniform silver nanoparticles for electronic applications. J. Mater. Sci. 2009, 44, 1076–1081. [Google Scholar] [CrossRef]
- Syed, S. Silver recovery aqueous techniques from diverse sources: Hydrometallurgy in recycling. Waste Manag. 2016, 50, 234–256. [Google Scholar] [CrossRef]
- Sánchez-Pomales, G.; Mudalige, T.K.; Lim, J.-H.; Linder, S.W. Rapid determination of silver in nanobased liquid dietary supplements using a portable X-ray fluorescence analyzer. J. Agric. Food Chem. 2013, 61, 7250–7257. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Chen, M.; Hu, L.; Chen, X.; Wang, J. Growth and stabilization of silver nanoparticles on carbon dots and sensing application. Langmuir 2013, 29, 16135–16140. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Bai, Z.; Zu, F.; Zhang, Y.; Sun, X.; Ma, T.; Chen, L. Yellow-emissive carbon dots with a large Stokes shift are viable fluorescent probes for detection and cellular imaging of silver ions and glutathione. Microchim. Acta 2019, 186, 113. [Google Scholar] [CrossRef] [PubMed]
- Correia, C.; Martinho, J.; Maçôas, E. A Fluorescent Nanosensor for Silver (Ag+) and Mercury (Hg2+) Ions Using Eu (III)-Doped Carbon Dots. Nanomaterials 2022, 12, 385. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.M.F.; Ginjom, I.R.; Ngu-Schwemlein, M.; Ng, S.M. Synthesis of yellow fluorescent carbon dots and their application to the determination of chromium (III) with selectivity improved by pH tuning. Microchim. Acta 2016, 183, 1899–1907. [Google Scholar] [CrossRef]
