Low-Temperature Synthesis Approach for Calcium Hydroxyapatite Coatings on Titanium Substrate
Abstract
1. Introduction
2. Results
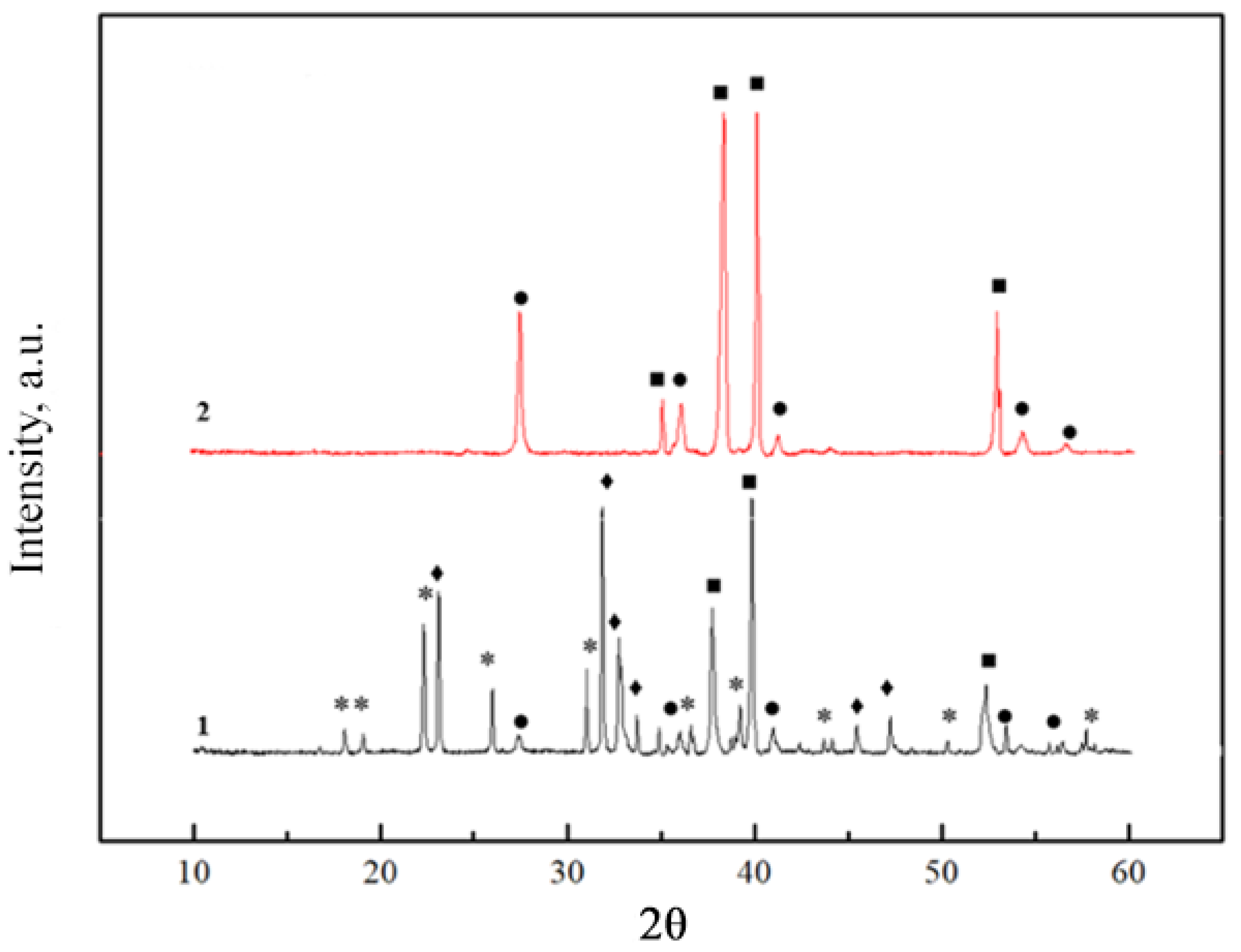
2.1. X-ray Diffraction Analysis
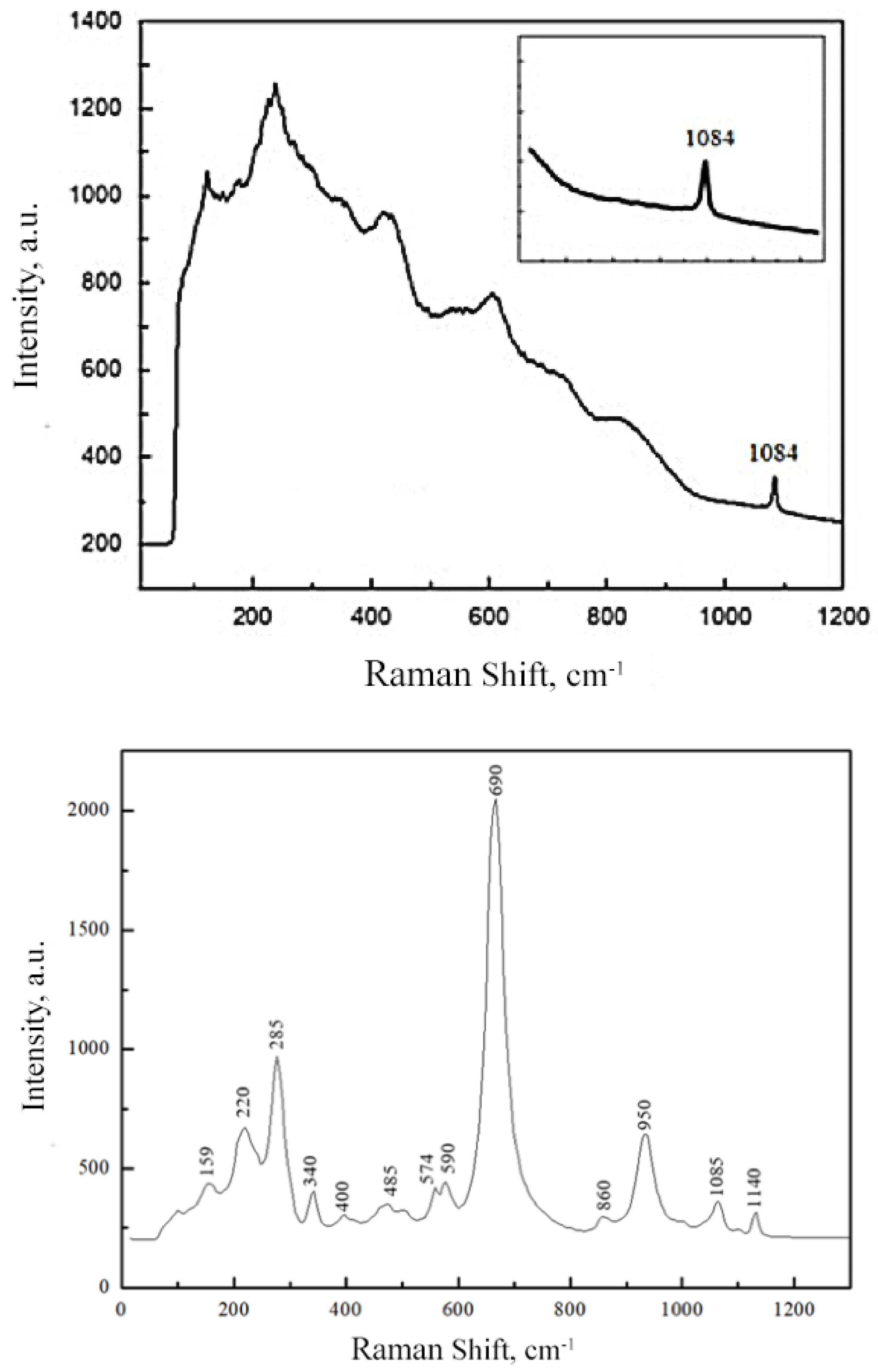
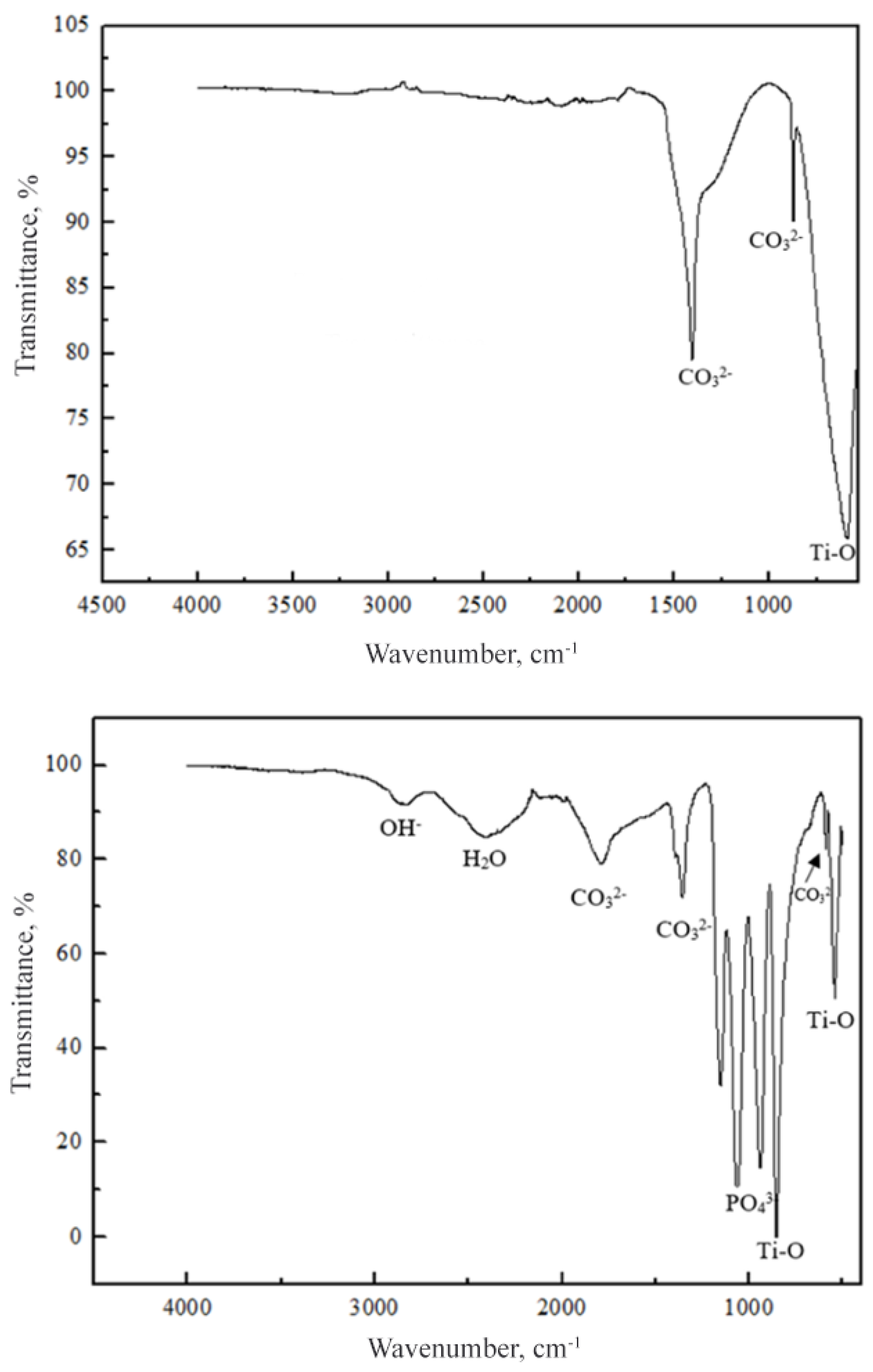
2.2. Raman and FTIR Spectroscopy
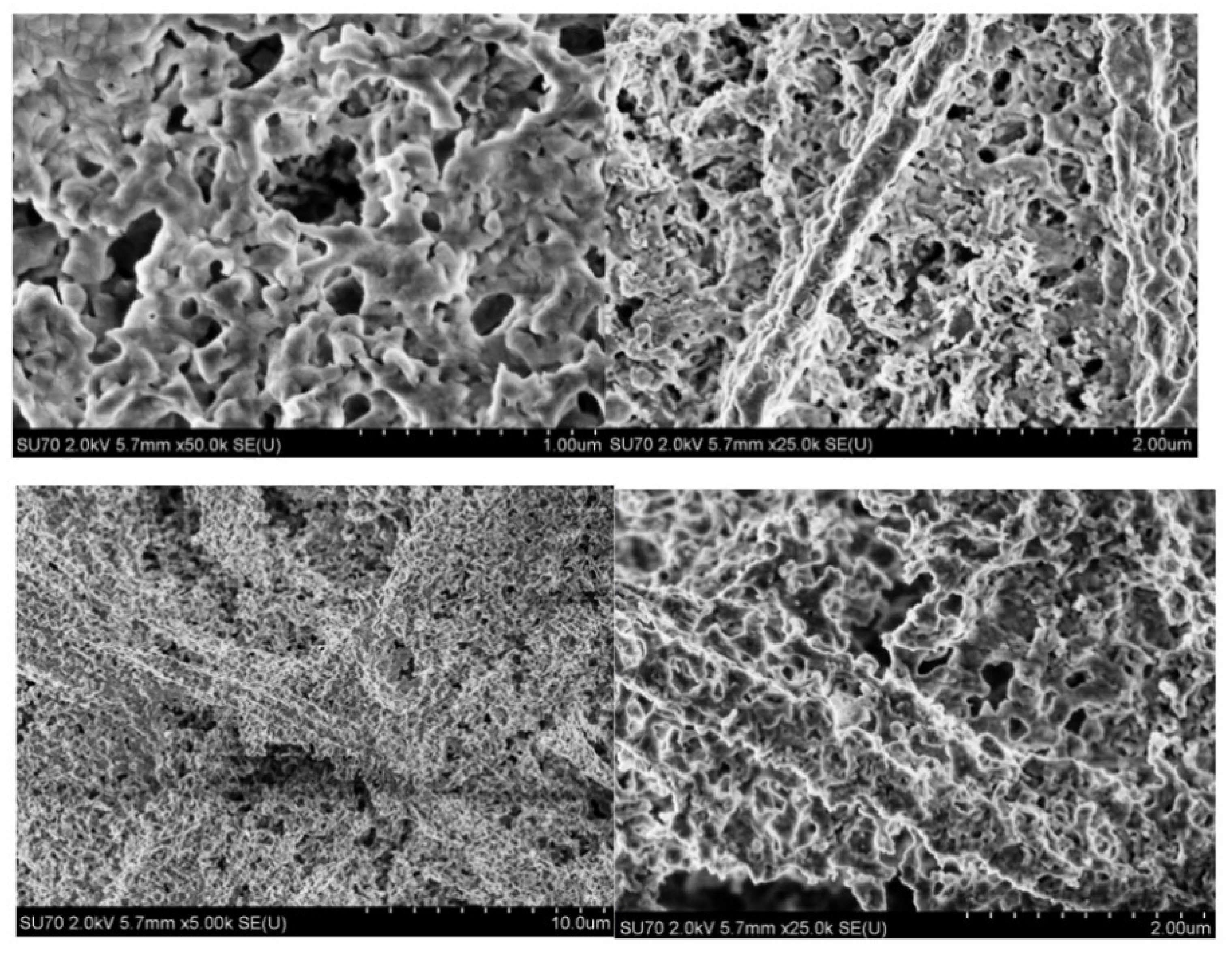
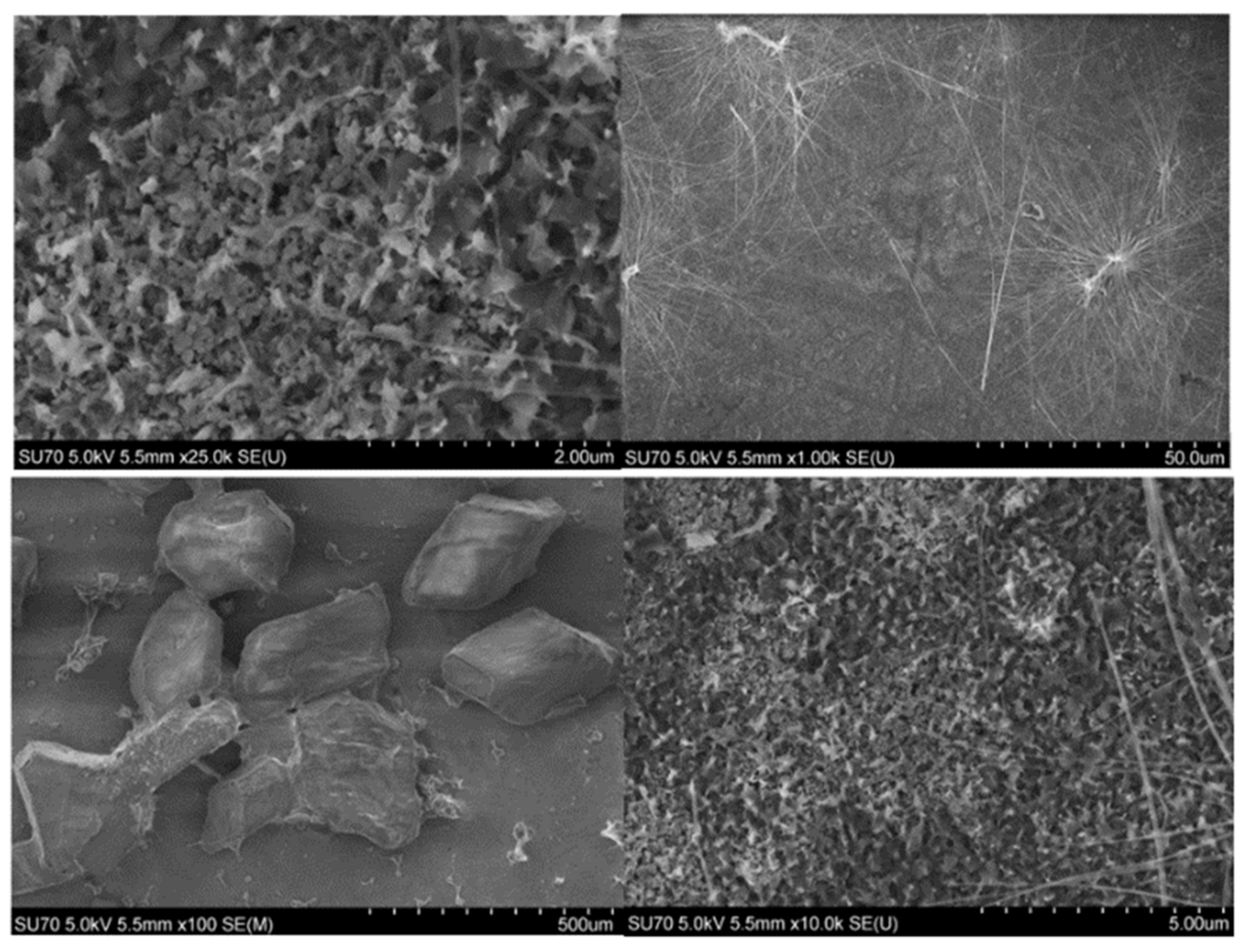
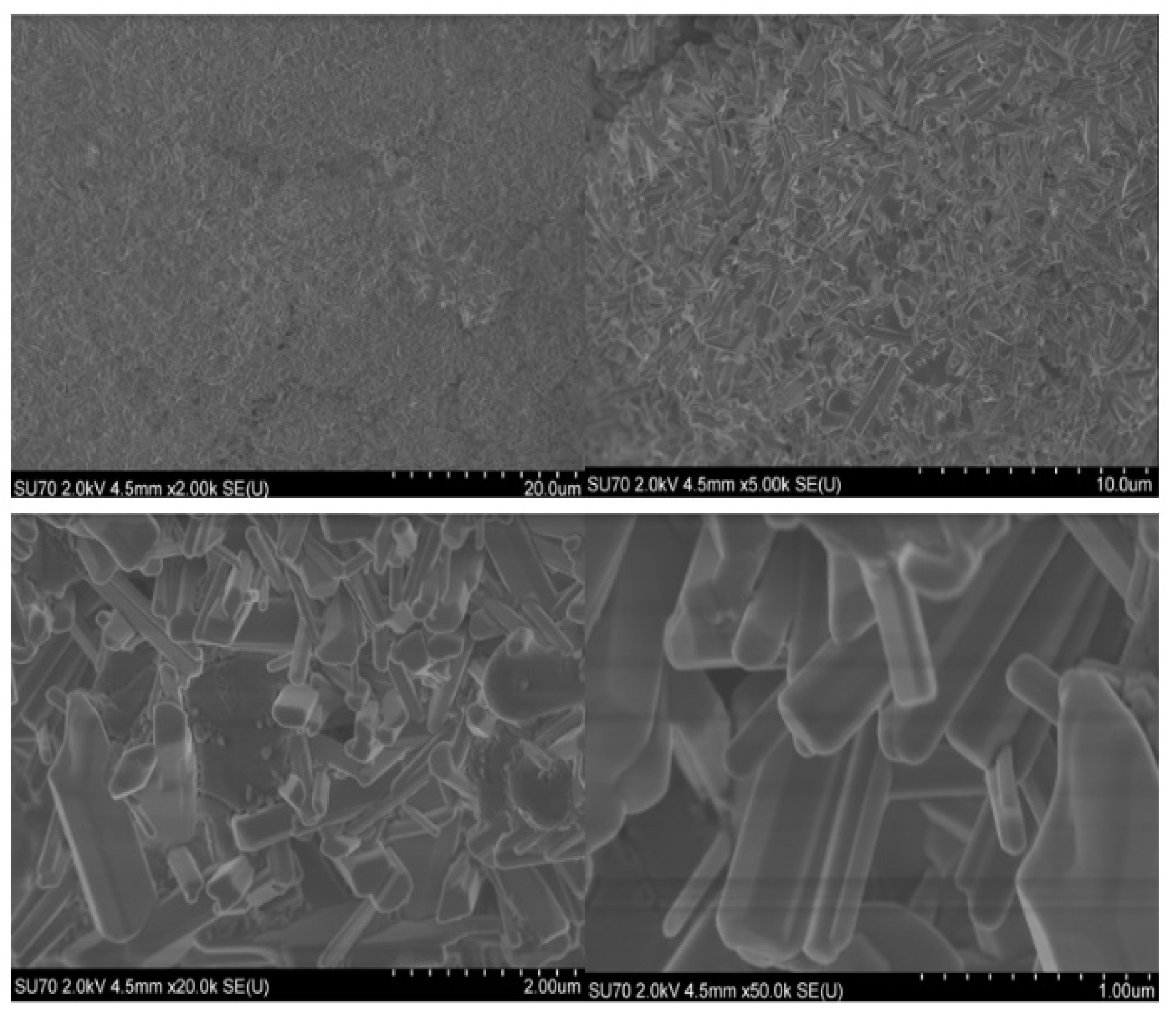
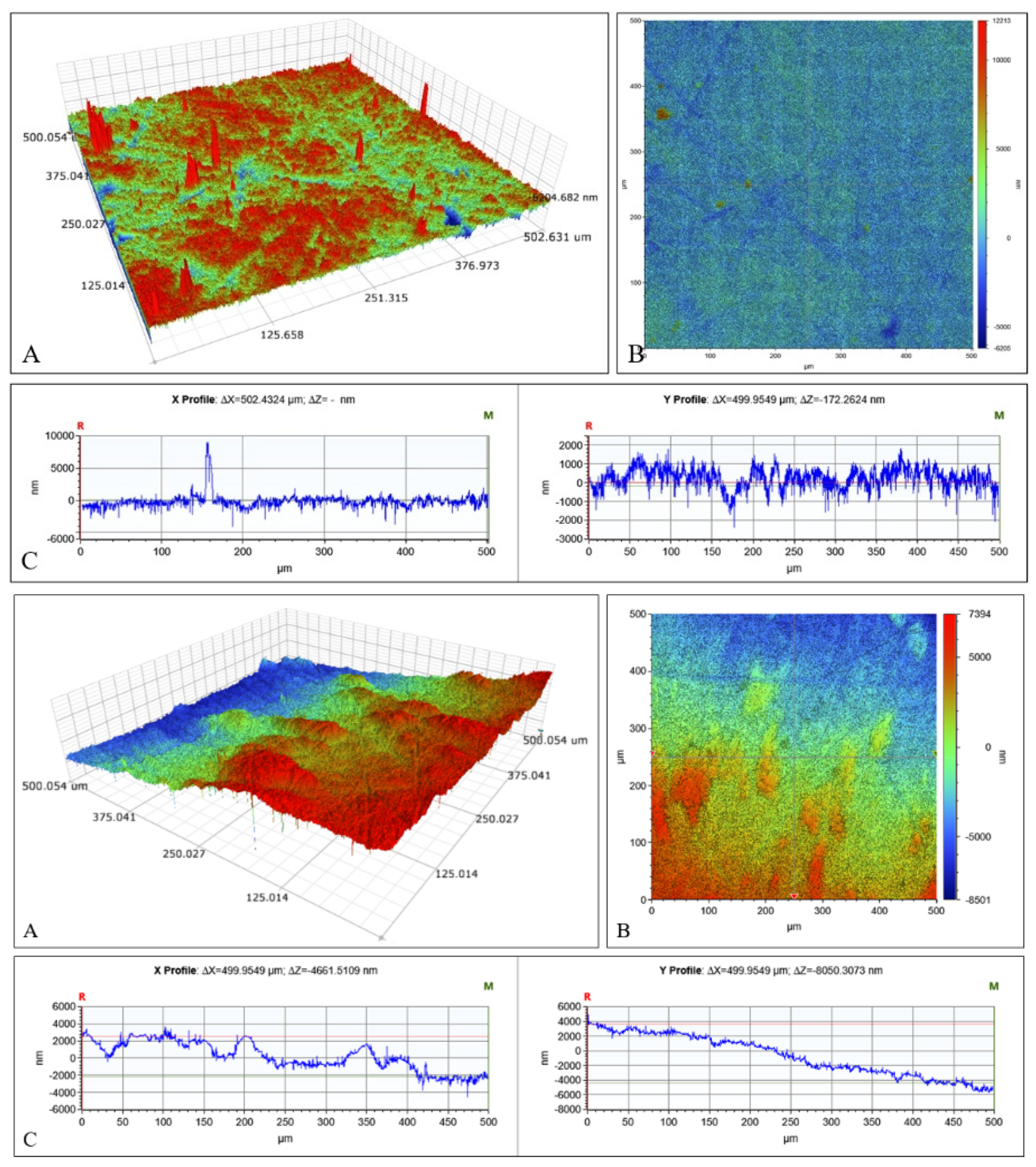
2.3. Microscopical Characterization
2.4. Electrochemical Characterization
3. Experimental
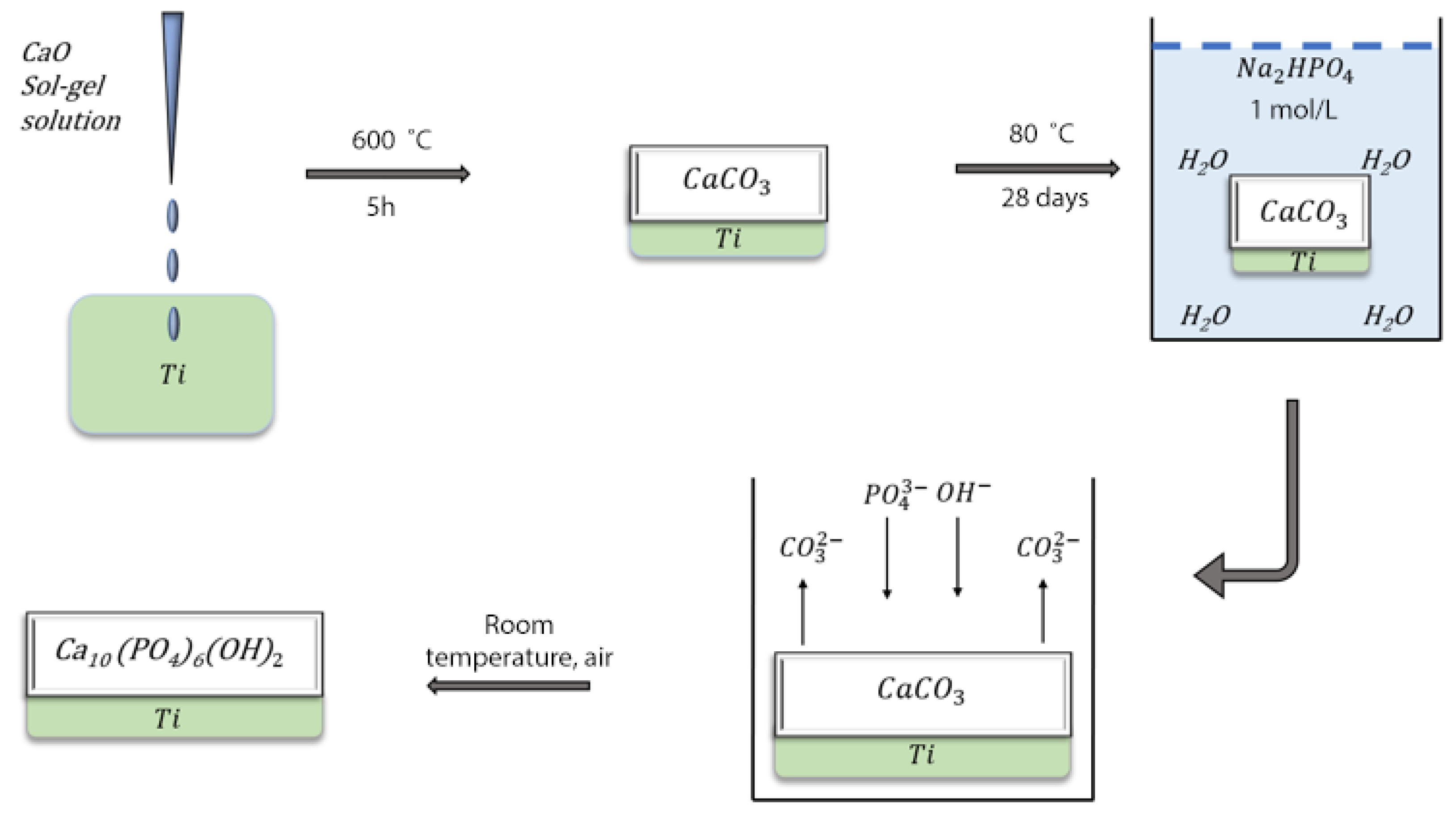
3.1. Materials and Synthesis
3.2. Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ishikawa, K.; Garskaite, E.; Kareiva, A. Sol–gel synthesis of calcium phosphate-based biomaterials—A review of environmentally benign, simple, and effective synthesis routes. J. Sol-Gel Sci. Technol. 2020, 94, 551–572. [Google Scholar] [CrossRef]
- Fox, K.; Tran, P.A.; Tran, N. Recent Advances in Research Applications of Nanophase Hydroxyapatite. ChemPhysChem 2012, 13, 2495–2506. [Google Scholar] [CrossRef] [PubMed]
- Nayak, B.; Samant, A.; Misra, P.K.; Saxena, M. Nanocrystalline Hydroxyapatite: A Potent Material for Adsorption, Biological and Catalytic Studies. Mater. Today Proc. 2019, 9, 689–698. [Google Scholar] [CrossRef]
