Abstract
This paper analyzes a surface plasmon resonance (SPR) sensor utilizing silver (Ag) and Zirconium Nitride (ZrN) for glucose concentration detection in urine samples by the transfer matrix method (TMM). For effective SP excitation, a high-RI BAF10 prism is thought to be used as the coupling layer in the suggested theoretical design. The performance of the proposed SPR biosensor is theoretically evaluated using the wavelength interrogation technique by analyzing wavelength sensitivity (WS), detection accuracy (DA), figure of merit (FoM), and penetration depth (PD) parameters. Glucose in urine samples serves as the sensing medium (SM) in this biosensor configuration. The sensor achieves a maximum wavelength sensitivity of 6416.66 nm/RIU with a penetration depth of 297.53 nm. The ZrN structure incorporated in the biosensor demonstrates enhanced wavelength sensitivity through its molecular recognition sites that provide strong binding with glucose molecules. The improved wavelength sensitivity is attributed to the greater resonance wavelength shift produced by ZrN, resulting in significant performance enhancement of the biosensor for glucose detection. Benefits of the proposed SPR biosensor include very small urine sample concentration requirements (usually 0 mg/dL to 10 g/dL), compatibility with compact prism-based configurations that support the development of portable and affordable point-of-care devices, and quick detection within a few seconds due to real-time plasmonic response. These features make the sensor ideal for rapid, minimally invasive, and field-deployable glucose monitoring in both home and clinical relevance.
1. Introduction
Chronic illnesses and cancer affect millions of people globally, representing the leading causes of mortality each year. Significant efforts have been devoted to developing accessible, accurate, and cost-effective diagnostic methods for various diseases [1,2,3]. Medical and engineering research has resulted in revolutionary advances in healthcare technology. While glucose serves as an essential biomolecule for metabolic processes and energy production, elevated glucose concentrations can lead to severe health complications, including blindness, renal disease, cardiovascular disorders, and other critical conditions [4,5,6,7]. Although blood glucose monitoring is commonly practiced, urine glucose level assessment is equally crucial for preventing chronic kidney diseases [8,9]. Elevated glucose concentrations in urine, known as renal glycosuria, require continuous monitoring for effective disease management. SPR represents an optical phenomenon that occurs when incident light interacts with free electrons at metal surfaces. Over the past few decades, SPR sensors have gained significant attention in medical diagnostics for detecting various blood components, including glucose, urea, cholesterol, and other biomarkers [10,11,12]. SPR biosensors function as highly effective analytical tools that enable real-time, label-free bioanalysis with exceptional sensitivity to biomolecular interactions [13,14]. These sensors utilize incident light and its interaction with free interface electrons between metal and dielectric media to generate surface plasmon waves. SPR demonstrates remarkable sensitivity to refractive index (RI) variations in sensing media, making it an ideal platform for medical diagnostics, environmental monitoring, and food safety applications [15,16,17]. The fundamental principle of SPR biosensors relies on real-time monitoring of RI variations caused by molecular interactions between liquid samples and pre-immobilized biomolecules on or near metal-dielectric interfaces. Currently, these biosensors can measure various parameters, including sample concentration, binding constants, and biomolecular interaction kinetics. Hundreds of studies are published annually detailing diverse biological applications of SPR sensors and recent developments in SPR-based platforms. Wavelength interrogation has emerged as the predominant SPR interrogation method due to its capability to deliver wide detection ranges with enhanced sensitivity features and adjustable operational wavelengths for specific applications [18]. Wavelength measurement of resonance enables analyte RI change detection through stable and reliable sensing operations [19]. The selection of appropriate plasmonic materials significantly determines SPR sensor application success. Gold (Au) and Ag are extensively utilized as plasmonic materials due to their favorable optical properties and ability to support surface plasmon waves (SPW) [20]. While Au maintains high sensitivity through broad reflectivity vs. wavelength responses despite stability issues related to toxicity, Ag exhibits moderate sensitivity but suffers from degradation during extended usage periods.
Because of their strong plasmonic response and high sensitivity, noble metal layers like Au, Ag and Cu are frequently used in SPR biosensors; however, their practical application is severely constrained by rapid oxidation and chemical instability in ambient and aqueous environments. Increased surface roughness, damping of surface plasmon waves, spectral broadening, and a notable decline in resonance sharpness and sensor performance are all consequences of oxidation of the metal layer [21]. Two-dimensional (2D) materials have been added to SPR sensor architectures as ultra-thin protective and functional layers to overcome these difficulties. Two-dimensional materials successfully inhibit metal oxidation while preserving effective plasmon–biomolecule coupling because of their atomic-scale thickness, high chemical inertness, and impermeability to moisture and oxygen [22]. Additionally, enhanced electromagnetic field confinement at the metal–analyte interface is made possible by the strong light–matter interaction, high carrier mobility, and large specific surface area of 2D materials. This improves SPR biosensors’ sensitivity, FoM, and long-term operational stability. Two-dimensional materials, such as transition metal dichalcogenides (TMDCs) [23], black phosphorus [24], MXenes [25], and other 2D nanomaterials, have shown promise recently for the development of highly sensitive SPR sensors. These materials’ exceptional surface area-to-volume ratios, which enhance biomolecule adsorption, along with their tunable optoelectronic properties, make them appealing. According to recent research that supports these findings, the large specific surface area of 2D materials encourages effective molecular adsorption, which is crucial for biological SPR applications. Furthermore, their adjustable optical properties improve light-matter interactions in RI-based SPR configurations, ultimately leading to enhanced sensing performance.
ZrN represents a relatively new and less-explored material in nonlinear optics, photonics, and plasmonics fields. The multifunctional characteristics of ZrN include high melting point, low electrical resistivity, minimal dielectric losses, excellent thermal conductivity, and low thermal expansion coefficient [26,27,28]. Additional properties encompass superior plasmonic qualities, outstanding mechanical strength, excellent phase stability, high hardness, corrosion resistance, and wear resistance. ZrN exhibits strong SPR within visible and infrared spectra, indicating promising optical and plasmonic properties. Furthermore, compared to conventional metals, ZrN possesses favorable characteristics, including low cost, non-toxicity, high chemical stability, and biocompatibility [29]. ZrN has been substituted for Au and Ag in numerous plasmonic applications. Researchers have investigated ZrN as an alternative plasmonic material due to its exceptional stability, high RI, and oxidation resistance [30]. The potential applications of ZrN for SPR sensors exceed current benchmarks by improving both performance and sensitivity while establishing itself as a leading material for biosensing technology development [28,31]. Numerous researchers have published manuscripts utilizing angular interrogation techniques [28,31]. For instance, Karki et al. discussed a glucose detection-based multilayer SPR sensor that achieved a maximum angular sensitivity of 133.5°/RIU [32]. Rahman et al. presented an RI glucose detection-based SPR sensor that attained a maximum sensitivity of 511°/RIU [12]. Rahad et al. designed an RI-based square ring-type resonator for glucose concentration detection, achieving a sensitivity of 3270.3 nm/RIU [33]. Salah et al. presented a wavelength interrogation technique of an RI-based SPR sensor with a maximum sensitivity of 3269.4 nm/RIU [34].