- Huang, Q.; Bao, Q.; Wu, C.; Hu, M.; Chen, Y.; Wang, L.; Chen, W. Carbon dots derived from Poria cocos polysaccharide as an effective “on-off” fluorescence sensor for chromium (VI) detection. J. Pharm. Anal. 2022, 12, 104–112. [Google Scholar] [CrossRef]
- Ganz, T.; Nemeth, E. Eminars in Hematology; Elsevier: Amsterdam, The Netherlands, 2009; pp. 387–393. [Google Scholar]
- Simcox, J.A.; McClain, D.A. Iron and diabetes risk. Cell Metab. 2013, 17, 329–341. [Google Scholar] [CrossRef]
- Liu, G.; Li, B.; Liu, Y.; Feng, Y.; Jia, D.; Zhou, Y. Rapid and high yield synthesis of carbon dots with chelating ability derived from acrylamide/chitosan for selective detection of ferrous ions. Appl. Surf. Sci. 2019, 487, 1167–1175. [Google Scholar] [CrossRef]
- Swarnkar, S.; Gupta, B.; Sekharan, R. Iron control in zinc plant residue leach solution. Hydrometallurgy 1996, 42, 21–26. [Google Scholar] [CrossRef]
- Shi, J.; Ni, G.; Tu, J.; Jin, X.; Peng, J. Green synthesis of fluorescent carbon dots for sensitive detection of Fe2+ and hydrogen peroxide. J. Nanoparticle Res. 2017, 19, 209. [Google Scholar] [CrossRef]