- Desbord, M.; Soulie, J.; Rey, C.; Combes, C. Tunable Behavior in Solution of Amorphous Calcium Ortho/ Pyrophosphate Materials: An Acellular In Vitro Study. ACS Biomater. Sci Eng. 2022, 8, 2363–2374. [Google Scholar] [CrossRef] [PubMed]
- Mahabole, M.P.; Aiyer, R.C.; Ramakrishna, C.V.; Sreedhar, B.; Khairnar, R.S. Synthesis, characterization and gas sensing property of hydroxyapatite ceramic. Bull. Mater. Sci. 2005, 28, 535–545. [Google Scholar] [CrossRef]
- Monkawa, A.; Ikoma, T.; Yunoki, S.; Yoshioka, T.; Tanaka, J.; Chakarov, D.; Kasemo, B. Fabrication of hydroxyapatite ultra-thin layer on gold surface and its application for quartz crystal microbalance technique. Biomaterials 2006, 27, 5748–5754. [Google Scholar] [CrossRef]
- Petrucelli, G.C.; Kawachi, E.Y.; Kubota, L.T.; Bertran, C.A. Hydroxyapatite-based electrode: A new sensor for phosphate. Anal. Commun. 1996, 33, 227–229. [Google Scholar] [CrossRef]
- Park, C.S.; Ha, T.H.; Kim, M.; Raja, N.; Yun, H.-S.; Sung, M.J.; Kwon, O.S.; Yoon, H.; Lee, C.-S. Fast and sensitive near-infrared fluorescent probes for ALP detection and 3d printed calcium phosphate scaffold imaging in vivo. Biosens. Bioelectron. 2018, 105, 151–158. [Google Scholar] [CrossRef]
- Lahrich, S.; El Mhammedi, M.A. Review—Application of Deficient Apatites Materials in Electrochemical Detection of Heavy Metals: Case of Mercury (II) in Seawater and Fish Samples. J. Electrochem. Soc. 2019, 166, B1567–B1576. [Google Scholar] [CrossRef]