2. Proposed Structure, Fabrication Feasibility, Refractive Indices, Modeling and Performance Matrices
2.1. Proposed Structure
This paper describes the use of wavelength interrogation to increase the wavelength sensitivity using a ZrN-based SPR biosensor to detect changes in analyte RI. With this technique, the incident light’s wavelength is changed using a tunable laser or a broadband source, but the angle of incidence of the light remains constant. A sharp dip in the intensity spectrum of reflected light is caused by a strong coupling between incident photons and surface plasmons at a specific wavelength, which is referred to as the resonance wavelength. When biomolecular interactions or environmental changes cause the sensing medium’s refractive index to change, the resonance wavelength also changes. A spectrometer can be used to track this wavelength shift, enabling extremely sensitive and accurate detection. A precise measurement of the analyte’s RI modifications at very low levels occurs because this sensor presents optimal characteristics for numerous applications, including glucose detection in biological materials such as urine [35]. Diagnosing and treating diabetes mellitus among millions of patients worldwide depends greatly on detecting glucose concentrations. Existing methods of glucose testing through enzymatic assays as well as electrochemical sensors demonstrate limitations during detection because they require regular calibration and have low stability and sensitivity [36]. A ZrN-based SPR sensor functions as a viable detection method that provides a fast and label-free measurement of glucose levels specific to urine samples with high sensitivity. A platform incorporating a BK-7 prism with a ZrN layer and glucose-responsive functionalized layer constitutes the proposed SPR sensor, as depicted in Figure 1a. Numerical finite element method (FEM) analysis demonstrates that the sensor achieves high sensitivity to wavelength changes along with excellent penetration depth. This paper addresses ZrN-based SPR sensor design alongside mathematical simulations of sensor performance and shows evidence of wavelength sensitivity improvement and its utilization for glucose analysis.
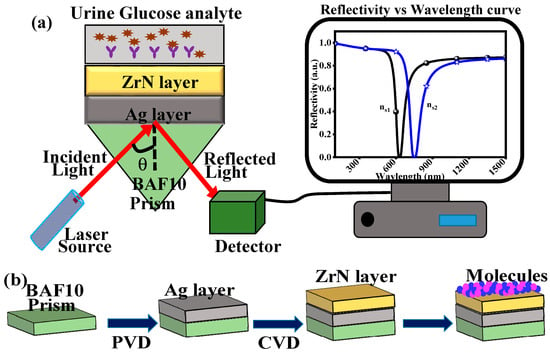
Figure 1.
(a) Suggested sensor (b) fabrication feasibility.
2.2. Fabrication Feasibility
Figure 1b illustrates the 3-layer fabrication process of the suggested biosensor using a prism BAF10, Ag, and ZrN layer. The BAF10 prism is cleaned using standard techniques like ultrasonic cleaning in acetone, isopropyl alcohol, and deionized water after the substrate has been prepared [37]. The substrate is then dried using a nitrogen gas stream. Next, physical vapor deposition (PVD) techniques are used to deposit a thin layer of Ag onto the BAF10 prism [37]. An ideal Ag layer must be deposited at a particular thickness to produce the required sensing capabilities. The Ag thickness of 30–38 nm is suitable for maximum wavelength sensitivity due to the complete transfer of the energy. Two techniques for creating the ZrN layer are mechanical exfoliation and chemical vapor deposition (CVD) [33]. The ZrN (2 nm) layer can be deposited on top of the Ag layer by chemical transfer. This technique involves moving the ZrN layer from a donor substrate to the Ag layer through a chemical reaction [38]. The method enables accurate ZrN layer deposition and is very controlled. A thorough characterization procedure is essential after biosensor fabrication. Several characterization techniques are used to confirm and ensure the intended film properties [39]. A prism-based SPR sensor setup may then be used to acquire and analyze the reflection intensity curves as a function of different incident angles. By adding a protective ZrN overlayer, which successfully reduces the well-known drawbacks of bare Ag, like oxidation, surface deterioration, and possible cytotoxicity, the use of Ag in the suggested sensor is justified. Ag has better plasmonic performance than Au, but if used alone, its instability in biological settings would limit practical application [40]. However, in our design, Ag is completely passivated by the chemically stable and biocompatible ZrN layer rather than being directly exposed to the biological fluid. Ag ion leaching and surface tarnishing are stopped by ZrN’s non-toxic, oxidation-resistant, and corrosion-resistant barrier [41]. In contrast to bare Ag, this configuration offers long-term stability and safety while maintaining the high field confinement of Ag. The Ag-ZrN bilayer was specifically selected to combine Ag’s high plasmonic sensitivity with ZrN’s strong passivation and compatibility for biosensing applications. Rather than being an experimentally realized device, the practical applicability of the proposed sensor is presented as a theoretical proof-of-concept. The main goal of this work is to use the TMM, a widely used and trustworthy method for predicting SPR behavior before fabrication, to numerically investigate and optimize the sensing performance of a Ag and ZrN layer structure for glucose detection in urine. The materials used in the design—Ag and ZrN—are well-documented in the literature and can be deposited using useful, well-established techniques like PVD and CVD, despite the lack of experimental results. This suggests that it is technically possible to build the proposed structure.
The goal of this work is to provide a solid theoretical foundation that identifies the potential sensitivity improvements provided by ZrN and to direct future experimental efforts. Compared to current other metal like Au-based sensors, the proposed Ag-ZrN combination offers a number of specific advantages. First, Ag has a stronger and sharper plasmon resonance than Au, which leads to larger resonance shifts and higher electromagnetic field confinement, both of which directly improve WS. Second, the main disadvantage of Ag’s oxidation susceptibility is overcome by the addition of ZrN, which provides better chemical stability and resistance to high temperatures than Ag alone. Additionally, ZrN has good biocompatibility and surface chemistry that can be functionalized, both of which are necessary for glucose-specific detection. Third, ZrN offers a cost-effective substitute for large-scale sensor fabrication because it is both more abundant and much less expensive than noble metals like Au. Additionally, ZrN can be deposited using conventional techniques, making integration with lab-on-a-chip or microfluidic platforms simpler. The Ag-ZrN multilayer structure offers a useful, stable, and economical substitute for traditional other metal-like Au-based SPR sensors by combining the high plasmonic performance of Ag with the chemical stability and affordability of ZrN.