- Shah, H.; Xin, Q.; Jia, X.; Gong, J.R. Single precursor-based luminescent nitrogen-doped carbon dots and their application for iron (III) sensing. Arab. J. Chem. 2019, 12, 1083–1091. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Chatterjee, S.; Prajapati, R.; Mukherjee, T.K. Size-dependent penetration of carbon dots inside the ferritin nanocages: Evidence for the quantum confinement effect in carbon dots. Phys. Chem. Chem. Phys. 2015, 17, 12833–12840. [Google Scholar] [CrossRef]
- Wu, H.; Pang, L.-F.; Fu, M.-J.; Guo, X.-F.; Wang, H. Boron and nitrogen codoped carbon dots as fluorescence sensor for Fe3+ with improved selectivity. J. Pharm. Biomed. Anal. 2020, 180, 113052. [Google Scholar] [CrossRef]
- Yan, F.; Zu, F.; Xu, J.; Zhou, X.; Bai, Z.; Ma, C.; Luo, Y.; Chen, L. Fluorescent carbon dots for ratiometric detection of curcumin and ferric ion based on inner filter effect, cell imaging and PVDF membrane fouling research of iron flocculants in wastewater treatment. Sens. Actuators B Chem. 2019, 287, 231–240. [Google Scholar] [CrossRef]
- Zhao, P.; Zhang, Q.; Cao, J.; Qian, C.; Ye, J.; Xu, S.; Zhang, Y.; Li, Y. Facile and Green Synthesis of Highly Fluorescent Carbon Quantum Dots from Water Hyacinth for the Detection of Ferric Iron and Cellular Imaging. Nanomaterials 2022, 12, 1528. [Google Scholar] [CrossRef] [PubMed]
- De Romaña, D.L.; Olivares, M.; Uauy, R.; Araya, M. Risks and benefits of copper in light of new insights of copper homeostasis. J. Trace Elem. Med. 2011, 25, 3–13. [Google Scholar] [CrossRef]