- Casado, G.E.; Ivanchenko, P.; Paul, G.; Bisio, C.; Marchese, L.; Ashrafi, A.M.; Milosavljevic, V.; Degli Esposti, L.; Iafisco, M.; Mino, L. Surface and structural characterization of Cu-exchanged hydroxyapatites and their application in H2O2 electrocatalytic reduction. Appl. Surf. Sci. 2022, 595, 153495. [Google Scholar] [CrossRef]
- Neacsu, I.A.; Arsenie, L.V.; Trusca, R.; Ardelean, I.L.; Mihailescu, N.; Mihailescu, I.N.; Ristoscu, C.; Bleotu, C.; Ficai, A.; Andronescu, E. Biomimetic Collagen/Zn2+-Substituted Calcium Phosphate Composite Coatings on Titanium Substrates as Prospective Bioactive Layer for Implants: A Comparative Study Spin Coating vs. MAPLE. Nanomaterials 2019, 9, 692. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Batra, U. Apatitic Nanopowders and Coatings: A Comprehensive Review. Surf. Rev. Lett. 2021, 29, 2230004. [Google Scholar] [CrossRef]
- Puurunen, R.L. Surface chemistry of atomic layer deposition: A case study for the trimethylaluminum/water process. J. Appl. Phys. 2005, 97, 121301. [Google Scholar] [CrossRef]
- Surmenev, R.A.; Surmeneva, M.A.; Grubova, I.Y.; Chernozem, R.V.; Krause, B.; Baumbach, T.; Loza, K.; Epple, M. RF magnetron sputtering of a hydroxyapatite target: A comparison study on polytetrafluorethylene and titanium substrates. Appl. Surf. Sci. 2017, 414, 335–344. [Google Scholar] [CrossRef]