Although the TMM framework is a well-known analytical tool that precisely forecasts resonance conditions, reflectivity vs. wavelength curve, and electromagnetic wave propagation in multilayer plasmonic structures, its results reflect an idealized environment devoid of material flaws, interfacial roughness, fabrication flaws, and biochemical interactions that arise in actual sensing conditions. In order to convert theoretical sensitivity into real-world performance, experimental validation is crucial. The Ag-ZrN multilayer can then be fabricated using conventional thin-film deposition techniques, such as PVD for Ag and CVD or sputtering for ZrN. This is followed by structural characterization using SEM, AFM, and XRD to verify layer thickness uniformity and crystallinity [42]. The measured resonance dips, linewidths, and sensitivity are compared with the simulated reflectivity vs. wavelength curve through optical characterization using a wavelength-interrogated SPR setup. Furthermore, ZrN surface functionalization using glucose-specific receptors (like: glucose oxidase or phenylboronic-acid derivatives) are enable binding tests to be carried out in real urine matrices and controlled glucose solutions to assess signal stability, cross-reactivity, and selectivity. These procedures ensure that elements like surface chemistry, biological fouling, noise, and environmental fluctuations are suitably considered and offer empirical support for the theoretical claims made. Therefore, in order to completely determine the practical viability of the suggested sensor design, experimental validation will be an essential next step. Baseline SPR measurements are taken in air and DI water to confirm resonance formation after the Kretschmann configuration is put together with a laser source, polarizer, rotation stage or spectrometer, and detector. After that, the sensor is calibrated using standard solutions with known refractive indices (e.g., glycerol–water mixtures), allowing sensitivity, and FoM to be extracted. Then, using artificial or spiked urine samples at physiologically relevant concentrations, glucose detection tests are carried out. Each measurement tracks the resonance shift brought on by variations in analyte RI. Specificity is measured through control experiments with blank urine, interferents, and non-functionalized surfaces; reproducibility and stability are evaluated through repeated measurements across several chips. A distinct, concentration-dependent SPR dip shift with good linearity, a low detection limit, and a stable response are the anticipated results, all of which confirm that the designed structure can be translated from simulation to a useful, lab-implementable sensing platform.
Even though the suggested SPR sensor shows promise, there are a number of practical issues that need to be considered for actual urine-based detection. Surface blocking layers or antifouling coatings like PEG, BSA, or self-assembled monolayers are usually needed to suppress undesired interactions because non-specific adsorption of biomolecules onto the metal or dielectric surface can introduce background noise, shift the baseline, and reduce measurement accuracy [43,44]. Furthermore, a number of substances found in urine, such as urea, creatinine, salts, proteins, and metabolites, may cause minor variations in RI or weak optical contributions, which could potentially affect glucose measurements if improperly managed. Surface functionalization, controlled sample preparation, and differential referencing all aid in reducing these effects. Another crucial issue is long-term stability, since metal films (e.g., Ag or Cu) may deteriorate due to oxidation or surface roughening, and functional layers may eventually become less active. Therefore, to ensure the dependable and stable sensor operation in real-world applications, encapsulation with ultrathin protective dielectrics, ideal storage conditions, and routine recalibration are required.
2.3. Refractive Index Used for the Proposed Structure
The FEM simulations were used to evaluate the sensor’s performance, including its wavelength sensitivity for urine glucose concentration detection. The first layer of the proposed sensor is BAF10 prism serves as a coupling device and has an RI of 1.6671 [45]. The optimal thickness for the 2nd layer, a Ag layer, is between 30 and 70 nm. To measure the RI of the Ag layer using the Drude–Lorentz model by Equation (1) [46].
where = 1.7614 × 10−5 m represents the collision wavelength and =1.4541 × 10−7 m represents plasma wavelength.
The 3rd layer is used as a ZrN material for a bio-recognition element (BRE) and which has a thickness of 0–2 nm with an RI of 0.63 + 2.75292i [26]. The last layer is used as a sensing layer to measure the glucose concentration in urine samples. Numerous interfering species, such as salts, urea, creatinine, proteins, and small metabolites, can produce RI variations in real-world biological environments like urine or blood that may overlap with or obscure the RI changes specifically brought on by glucose. Molecularly imprinted polymers, phenylboronic acid derivatives, or glucose oxidase are examples of glucose-specific recognition layers that can be immobilized on the sensing surface to produce a localized RI change only when glucose is present. To reduce nonspecific adsorption from proteins, salts, and metabolites found in urine, antifouling surface modification is also crucial. While zwitterionic materials (like sulfobetaine or carboxybetaine polymers) achieve antifouling through strong ionic hydration and overall charge neutrality, poly (ethylene glycol) (PEG)-based coatings produce a highly hydrated, sterically repulsive layer that inhibits protein adhesion. By preventing nonspecific interactions and preserving a steady optical response, both approaches greatly reduce biofouling, enhancing signal dependability and long-term sensor performance in complex biofluids like urine [47,48]. The cyclic α- and β-anomers interconvert through an open-chain form during mutarotation, a dynamic equilibrium that occurs in free glucose in aqueous media. The fraction of molecules displaying the reactive cis-1,2-diol—necessary for boronic-acid binding—is not constant because these anomers vary in the orientation and accessibility of their hydroxyl groups. At equilibrium, the β-anomer is typically more abundant; however, pH, temperature, and ionic strength all affect the rate and final ratio of α/β forms. This implies that during equilibration, the binding constant and sensor response are affected by changes in the effective concentration of glucose available for boronate complexation. Mutarotation effects must therefore be taken into consideration when interpreting affinity, selectivity, and time-dependent signal behavior in boronic acid-based sensing schemes [49,50]. To verify that the high theoretical sensitivity predicted in this study can be consistently attained in complex biological fluids, including cross-reactivity testing with common interferents, spiking-and-recovery analysis in actual urine samples, and repeatability evaluations, will be crucial.
There are a number of useful and proven techniques for biomolecule immobilization for glucose recognition that ensure the stable attachment and efficient interaction with the sensing surface. In enzyme-based methods, glucose oxidase (GOx) is typically immobilized by covalent coupling using carbodiimide chemistry (EDC/NHS) [51]. This involves activating and connecting carboxyl-functionalized surfaces—typically created by thiol self-assembled monolayers on Ag or adhesive polydopamine layers—to the amine groups of the enzyme, then blocking the surface to prevent non-specific adsorption. As an alternative, amine-terminated interfaces can benefit from glutaraldehyde crosslinking, which offers strong enzyme attachment with few processing steps. Affinity-based techniques like His-tag/Ni–NTA chemistry or biotin–streptavidin interactions can be used for better stability and orientation. Boronic acid derivatives, which selectively bind to glucose’s cis-diol groups and can be immobilized onto metal surfaces via thiol chemistry or covalent coupling, can also be used to achieve non-enzymatic glucose recognition. Furthermore, by attaching thiolated or amine-modified DNA aptamers to functionalized sensor surfaces, aptamer-based immobilization provides improved chemical stability and high specificity. Effective glucose recognition is made possible by these useful immobilization techniques, which also preserve compatibility with SPR sensing, real-time detection, and operation in complex media like urine samples. Urine’s RI gradually rises as glucose concentration rises, suggesting a significant relationship between optical response and glucose content. Higher glucose levels cause detectable RI shifts from 1.336 to 1.347, whereas the normal range (0–15 mg/dL) exhibits a nearly constant RI of 1.335 [51]. Using optical sensing methods like SPR-based biosensors, this monotonic increase allows for the sensitive detection of abnormal glucose levels. The plotted trend demonstrates that RI measurement is a suitable and trustworthy indicator for non-invasive glucose monitoring. The RI of the glucose concentration in the urine sample is shown in Table 1 and Figure 2.

Table 1.
RI of urine glucose concentration [51].