- Wang, P.; Yuan, Y.; Xu, K.; Zhong, H.; Yang, Y.; Jin, S.; Yang, K.; Qi, X. Biological applications of copper-containing materials. Bioact. Mater. 2021, 6, 916–927. [Google Scholar] [CrossRef]
- Salinas-Castillo, A.; Ariza-Avidad, M.; Pritz, C.; Camprubí-Robles, M.; Fernández, B.; Ruedas-Rama, M.J.; Megia-Fernández, A.; Lapresta-Fernández, A.; Santoyo-Gonzalez, F.; Schrott-Fischer, A.; et al. Carbon dots for copper detection with down and upconversion fluorescent properties as excitation sources. Chem. Commun. 2013, 49, 1103–1105. [Google Scholar] [CrossRef]
- Dang, V.D.; Ganganboina, A.B.; Doong, R.-A. Bipyridine-and Copper-Functionalized N-doped Carbon Dots for Fluorescence Turn Off–On Detection of Ciprofloxacin. ACS Appl. Mater. 2020, 12, 32247–32258. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Liu, W.; Gai, Q.; Tian, Z.; Ren, S. A carbon-dot-based fluorescent probe for the sensitive and selective detection of Copper (II) Ions. ChemistrySelect 2019, 4, 2392–2397. [Google Scholar] [CrossRef]
- Li, X.; Zhang, S.; Kulinich, S.A.; Liu, Y.; Zeng, H. Engineering surface states of carbon dots to achieve controllable luminescence for solid-luminescent composites and sensitive Be2+ detection. Sci. Rep. 2014, 4, 4976. [Google Scholar] [CrossRef]