- Mostafa, N.Y.; Kamel, M.M. Enhancement of adhesion bonding between titanium metal and electrodeposited calcium phosphate. Surf. Eng. Appl. Electrochem. 2016, 52, 520–523. [Google Scholar] [CrossRef]
- Ishikawa, K.; Kareiva, A. Sol–gel synthesis of calcium phosphate-based coatings–A review. CHEMIJA 2020, 31, 25–41. [Google Scholar] [CrossRef]
- Munir, M.U.; Salman, S.; Ihsan, A.; Elsaman, T. Synthesis, Characterization, Functionalization and Bio-Applications of Hydroxyapatite Nanomaterials: An Overview. Int. J. Nanomed. 2022, 17, 1903–1925. [Google Scholar] [CrossRef] [PubMed]
- Chai, C.S.; Ben-Nissan, B. Bioactive nanocrystalline sol-gel hydroxyapatite coatings. J. Mater. Sci. Mater. Electron. 1999, 10, 465–469. [Google Scholar] [CrossRef]
- Usinskas, P.; Stankeviciute, Z.; Beganskiene, A.; Kareiva, A. Sol-gel derived porous and hydrophilic calcium hydroxyapatite coating on modified titanium substrate. Surf. Coatings Technol. 2016, 307, 935–940. [Google Scholar] [CrossRef]
- Ishikawa, K. Carbonate apatite bone replacement: Learn from the bone. J. Ceram. Soc. Jpn. 2019, 127, 595–601. [Google Scholar] [CrossRef]