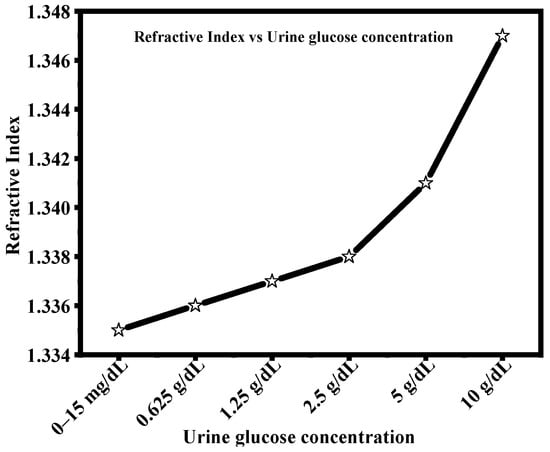
Figure 2.
Refractive Index vs. Urine glucose concentration.
2.4. Mathematical Modeling
The TMM is utilized to calculate the reflectivity of p-polarized light in an N-layer SPR structure without introducing any approximations. In this method, the tangential components of the electric and magnetic fields at the first and last interfaces of the multilayer stack are related through the characteristic transfer matrix , as expressed in Equation (2). The overall transfer matrix for the entire N-layer sensor is obtained by multiplying the individual characteristic matrices Mk of each intermediate layer, as shown in Equation (3) [18].
The overall transfer matrix connects the electromagnetic fields at the multilayer structure’s first interface (E1, B1) to the fields at the final interface (EN−1, BN−1). The characteristic matrices of each intermediate layer are multiplied to create this matrix. It explains how the incident field changes physically as it travels through the stacked layers, including phase shifts and impedance changes. The reflectivity and resonance conditions in the SPR sensor are computed using this relationship.
Here,
where δk is the layer’s optical admittance parameter and βk is its phase thickness. The phase accumulation through the layer is represented by the diagonal terms cos βk, whereas the off-diagonal terms (involving sin βk). the optical impedance of the layer causes the electric and magnetic fields to couple. Mk collectively establishes how each layer affects the SPR device’s overall reflectivity and resonance behavior.
It demonstrates that the dielectric constant of the layer and the angle of incidence in the first medium determine δk. Physically, as light moves through each layer, δk controls how the impedance and electric field change.
The coefficients θ1, dk, εk, and μk are considered as the incident angle, thickness, permittivity, and permeability for the layers, respectively. The following is an expression for the reflectivity intensity (Rp) of the incident TM wave, which is crucial for the suggested sensor.
Here, is the reflection coefficient.
2.5. Performance Parameters
The wavelength corresponding to the minimum reflectivity (Rmin) is known as the resonance wavelength (λres), and the WS is defined as the ratio between the change in resonance wavelength (Δλres) to the change in SM (Δns) [19]:
Additionally, the DA can be expressed as:
where FWHM represents the full width at half maximum and is calculated from the reflectivity curve at 50% (i.e., 0.5 a.u.) of reflected intensity, furthermore, the FoM is another parameter and can be expressed as:
Under different conditions, the electric field normalized of p-polarized light within each layer demonstrated by the sensor structure, enhances the evanescent field. The structure’s P-polarized light is described and explained by reflection, which also establishes the field’s distribution [52].
where is measured as:
The electric and magnetic field distribution within layers j ≥ 2 is determined by the following expression.
Here, is measured as:
3. Results and Discussion
3.1. Reflectivity Spectrum and Reflectivity vs. Wavelength Curves Analysis
Figure 3 shows the performance parameters such as WS, Rmin, and the reflectivity vs. wavelength curve at an optimized thickness of the Ag and ZrN layers. All parameters have been observed by the wavelength interrogation at a fixed resonance angle of 58°. When the resonance angle is decreased or increased, the reflectivity vs. wavelength curves do not show significant changes. Figure 3a shows the variation in WS at an optimized thickness of the Ag layer with several thicknesses of 0 nm, 1 nm, and 2 nm ZrN layer. The sensitivity decreases with an increase in Ag thickness due to the higher probability of electron tunneling and the increased number of Ag atoms at the interface, which raises the probability of electron transfer [53]. The maximum WS is obtained at a 30 nm Ag layer thickness with 0 nm, 1 nm, and 2 nm ZrN layers. Furthermore, the Rmin is observed at an optimized Ag layer thickness, as depicted in Figure 3b,c at RI of SM 1.335 and 1.347. The Rmin value initially decreases at a certain thickness and then increases. For sensor technologies, where precise reflectivity levels are necessary for precise detection, these variable Rmin values can be very important when designing optical coatings. They can also be used in energy systems to reduce reflective losses and increase the efficiency of light absorption. Furthermore, at a remarkable Rmin value (below: 0.30 a.u.), the reflectivity vs. wavelength curve at an optimal thickness of Ag and ZrN layer is shown in Figure 3d. Due to the remarkable Rmin value, we have selected the Ag thicknesses 37 nm, 31 nm, and 30 nm with 0 nm, 1 nm, and 2 nm thickness of the ZrN layer for the reflectivity vs. wavelength curve and maximal WS. The resonance wavelength (λres) is observed at 622 nm, 650 nm, and 669 nm for an RI of 1.335 and at 682 nm, 722 nm, and 746 nm for an RI of 1.347 in a glucose-containing urine sample. The change in resonance wavelength (Δλres) of 60 nm, 72 nm, and 77 nm is attained between 1.335 and 1.347 RI of SM. Table 2 shows the performance parameters at a remarkable Rmin value.
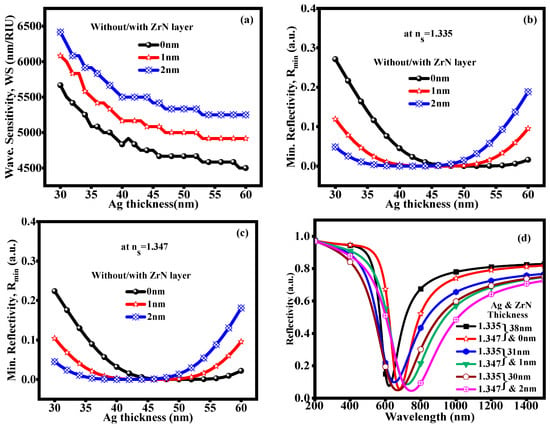
Figure 3.
(a) Wavelength sensitivity (b) Rmin at ns = 1.335 (c) Rmin at ns = 1.347: at optimized thickness of Ag layer (d) Reflectivity vs. wavelength curve with various thickness of Ag and ZrN layer at remarkable Rmin value.

Table 2.
Display the performance parameter at a remarkable Rmin value.
Further analysis, including plotting the reflectivity vs. wavelength curves for the biosensor structure with/without the ZrN layer, is depicted in Figure 4. The normal range of glucose concentrations, which is 0 to 15 mg/dL, is taken into consideration in this plot for the ZrN layer. The Rmin value is shifted to the horizontal line by increasing the Ag and ZrN layer. Adding Ag and ZrN layers lowers the Rmin value, increasing the biosensor’s sensitivity. More precise measurements of glucose concentration are made possible by this shift, which suggests enhanced signal detection capabilities. A more effective biosensing platform is produced by the multilayer structure, which maximizes the plasmonic response. In this plot, glucose concentrations of 0 to 15 mg/dL (normal range) are considered. In Figure 4a, the resonance wavelength dip is attained at 647 nm, 618 nm, 611 nm, and 609 nm. The resonance wavelength dips of 655 nm, 633 nm, 627 nm, and 625 nm with 1 nm thickness of ZrN layer as seen in Figure 4b, and 669 nm, 650 nm, 644 nm, and 641 nm with 2 nm thickness of ZrN layer as seen in Figure 4c, respectively. These changes in resonance wavelength on the lower side show that the sensitivity and functionality of the biosensor are greatly impacted by changes in the ZrN layer’s thickness. This can improve the detection capabilities for various glucose concentrations because the resonance wavelength changes as the thickness increases. In medical diagnostics, this implies that obtaining accurate and consistent sensor readings necessitates optimizing the ZrN layer thickness.