- Gao, H.; Pang, Y.; Li, L.; Zhu, C.; Ma, C.; Gu, J.; Wu, Y.; Chen, G. One-step synthesis of the nitrogen and sulfur codoped carbon dots for detection of lead and copper ions in aqueous solution. J. Sens. 2020, 2020, 8828456. [Google Scholar] [CrossRef]
- Deng, M.; Wang, S.; Liang, C.; Shang, H.; Jiang, S. A FRET fluorescent nanosensor based on carbon dots for ratiometric detection of Fe3+ in aqueous solution. Rsc Adv. 2016, 6, 26936–26940. [Google Scholar] [CrossRef]
- Shabbir, H.; Tokarski, T.; Ungor, D.; Wojnicki, M. Eco Friendly Synthesis of Carbon Dot by Hydrothermal Method for Metal Ions Salt Identification. Materials 2021, 14, 7604. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.Q.; Barzani, R.K.; Omer, K.M.; Al-Hashimi, B.R.; Mohammadi, S.; Salimi, A. Dual-emitter polymer carbon dots with spectral selection towards nanomolar detection of iron and aluminum ions. Arab. J. Chem. 2021, 14, 103452. [Google Scholar] [CrossRef]
- Shabbir, H.; Wojtaszek, K.; Rutkowski, B.; Csapó, E.; Bednarski, M.; Adamiec, A.; Głuch-Lutwin, M.; Mordyl, B.; Druciarek, J.; Kotańska, M.; et al. Milk-Derived Carbon Quantum Dots: Study of Biological and Chemical Properties Provides Evidence of Toxicity. Molecules 2022, 27, 8728. [Google Scholar] [CrossRef] [PubMed]
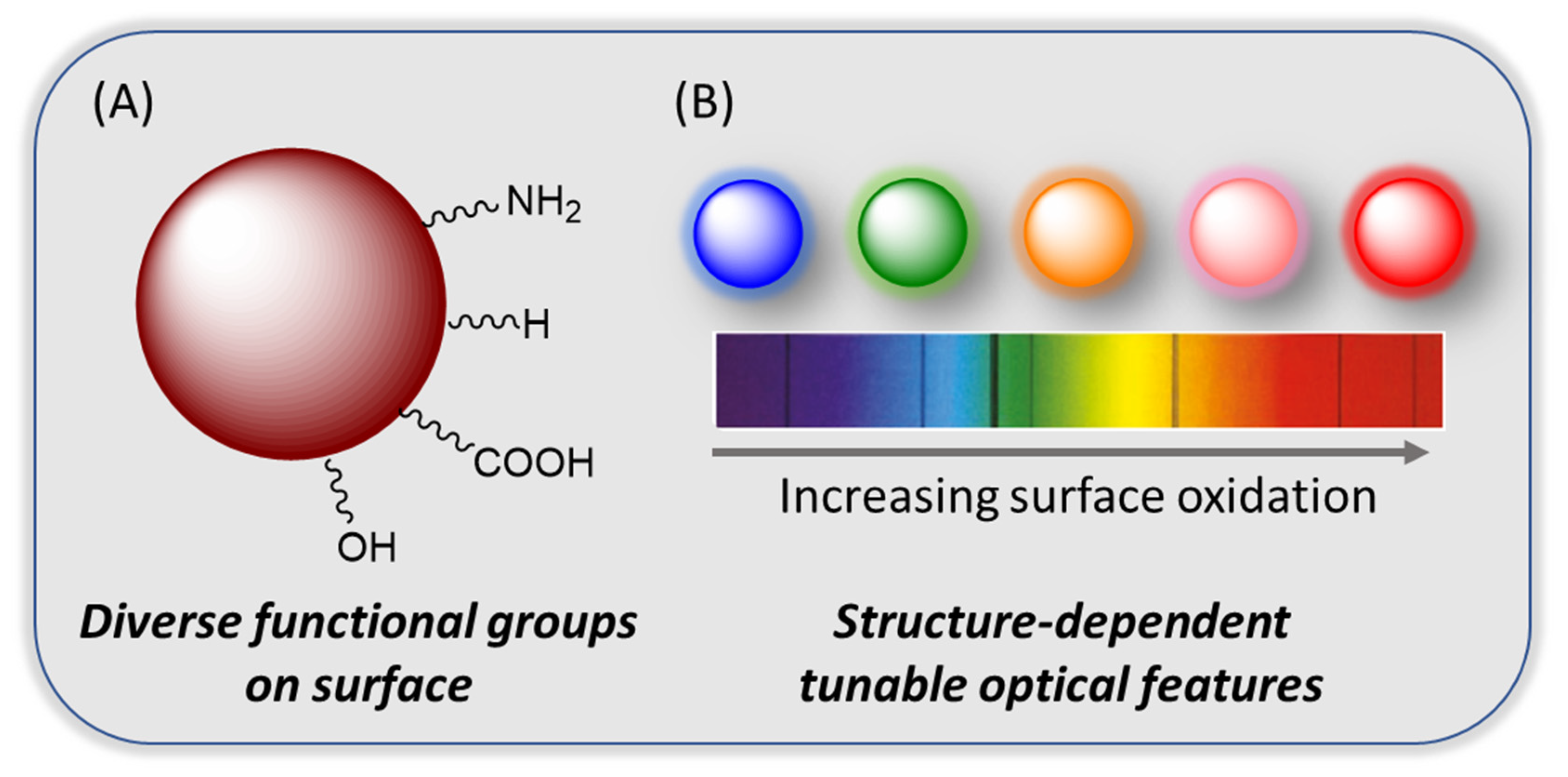
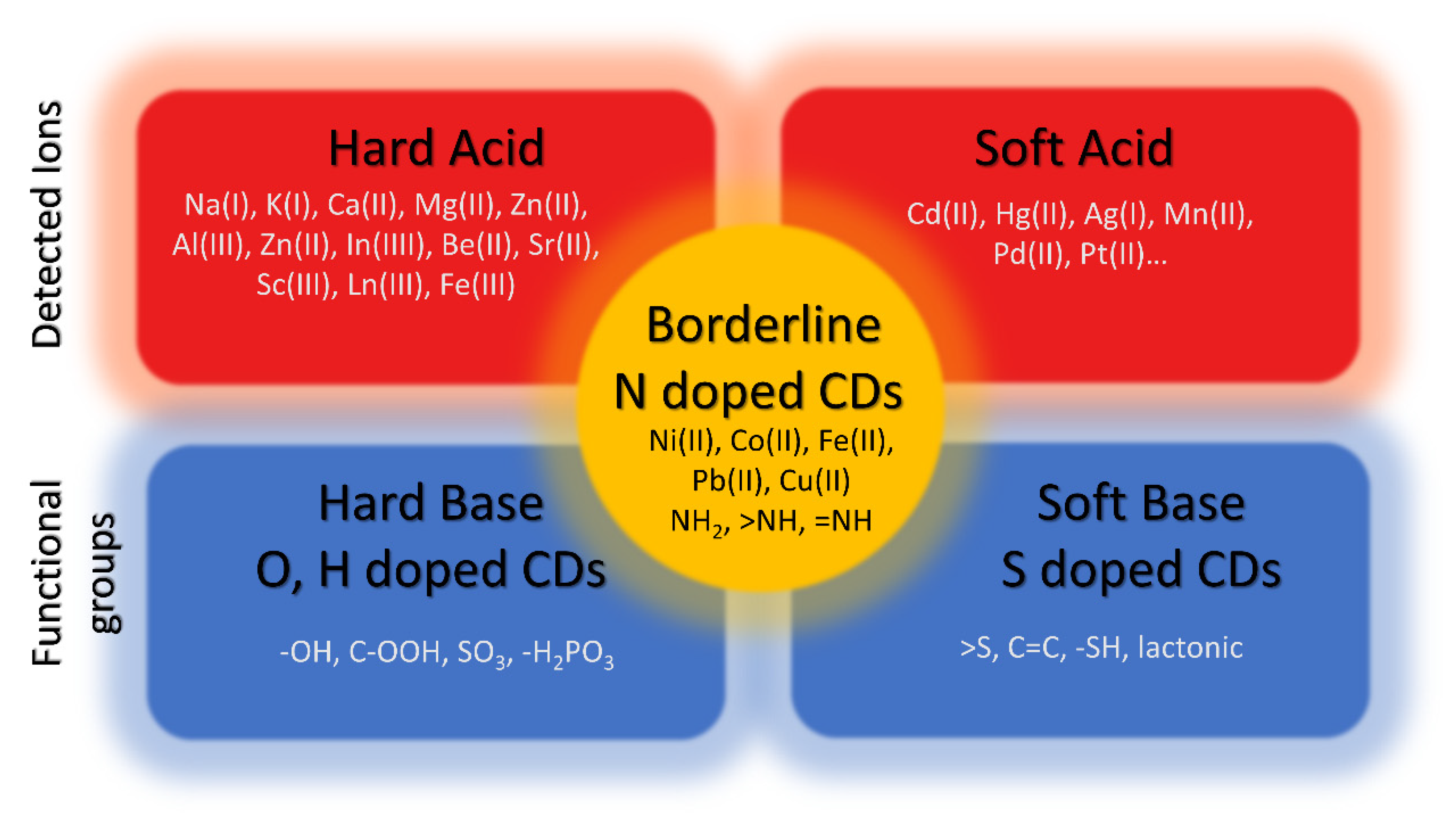
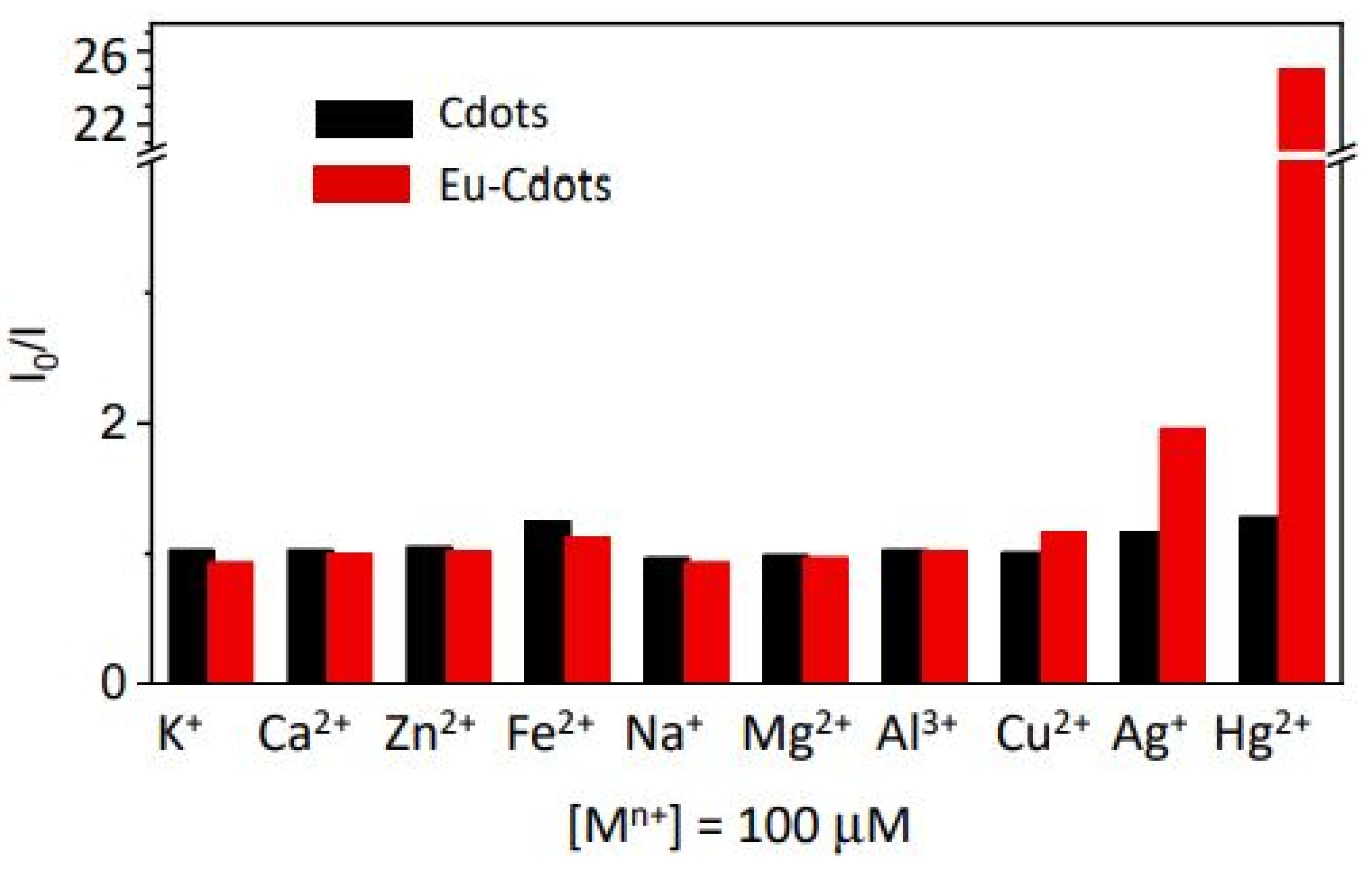