- Grigoraviciute-Puroniene, I.; Tanaka, Y.; Vegelyte, V.; Nishimoto, Y.; Ishikawa, K.; Kareiva, A. A novel synthetic approach to low-crystallinity calcium deficient hydroxyapatite. Ceram. Int. 2019, 45, 15620–15623. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, G.; Chen, X.; Li, W.; Umemoto, S.; Tajika, M.; Osaka, A. In vitro Assessment of Calcite-Hydroxyapatite Conversion of 3D-Printed Cube Honeycombs in Dilute Phosphate Solutions in the Neutral pH Range. J. Mater. Res. Technol. 2022, in press. [Google Scholar] [CrossRef]
- Lukong, V.T.; Ukoba, K.; Jen, T.-C. Review of self-cleaning TiO2 thin films deposited with spin coating. Int. J. Adv. Manuf. Technol. 2022, 122, 3525–3546. [Google Scholar] [CrossRef]
- Choi, G.; Choi, A.H.; Evans, L.A.; Akyol, S.; Ben-Nissan, B. A review: Recent advances in sol-gel-derived hydroxyapatite nanocoatings for clinical applications. J. Am. Ceram. Soc. 2020, 103, 5442–5453. [Google Scholar] [CrossRef]
- Karalkevičienė, R.; Briedytė, G.; Murauskas, T.; Norkus, M.; Žarkov, A.; Yang, J.-C.; Kareiva, A. A novel method for the formation of bioceramic nano-calcium hydroxyapatite coatings using sol-gel and dissolution-precipitation processing. CHEMIJA 2022, 33, 27–34. [Google Scholar] [CrossRef]
- Pawlewicz, W.T.; Exarhos, G.J.; Conaway, W.E. Structural characterization of TiO2 optical coatings by Raman spectroscopy. Appl. Opt. 1983, 22, 1837–1840. [Google Scholar] [CrossRef]