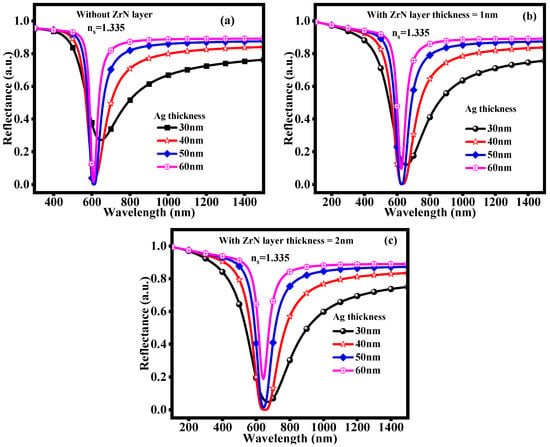
Figure 4.
Reflectivity vs. Wavelength curves (a) Without ZrN layer (b) With ZrN layer thickness = 1 nm (c) With ZrN layer thickness = 2 nm.
For better observation, Figure 5 plots the FWHM, DA, and FoM at glucose concentrations of 0 to 15 mg/dL (RI: 1.335). It shows the variation in FWHM in terms of graphical representation as depicted in Figure 5a with/without the ZrN layer. As the thickness of Ag increases, the FWHM decreases. The variation in FWHM is significant because it indicates changes in the reflectivity vs. wavelength curve width. A decrease in FWHM suggests improved structure, while an increase may imply defects within the layers. The DA variation is shown in Figure 5b. The Ag thickness layers increase as the DA increases. Because of the enhanced surface plasmon resonance effect, DA increases with Ag thickness. Greater plasmonic interactions are supported by a thicker Ag layer, which raises the DA value and increases optical absorption. The local electromagnetic field is strengthened by the plasmonic interactions at the metal-dielectric interface, which raises the material’s optical absorption. This can have a major impact on the system’s overall optical properties since it produces a more noticeable absorption peak and better light trapping within the layer. Consequently, the system can demonstrate improved sensitivity and effectiveness in applications such as sensors. Next, showing the FoM variation at an optimized thickness of the Ag layer with/without the ZrN layer, as depicted in Figure 5c. The FoM increases with an increase in Ag thickness. Due to its ability to balance sensitivity and resolution, the FoM is crucial for creating high-performance SPR sensors. A higher FoM is necessary for applications in environmental monitoring, biochemical sensing, and medical diagnostics since it produces more precise detection. At a 10 g/dL concentration of glucose in a urine sample (RI: 1.347), the variation in FWHM, DA, and FoM is displayed as depicted in Figure 5 with optimized Ag thickness for the 0 nm, 1 nm, and 2 nm thicknesses of the ZrN layer.
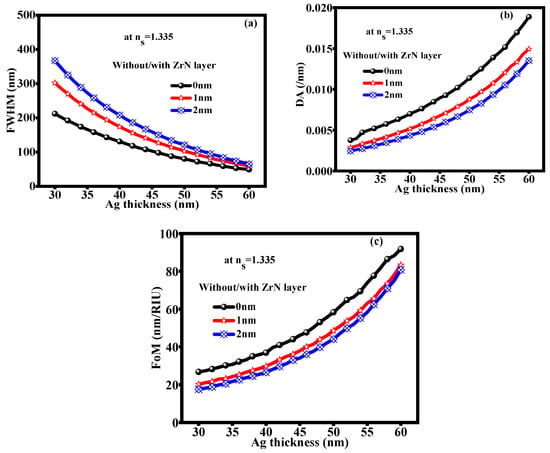
Figure 5.
(a) FWHM (b) DA (c) FoM: at an optimized thickness of Ag layer with 0 nm, 1 nm, and 2 nm thickness of ZrN layer at ns = 1.347.
The presence of diabetes causes fluctuations in the patient’s urine glucose level. Similarly, we must place biological urine samples on the ZrN layer’s surface to measure the glucose content of the samples. It shows the WS variation at glucose concentration, as depicted in Figure 6a. The WS increases with an increase in glucose concentration in the urine sample. The maximum WS is obtained at 0–15 mg/dL to 0.625 g/dL of glucose concentration due to small changes in the RI. In urine samples, an increase in glucose concentration causes a rise in the RI as shown in Figure 6b, which the sensor would detect by moving the wavelength to the right. Table 3 demonstrates how the RI change for the corresponding glucose concentration variation is used to detect the glucose levels in the urine sample. In urine samples, we have once more measured the resonance wavelength shift caused by variations in glucose concentration levels. The RI rises in urine samples with higher glucose concentrations, which causes an SPR wavelength shift. As the glucose concentration increases in urine samples, the RI increases, resulting in a wavelength shift. The WS of 7000 nm/RIU, 6500 nm/RIU, 6333.33 nm/RIU, 6166.66 nm/RIU, and 6416.66 nm/RIU is achieved at various glucose concentrations of 0–15 mg/dL, 0.625 g/dL, 1.25 g/dL, 2.5 g/dL, 5 g/dL, and 10 g/dL, respectively. The WS decreases and then increases the glucose concentration. The main causes of the wavelength sensitivity decrease with increasing glucose concentration are increased absorption/scattering effects, multilayer optical interactions, nonlinear RI changes, and SPR saturation. So, the FoM is calculated for the following particular change in glucose concentration and displayed in Figure 6c. The FoM increases due to an increase in the glucose concentration in a urine sample. This rise in the FoM suggests that the detection method is more sensitive and accurate to changes in glucose concentration. For the early diagnosis and treatment of diseases like diabetes, a higher FoM indicates that the system can more accurately track variations. As a result, it highlights how well the approach provides accurate and useful health information.
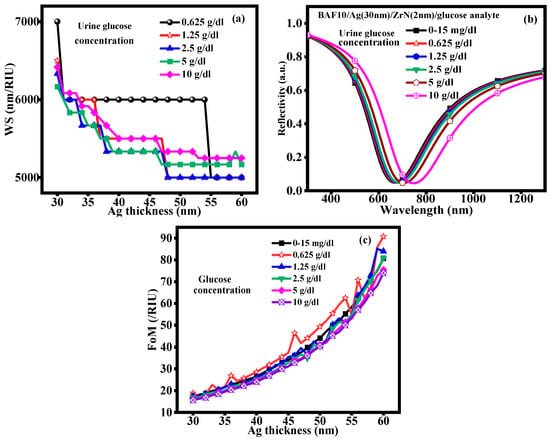
Figure 6.