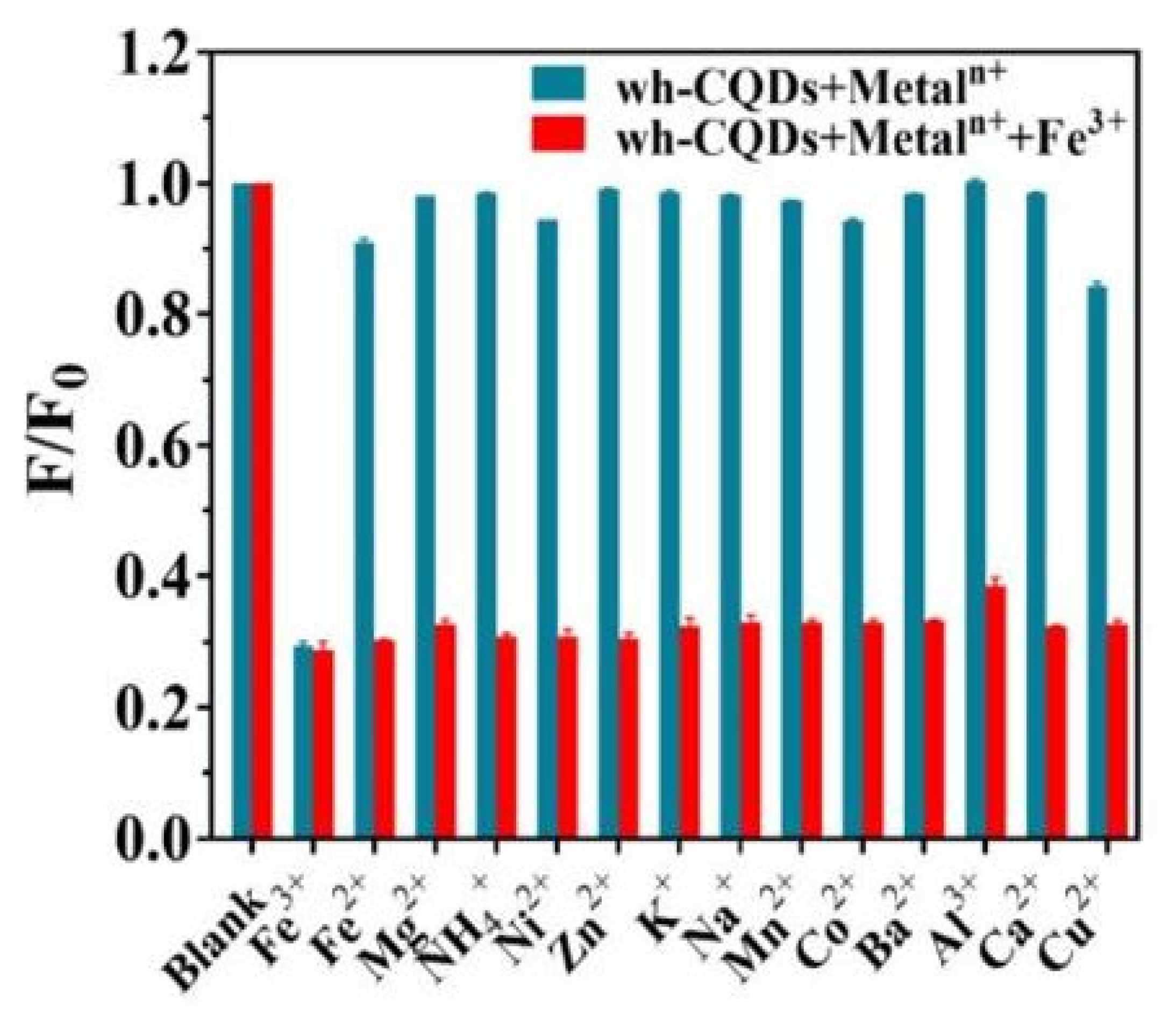
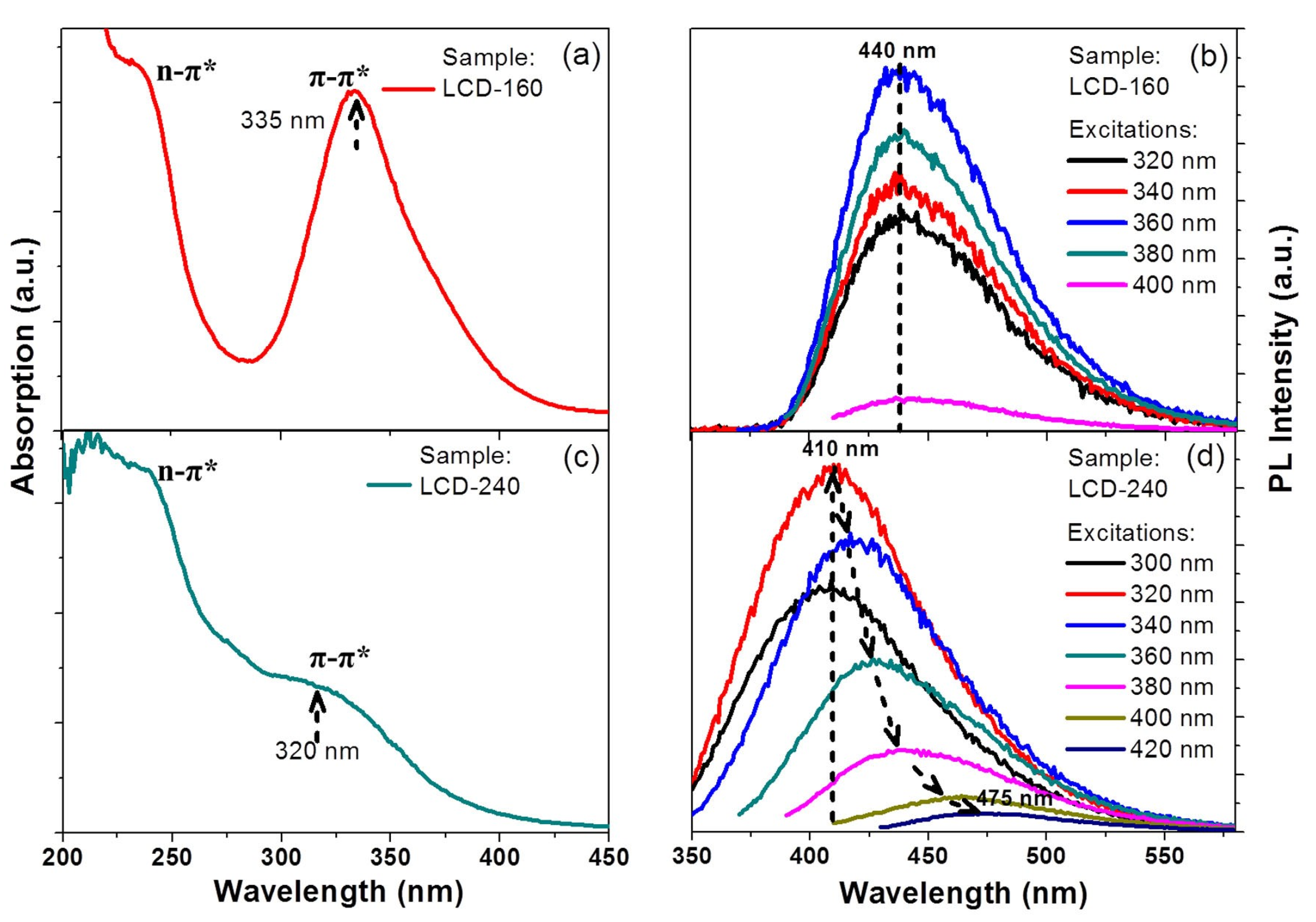
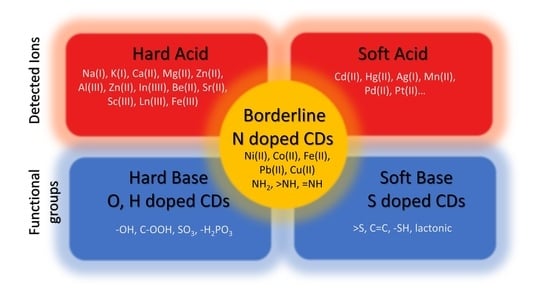
| Precursor | Metal to Detect | Other Metal Ions Used for Detection | Reference |
|---|---|---|---|
| L-lysine and L-glutathione | Pb2+ | Ag+, Ca2+, Cd2+, Fe2+, Hg2+, In2+, Pb2+, Mn2+, Ni2+, Zn2+ and Fe3+ | [82] |
| Seeds of pearl millet | Pb2+ | Ag+, Cd2+, Cu2+, Fe3+, Co2+, Pb2+, Zn2+,Mg2+, Ni2+, Ca2+, Fe2+, Ba2+, NH4+, Na+ and K+ | [93] |
| Coccinia indica | Hg2+, Cu2+, Pb2+ and Fe3+ | Ag+, K+, Ca2+, Cu2+, Ni2+, Ba2+, Pb2+, Hg2+, Cd2+, Co2+, Fe2+ and As3+ | [94] |
| Table sugar | Pb2+ | Cd2+, Hg2+, Cu2+, Fe3+, K+, Na+, Ni2+, Co2+, Cr6+, Mn2+, Ca2+ and Zn2+ | [95] |
| L-cysteine | Pb2+, Cu2+ | Ca2+, Fe2+, Al3+, Pb2+, Mg2+, Zn2+, Fe3+, K+,Cu2+ and Na+ | [124] |
| Chocolate | Pb2+ | Hg2+, Fe3+, Cu2+, As3+, As5+, Mn2+, Zn2+, Al3+, Mg2+, Ni2+, Cd2+, Co2+, Ba2+, Ca2+, Sn2+, Fe2+, Ag+, Na+ and K+ | [97] |
| Tapioca flour | Cd2+, Pb2+ and Cu2+ | Mg2+, K+, Na+, NO3− and SO42− | [98] |
| Citric acid and phenazine diamine | Ag+ | K+, Na+, Zn2+, Mg2+, Ba2+, Co2+, Ni2+, Cu2+, Hg2+, Pb2+, Fe3+, Al3+, Cr3+, As3+ and Ag2+ | [104] |
| N-(2-hydroxyethyl) ethylenediamine triacetic acid (HEDTA) | Fe3+ | Fe3+, Fe2+, Ca2+, Co2+, Cu2+, Mg2+, Mo2+,Zn2+, Ni2+, Na+ and K+ | [113] |
| Sucrose | Cr3+ | Al3+, Ca2+, Mg2+,Co2+, Cu2+, Cr3+, Pb2+, Hg2+, Ni2+, Sn2+ and Zn2+ | [106] |