- Legodi, M.; de Waal, D.; Potgieter, J.; Potgieter, S. Rapid determination of CaCO3 in mixtures utilising FT—IR spectroscopy. Miner. Eng. 2001, 14, 1107–1111. [Google Scholar] [CrossRef]
- Grigoraviciute-Puroniene, I.; Stankeviciute, Z.; Ishikawa, K.; Kareiva, A. Formation of calcium hydroxyapatite with high concentration of homogeneously distributed silver. Microporous Mesoporous Mater. 2019, 293, 109806. [Google Scholar] [CrossRef]
- Garskaite, E.; Gross, K.-A.; Yang, S.-W.; Yang, T.C.-K.; Yang, J.-C.; Kareiva, A. Effect of processing conditions on the crystallinity and structure of carbonated calcium hydroxyapatite (CHAp). Crystengcomm 2014, 16, 3950–3959. [Google Scholar] [CrossRef]
- Nguyen, T.-L.; Tseng, C.-C.; Cheng, T.-C.; Nguyen, V.-T.; Chang, Y.-H. Formation and characterization of calcium phosphate ceramic coatings on Ti-6Al-4V alloy. Mater. Today Commun. 2022, 31, 103686. [Google Scholar] [CrossRef]
- Gao, F.; Chen, X.; Tanaka, H.; Nishitani, A.; Wang, Q. Alkaline phosphatase mediated synthesis of carbon nanotube–hydroxyapatite nanocomposite and its application for electrochemical determination of luteolin. Adv. Powder Technol. 2016, 27, 921–928. [Google Scholar] [CrossRef]
- Ortiz, S.L.; Lugo, V.R.; Salado-Leza, D.; Reyes-Valderrama, M.I.; Alcántara-Quintana, L.E.; González-Martínez, P.; Anaya, D.M. Dy2O3-unpurified hydroxyapatite: A promising thermoluminescent sensor and biomimetic nanotherapeutic. Appl. Phys. A 2021, 127, 893. [Google Scholar] [CrossRef]