(a) Wavelength sensitivity, (b) Reflectivity vs. Wavelength curve, (c) FoM with various glucose concentrations.

Table 3.
RI-based measured the performance parameters with glucose concentration in the urine sample at structure: BAF10/Ag (30 nm)/ZrN (2 nm)/Analyte.
3.2. Electric Field Analysis
Through performance analysis using FEM based on COMSOL Multiphysics 6.1 software, we have identified biological samples with the RI variation in urine glucose concentration levels. In order to improve our numerical analysis’s reproducibility and transparency, more information about the simulation workflow is provided. The reflectivity vs. wavelength curve and plasmonic field distributions are accurately calculated thanks to the theoretical model’s integration of the TMM and FEM. Periodic boundary conditions were imposed laterally to simulate an extended multilayer structure, and perfectly matched layer (PML) boundaries were applied at the top and bottom of the computational domain to eliminate non-physical reflections in the FEM simulations. A non-uniform mesh was used, with coarser meshing away from metal–dielectric interfaces to minimize computational load and denser meshing (minimum element size ≈ 1–2 nm) close to these areas where strong field confinement is anticipated. Mesh convergence was confirmed by making sure that additional refinement resulted in a resonance wavelength change of less than 0.5%. The optical constants (complex RIs) of Ag and ZrN were derived from experimentally reported literature for both TMM and FEM analyses. Standard empirical relations found in studies on glucose–water mixtures were used to calculate the RI of urine with different glucose concentrations. The simulation framework guarantees that the results can be consistently replicated and verified by other researchers by clearly defining boundary conditions, mesh settings, and source optical parameters. The electric field (EF) distribution at the sensing interface can be greatly increased by depositing the BAF10 prism and the ZrN layer on the Ag layer, as depicted in Figure 7a. The evanescent wave generated by the induced electric field on the surface of Ag is extremely sensitive to variations in the sensing environment’s RI. Higher sensitivity can be attained with a stronger electric field, making it possible to detect even tiny changes in the sensing analyte. It shows that the EF is normalized, as depicted in Figure 7b, inside all layers. The maximum peak amplitude is obtained at the interface between ZrN and the glucose-sensing analyte. The maximal EF intensity of 1.07 × 105 V/m is attained. The PDs of 233 nm and 297.53 nm are obtained at RI of 1.335 and 1.347, respectively. For this analysis, we have considered the fixed resonance angle (58°) to maintain the Rmin value. If the resonance angle, the Rmin value is shifted upward from the horizontal surface (reflectivity vs. wavelength curves). The field penetration in the sample will, therefore, be significantly greater than that of the metal. Within the metal at both interfaces in Figure 7b, the magnetic field’s magnitude rapidly and exponentially decreases due to the metal’s exceptionally high extinction coefficient. The SPWs are generated at the metal–sample interface by this exponentially decaying field in the metal at the substrate–metal interface, which can be seen in Figure 7b. It can be seen that by increasing or decreasing the wavelength concerning the resonance wavelength, the field peak at the metal–sample interface decreases because the incident wave’s wave vector matches that of SPW at the resonance wavelength. The SPR sensor structure’s p-polarized magnetic field (Hz) variation with wavelength (λres) is displayed in Figure 7c. The surface is supported by the observation that field oscillation is present in the metal film at a fixed wavelength (669 nm for RI: 1.335). Nonetheless, the metal may have an evanescent field close to its boundaries with other materials. PD is the distance normal to the interface that SPW travels between its maximum intensity at the interface and the distance at which it decays to 1/e (37%) of its maximum intensity. The 3D plot of the SPPs mode is presented in Figure 7d.
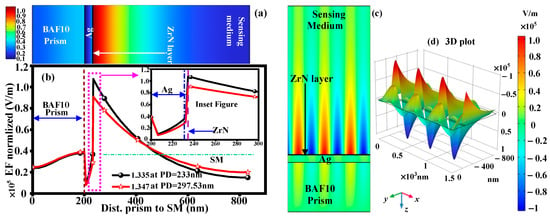
Figure 7.
(a) EF distribution (b) EF normalized (c) 2D SPPs mode (d) 3D plot of SPP mode.
The sensor’s performance is finally contrasted with that of recent SPR sensors using the wavelength interrogation technique in Table 4. Table 4 shows that the suggested sensor’s sensitivity is at its highest.

Table 4.
Comparative analysis in terms of sensitivity with existing sensors at various applications.
4. Conclusions
Using a ZrN layer, we have presented an SPR sensor that improves the wavelength sensitivity for the detection of glucose concentration in urine samples. A high-RI BAF10 prism, serving as the coupling layer in the proposed theoretical design, is considered to be positioned below the Ag layer for efficient excitation of SPs. The outcome demonstrates a significant rise in WS due to the enhanced electric field. After integrating the ZrN layer, the electric field and wavelength sensitivity are improved. The proposed sensor has a WS of 6416.66 nm/RIU. For the various concentrations of glucose (0–15 mg/dL to 10 g/dL), the WS of 7000, 6500, 6333.33, 6166.66, and 6416.66 nm/RIU, respectively, is indicated by the numerical results at 30 nm and 2 nm thicknesses of Ag and ZrN layer. The PD of 233 nm and 297.53 nm are attained for an RI of 1.335 and 1.347, respectively. All results are based on an RI of glucose concentration. The proposed sensor is a suitable candidate for the biosensing application to maintain the Rmin value.