| Alkali-soluble Poria and Cocos polysaccharide | Cr6+ | Ag+, Ba2+, Ca2+, Cr6+, Cu2+, Fe2+, Fe3+, K+, Mg2+, Mn2+, Na+, Ni2+, Zn2+ and Cr3+ | [107] |
| o-phenylenediamine (OPA) and 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl) benzylchloroformate | Fe3+ | Ag+, Al3+, Ba2+, Ca2+, Cu2+, Fe2+, Cd2+, Co2+, Mg2+, Mn2+, Na+, Pb2+, Sn2+,Zn2+, Hg2+ and K+ | [115] |
| Anhydrous citric acid(CA) as a source of carbon while diethylenetriamine (DETA) | Fe3+ | Ag+, K+, Pb2+, Cu2+, Mn2+, Ba2+, Ca2+, Zn2+, Mg2+, Hg2+, Al3+, Fe2+, Co2+, Ni2+, MnO4− and Cr2O72− | [116] |
| Glucose | Fe3+ | K+, Na+, Ag+, Ca2+, Ba2+, Cd2+, Co2+, Cu2+, Fe2+, Mn2+, Ni2+, Pb2+, Zn2+, Hg2+, Al3+ and Cr3+ | [125] |
| Ascorbic acid | Fe3+ | Ni2+, Co2+, Zn2+, Mg2+, Li+, Fe3+, Cu2+ and Al3+ | [126] |
| Urea | Fe3+ | C, sugars, Na+, Mg2+,Ca2+, and Cl− | [127] |
| Citric acid and ethylenediamine | Cu2+ | Cr3+, Mn2+, Ni2+ and Pb2+ | [121] |
| Citric acid and Polyethyleneimine | Cu2+ | Na+, Al3+, Mg2+, Mn2+, Li+, K+, Co2+, Sb3+, Cd2+, Zn2+, Hg+, Fe2+, Fe3+ and Cr3+ | [122] |
| Citric acid and urea | Be2+ | K+, Zn2+, Al3+, Mn2+, Mg2+, Cu2+, Na+ and Ca2+ | [123] |
| Metal Ion Name and Type | Frequency of Occurrence | Functional Group |
|---|---|---|
| Be2+ (Hard) | 1 | Amino-groups |
| Fe3+(Hard) | 7 | Nitrogen, Carbon and oxygen based functional group |