- Kollenda, S.; Kopp, M.; Wens, J.; Koch, J.; Schulze, N.; Papadopoulos, C.; Pöhler, R.; Meyer, H.; Epple, M. A pH-sensitive fluorescent protein sensor to follow the pathway of calcium phosphate nanoparticles into cells. Acta Biomater. 2020, 111, 406–417. [Google Scholar] [CrossRef]
- Prichodko, A.; Enrichi, F.; Stankeviciute, Z.; Benedetti, A.; Grigoraviciute-Puroniene, I.; Kareiva, A. Study of Eu3+ and Tm3+ substitution effects in sol–gel fabricated calcium hydroxyapatite. J. Sol-Gel Sci. Technol. 2016, 81, 261–267. [Google Scholar] [CrossRef]
- Merkl, P.; Aschtgen, M.-S.; Henriques-Normark, B.; Sotiriou, G.A. Biofilm interfacial acidity evaluation by pH-Responsive luminescent nanoparticle films. Biosens. Bioelectron. 2021, 171, 112732. [Google Scholar] [CrossRef]
- Li, Y.; Fu, Y.; Zhang, H.; Song, J.; Yang, S. FITC-Labeled Alendronate as an In Vivo Bone pH Sensor. BioMed Res. Int. 2020, 2020, 4012194. [Google Scholar] [CrossRef]
- Zarkov, A.; Stanulis, A.; Sakaliuniene, J.; Butkute, S.; Abakeviciene, B.; Salkus, T.; Tautkus, S.; Orliukas, A.F.; Tamulevicius, S.; Kareiva, A. On the synthesis of yttria-stabilized zirconia: A comparative study. J. Sol-Gel Sci. Technol. 2015, 76, 309–319. [Google Scholar] [CrossRef]


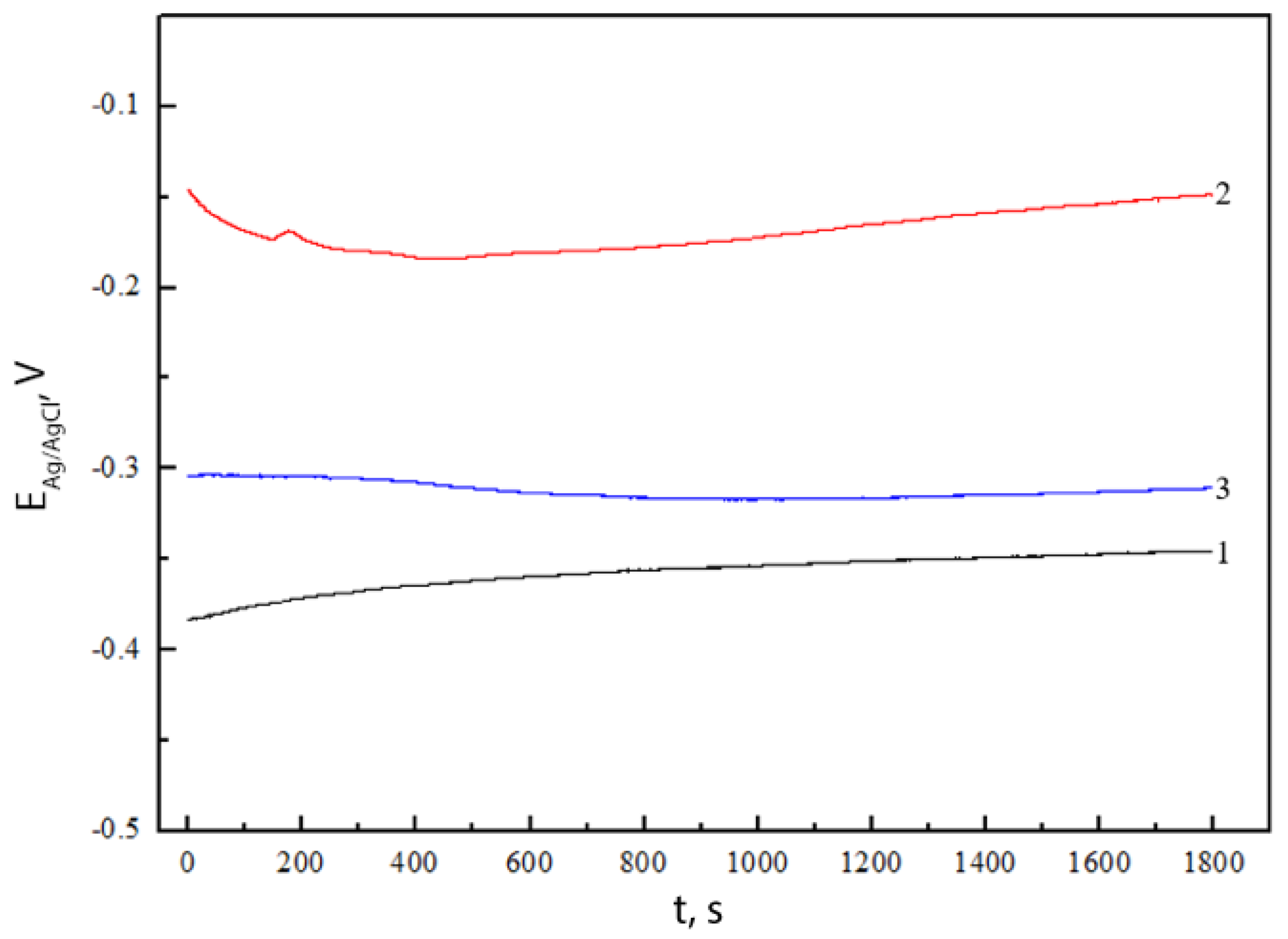