Author Contributions
Conceptualization, R.K., L.G. and M.S.; Methodology, R.K. and L.G., software, L.G. and T.S.Y.; validation, R.K. and M.S.; formal analysis, R.K.; investigation, T.S.Y.; resources, T.S.Y. and M.S.; data curation, visualization, R.K. and L.G.; Writing—original draft preparation, R.K. and L.G.; Writing—review and editing, M.S. and T.S.Y.; Supervision M.S., project administration, T.S.Y. and M.S.; funding acquisition, T.S.Y. and M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Dongseo University, “Dongseo Cluster Project (type 1)” Research Fund of 2025 (DSU-20250011, Advanced Arcade Game Regional Innovation Centre).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Paliwal, A.; Gaur, R.; Sharma, A.; Tomar, M.; Gupta, V. Sensitive Optical Biosensor Based on Surface Plasmon Resonance Using ZnO/Au Bilayered Structure. Optik 2016, 127, 7642–7647. [Google Scholar] [CrossRef]
- Du, W.; Zhao, F. Silicon Carbide Based Surface Plasmon Resonance Waveguide Sensor with a Bimetallic Layer for Improved Sensitivity. Mater. Lett. 2017, 186, 224–226. [Google Scholar] [CrossRef]
- Chowdhury, A.S.; Islam, M.A.; Islam, M.S.; Dey, B.; Park, J. Design and Analysis of PtSe2 and Blue Phosphorus/MoS2 Heterostructure-Based SPR Biosensor. ACS Appl. Opt. Mater. 2024, 2, 1046–1059. [Google Scholar] [CrossRef]
- Rahad, R.; Rakib, A.K.M.; Haque, M.A.; Sharar, S.S.; Sagor, R.H. Plasmonic Refractive Index Sensing in the Early Diagnosis of Diabetes, Anemia, and Cancer: An Exploration of Biological Biomarkers. Results Phys. 2023, 49, 106478. [Google Scholar] [CrossRef]
- Kaur, B.; Kumar, S.; Kaushik, B.K. Trends, Challenges, and Advances in Optical Sensing for Pathogenic Bacteria Detection (PathoBactD). Biosens. Bioelectron. X 2023, 14, 100352. [Google Scholar] [CrossRef]
- Karki, B.; Salah, N.H.; Srivastava, G.; Muduli, A.; Yadav, R.B. A Simulation Study for Dengue Virus Detection Using Surface Plasmon Resonance Sensor Heterostructure of Silver, Barium Titanate, and Cerium Oxide. Plasmonics 2023, 18, 2031–2040. [Google Scholar] [CrossRef]
- Nurrohman, D.T.; Chiu, N.F. A Review of Graphene-Based Surface Plasmon Resonance and Surface-Enhanced Raman Scattering Biosensors: Current Status and Future Prospects. Nanomaterials 2021, 11, 216. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Kumar, A.; Sharan, P. Sensitivity Enhancement of a Plasmonic Biosensor for Urine Glucose Detection by Employing Black Phosphorous. J. Opt. Soc. Am. B 2022, 39, 200. [Google Scholar] [CrossRef]
- Karki, B.; Uniyal, A.; Sarkar, P.; Pal, A.; Yadav, R.B. Sensitivity Improvement of Surface Plasmon Resonance Sensor for Glucose Detection in Urine Samples Using Heterogeneous Layers: An Analytical Perspective. J. Opt. 2023, 53, 2567–2577. [Google Scholar] [CrossRef]
- Yadav, A.; Kumar, A.; Sharan, P.; Mishra, M. Highly Sensitive Bimetallic-Metal Nitride SPR Biosensor for Urine Glucose Detection. IEEE Trans. Nanobiosci. 2023, 22, 897–903. [Google Scholar] [CrossRef]
- Bisht, S.; Singh, A.; Kushwah, H.; Pratap, Y. Advancement in Glucose Concentration Measurement in Human Blood: A Surface Plasmon Resonance-Based Optical Sensing Approach. J. Opt. 2024, 54, 3479–3489. [Google Scholar] [CrossRef]
- Mustafizur Rahman, K.; Nayan, F.; Ahmed, R.; Rahman, M. Design and Development of High Sensitive Surface Plasmon Resonance Biosensors for Glucose Detection. Plasmonics 2024, 20, 2305–2319. [Google Scholar] [CrossRef]
- Kumar, R.; Pal, S.; Pal, N.; Mishra, V.; Prajapati, Y.K. High-Performance Bimetallic Surface Plasmon Resonance Biochemical Sensor Using a Black Phosphorus–MXene Hybrid Structure. Appl. Phys. A Mater. Sci. Process. 2021, 127, 259. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, M.K.; Garia, L.; Patel, B.D.; Mridula; Singh, B.M. Refractive Index Sensing–Based Ultra Sensitive Black Phosphorus Configured Surface Plasmon Resonance Sensor for the Detection of Glucose Level. Plasmonics 2024, 19, 203–213. [Google Scholar] [CrossRef]
- Kretschmann, E.; Raether, H. Radiative Decay of Non Radiative Surface Plasmons Excited by Light. Z. Naturforsch. Sect. A J. Phys. Sci. 1968, 23, 2135–2136. [Google Scholar] [CrossRef]
- Homola, J.; Yee, S.S.; Gauglitz, G. Surface Plasmon Resonance Sensors: Review. Sens. Actuators B Chem. 1999, 54, 3–15. [Google Scholar] [CrossRef]
- Piliarik, M.; Párová, L.; Homola, J. High-Throughput SPR Sensor for Food Safety. Biosens. Bioelectron. 2009, 24, 1399–1404. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, S.; Bouandas, H.; Alam, J. Detection of COVID-19 Using Surface Plasmon Resonance Sensor for Sensitivity Enhancement: Theoretical Analysis. Plasmonics 2025, 20, 7049–7059. [Google Scholar] [CrossRef]
- Li, J.; Han, D.; Zeng, J.; Deng, J.; Hu, N.; Yang, J. Multi-Channel Surface Plasmon Resonance Biosensor Using Prism-Based Wavelength Interrogation. Opt. Express 2020, 28, 14007. [Google Scholar] [CrossRef]
- Kumar, R.; Pal, S.; Prajapati, Y.K.; Kumar, S.; Saini, J.P. Sensitivity Improvement of a MXene- Immobilized SPR Sensor With Ga-Doped-ZnO for Biomolecules Detection. IEEE Sens. J. 2022, 22, 6536–6543. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, P.; Yun, T.S.; Sain, M. Performance Evaluation of Black Phosphorus and Graphene Layers Using Surface Plasmon Resonance Biosensor for the Detection of CEA Antigens. Photonics 2025, 12, 1105. [Google Scholar] [CrossRef]
- Almawgani, A.H.M.; Surve, J.; Parmar, T.; Armghan, A.; Aliqab, K.; Ali, G.A.; Patel, S.K. A Graphene-Metasurface-Inspired Optical Sensor for the Heavy Metals Detection for Efficient and Rapid Water Treatment. Photonics 2023, 10, 56. [Google Scholar] [CrossRef]
- Han, L.; He, X.; Ge, L.; Huang, T.; Ding, H.; Wu, C. Comprehensive Study of SPR Biosensor Performance Based on Metal-ITO-Graphene/TMDC Hybrid Multilayer. Plasmonics 2019, 14, 2021–2030. [Google Scholar] [CrossRef]
- Maurya, J.B.; Prajapati, Y.K.; Raikwar, S.; Saini, J.P. A Silicon-Black Phosphorous Based Surface Plasmon Resonance Sensor for the Detection of NO2 Gas. Optik 2018, 160, 428–433. [Google Scholar] [CrossRef]