| Cr6+ (Hard) | 1 | Nitrogen, Carbon and oxygen based functional group |
| Cr3+ (Hard) | 1 | Nitrogen, Carbon and oxygen based functional group |
| Pb2+ (Borderline) | 7 | Amine, Carboxyl and Thiol, Carboxylate, Hydroxyl and Epoxy |
| Cu2+ (Borderline) | 5 | Hydroxy and Amino groups |
| Ag+ (Soft) | 1 | Nitrogen, Carbon and oxygen based functional group |
| Cd2+ (Soft) | 1 | Nitrogen, Carbon and oxygen based functional group |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shabbir, H.; Csapó, E.; Wojnicki, M. Carbon Quantum Dots: The Role of Surface Functional Groups and Proposed Mechanisms for Metal Ion Sensing. Inorganics 2023, 11, 262. https://doi.org/10.3390/inorganics11060262
Shabbir H, Csapó E, Wojnicki M. Carbon Quantum Dots: The Role of Surface Functional Groups and Proposed Mechanisms for Metal Ion Sensing. Inorganics. 2023; 11(6):262. https://doi.org/10.3390/inorganics11060262
Chicago/Turabian StyleShabbir, Hasan, Edit Csapó, and Marek Wojnicki. 2023. "Carbon Quantum Dots: The Role of Surface Functional Groups and Proposed Mechanisms for Metal Ion Sensing" Inorganics 11, no. 6: 262. https://doi.org/10.3390/inorganics11060262
APA StyleShabbir, H., Csapó, E., & Wojnicki, M. (2023). Carbon Quantum Dots: The Role of Surface Functional Groups and Proposed Mechanisms for Metal Ion Sensing. Inorganics, 11(6), 262. https://doi.org/10.3390/inorganics11060262