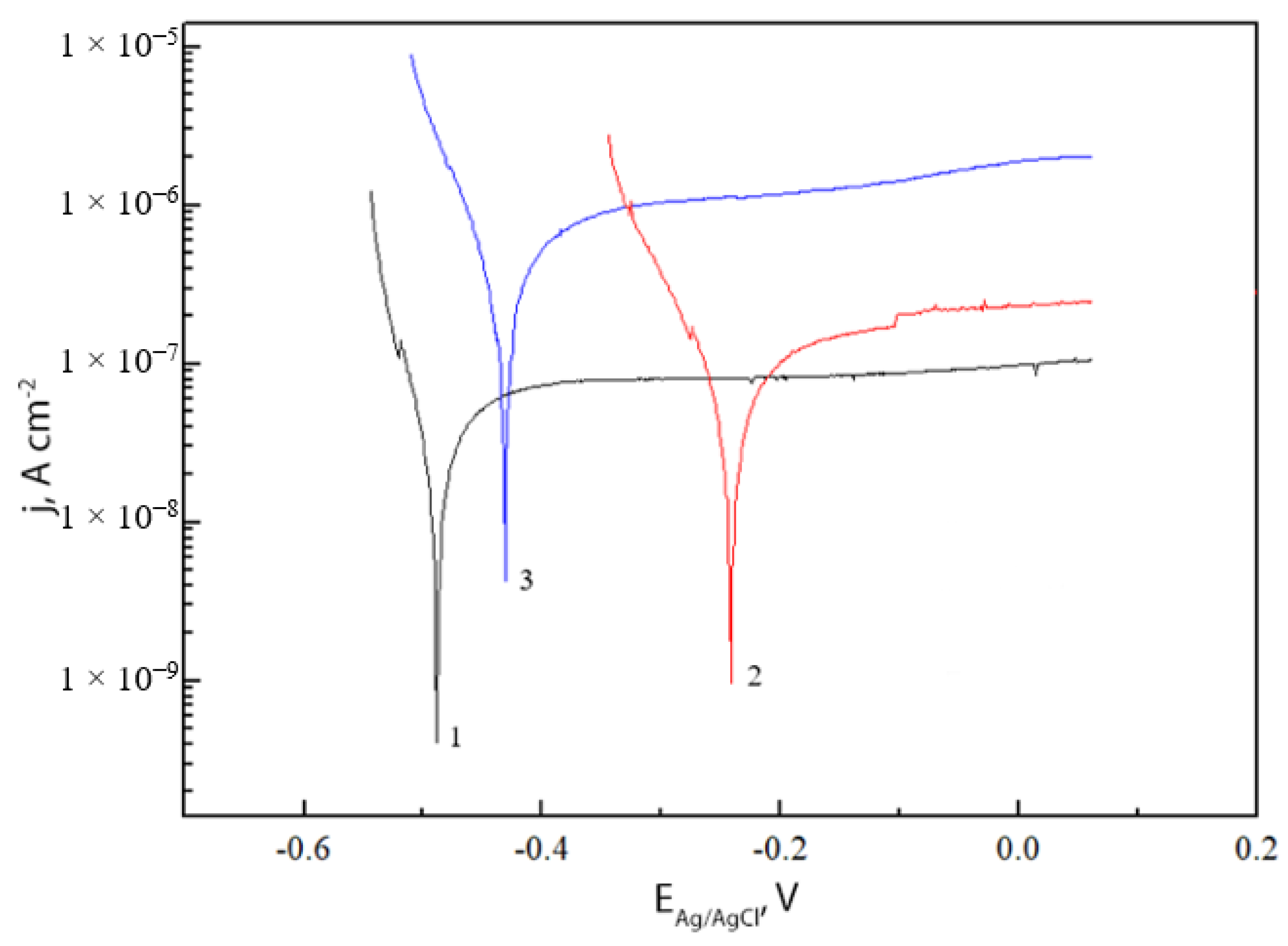
| Sample | bc, V | ba, V | jcorr, A·cm−2 |
|---|---|---|---|
| Ti substrate | 0.0209 | 0.0163 | 2.27 × 10−8 |
| CHAp coating | 0.0302 | 0.0154 | 3.34 × 10−8 |
| CHAp coating annealed at 900 °C | 0.0420 | 0.0106 | 1.82 × 10−7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karalkeviciene, R.; Briedyte, G.; Popov, A.; Tutliene, S.; Zarkov, A.; Kareiva, A. Low-Temperature Synthesis Approach for Calcium Hydroxyapatite Coatings on Titanium Substrate. Inorganics 2023, 11, 33. https://doi.org/10.3390/inorganics11010033
Karalkeviciene R, Briedyte G, Popov A, Tutliene S, Zarkov A, Kareiva A. Low-Temperature Synthesis Approach for Calcium Hydroxyapatite Coatings on Titanium Substrate. Inorganics. 2023; 11(1):33. https://doi.org/10.3390/inorganics11010033
Chicago/Turabian StyleKaralkeviciene, Rasa, Greta Briedyte, Anton Popov, Skirmante Tutliene, Aleksej Zarkov, and Aivaras Kareiva. 2023. "Low-Temperature Synthesis Approach for Calcium Hydroxyapatite Coatings on Titanium Substrate" Inorganics 11, no. 1: 33. https://doi.org/10.3390/inorganics11010033
APA StyleKaralkeviciene, R., Briedyte, G., Popov, A., Tutliene, S., Zarkov, A., & Kareiva, A. (2023). Low-Temperature Synthesis Approach for Calcium Hydroxyapatite Coatings on Titanium Substrate. Inorganics, 11(1), 33. https://doi.org/10.3390/inorganics11010033