- Dai, X.; Song, C.; Qiu, C.; Wu, L.; Xiang, Y. Theoretical Investigation of Multilayer Ti3C2Tx MXene as the Plasmonic Material for Surface Plasmon Resonance Sensors in near Infrared Region. IEEE Sens. J. 2019, 19, 11834–11838. [Google Scholar] [CrossRef]
- Shabani, A.; Tsegay Korsa, M.; Petersen, S.; Khazaei Nezhad, M.; Kumar Mishra, Y.; Adam, J. Zirconium Nitride: Optical Properties of an Emerging Intermetallic for Plasmonic Applications. Adv. Photonics Res. 2021, 2, 2100178. [Google Scholar] [CrossRef]
- Rakib, A.K.M.; Rahad, R.; Faruque, M.O.; Sagor, R.H. ZrN-Based Plasmonic Sensor: A Promising Alternative to Traditional Noble Metal-Based Sensors for CMOS-Compatible and Tunable Optical Properties. Opt. Express 2023, 31, 25280. [Google Scholar] [CrossRef]
- Naik, G.V.; Kim, J.; Boltasseva, A. Oxides and Nitrides as Alternative Plasmonic Materials in the Optical Range [Invited]. Opt. Mater. Express 2011, 1, 1090. [Google Scholar] [CrossRef]
- Rahad, R.; Haque, M.A.; Mahadi, M.K.; Mohsin, A.S.M.; Faruque, M.O.; Afrid, S.M.T.S.; Emon, M.J.H.; Sagor, R.H. Highly Sensitive Optically Tunable Transition Metal Nitride-Based Plasmonic Pressure Sensor with CMOS-Compatibility at Compact Subwavelength Dimensions. IEEE Sens. J. 2024, 24, 22271–22278. [Google Scholar] [CrossRef]
- Zhao, S.; Song, J.; Xu, R.; Nie, L.; Ma, J.; Deng, C.; Cheng, X.; Zhao, X.; Hao, S.; Li, J. Fabrication of Zirconium Nitride Nanopowder with a High Specific Surface Area by Introducing Fructose as a Double-Function Additive. Ceram. Int. 2021, 47, 23267–23274. [Google Scholar] [CrossRef]
- Lalisse, A.; Tessier, G.; Plain, J.; Baffou, G. Plasmonic Efficiencies of Nanoparticles Made of Metal Nitrides (TiN, ZrN) Compared with Gold. Sci. Rep. 2016, 6, 38647. [Google Scholar] [CrossRef]
- Karki, B.; Jha, A.; Pal, A.; Srivastava, V. Sensitivity Enhancement of Refractive Index-Based Surface Plasmon Resonance Sensor for Glucose Detection. Opt. Quantum Electron. 2022, 54, 595. [Google Scholar] [CrossRef]
- Rahad, R.; Rakib, A.K.M.; Mahadi, M.K.; Faruque, M.O. Fuel Classification and Adulteration Detection Using a Highly Sensitive Plasmonic Sensor. Sens. Bio-Sens. Res. 2023, 40, 100560. [Google Scholar] [CrossRef]
- Hma Salah, N.; Pal, A.; Uniyal, A. Enhancing Precision in Fuel Adulteration Detection: Utilizing a Wavelength Interrogation Surface Plasmon Resonance Approach. Plasmonics 2024, 20, 925–934. [Google Scholar] [CrossRef]
- Mostufa, S.; Paul, A.K.; Chakrabarti, K. Detection of Hemoglobin in Blood and Urine Glucose Level Samples Using a Graphene-Coated SPR Based Biosensor. OSA Contin. 2021, 4, 2164–2176. [Google Scholar] [CrossRef]
- Chen, J.-C.; Kumar, A.S.; Chung, H.-H.; Chien, S.-H.; Kuo, M.-C.; Zen, J.-M. An Enzymeless Electrochemical Sensor for the Selective Determination of Creatinine in Human Urine. Sens. Actuators B Chem. 2006, 115, 473–480. [Google Scholar] [CrossRef]
- Agarwal, S.; Prajapati, Y.K.; Singh, V. Influence of Metal Roughness on SPR Sensor Performance. Opt. Commun. 2017, 383, 113–118. [Google Scholar] [CrossRef]
- Prasad, R.D.; Teli, B.; Prasad, R.S.; Prasad, R.B.; Prasad, S.R.; Sinha, P.; Sinha, A.; Sinha, P.; Saxena, M.; Prasad, R.R.; et al. A Review on Thin Film Technology and Nanomaterial Characterization Techniques. ES Mater. Manuf. 2024, 25, 1198. [Google Scholar]
- Chen, M.; He, Y.; Huang, J.; Zhu, J. Synthesis and Solar Photo-Thermal Conversion of Au, Ag, and Au-Ag Blended Plasmonic Nanoparticles. Energy Convers. Manag. 2016, 127, 293–300. [Google Scholar] [CrossRef]
- Dohm, J.C.; Schmidt, S.; Puente Reyna, A.L.; Richter, B.; Santana, A.; Grupp, T.M. Comparative Study of Zirconium Nitride Multilayer Coatings: Crystallinity, In Vitro Oxidation Behaviour and Tribological Properties Deposited via Sputtering and Arc Deposition. J. Funct. Biomater. 2024, 15, 223. [Google Scholar] [CrossRef]
- Fellah, M.; Aissani, L.; Samad, M.A.; Mechacheti, S.; Touhami, M.Z.; Montagne, A.; Iost, A. Characterisation of RF Magnetron Sputtered Cr-N, Cr-Zr-N and Zr-N Coatings. Trans. IMF 2017, 95, 261–268. [Google Scholar] [CrossRef]
- Postma, E.J.; Scheres, L.; de Beer, S.; Kuzmyn, A.R.; Zuilhof, H. Functionalized Antifouling Polymer Brushes for Biospecific Surfaces. Adv. Mater. Interfaces 2025, 2025, 2400955. [Google Scholar] [CrossRef]
- Sheng, J.C.; De La Franier, B.; Thompson, M. Assembling Surface Linker Chemistry with Minimization of Non-Specific Adsorption on Biosensor Materials. Materials 2021, 14, 472. [Google Scholar] [CrossRef]
- Gaur, S.S.; Singh, A.P. Theoretical Analysis and Optimization of Sensing Parameters of Surface Plasmon Resonance Sensor. Z. Naturforsch. Sect. A J. Phys. Sci. 2023, 78, 89–95. [Google Scholar] [CrossRef]
- Wu, L.; Guo, J.; Wang, Q.; Lu, S.; Dai, X.; Xiang, Y.; Fan, D. Sensitivity Enhancement by Using Few-Layer Black Phosphorus-Graphene/TMDCs Heterostructure in Surface Plasmon Resonance Biochemical Sensor. Sens. Actuators B Chem. 2017, 249, 542–548. [Google Scholar] [CrossRef]
- Li, Q.; Wen, C.; Yang, J.; Zhou, X.; Zhu, Y.; Zheng, J.; Cheng, G.; Bai, J.; Xu, T.; Ji, J. Zwitterionic Biomaterials. Chem. Rev. 2022, 122, 17073–17154. [Google Scholar] [CrossRef]
- Jeon, S.I.; Lee, J.H.; Andrade, J.D.; De Gennes, P. Protein—Surface Interactions in the Presence of Polyethylene Oxide: I. Simplified Theory. J. Colloid Interface Sci. 1991, 142, 149–158. [Google Scholar] [CrossRef]
- Nan, K.; Jiang, Y.-N.; Li, M.; Wang, B. Recent Progress in Diboronic-Acid-Based Glucose Sensors. Biosensors 2023, 13, 618. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Kaur, G.; Wang, B. Progress in Boronic Acid-Based Fluorescent Glucose Sensors. J. Fluoresc. 2004, 14, 481–489. [Google Scholar] [CrossRef]
- Chia, H.L.; Mayorga-Martinez, C.C.; Antonatos, N.; Sofer, Z.; Gonzalez-Julian, J.J.; Webster, R.D.; Pumera, M. MXene Titanium Carbide-Based Biosensor: Strong Dependence of Exfoliation Method on Performance. Anal. Chem. 2020, 92, 2452–2459. [Google Scholar] [CrossRef]
- Robinson, S.; Dhanlaksmi, N. Photonic Crystal Based Biosensor for the Detection of Glucose Concentration in Urine. Photonic Sens. 2017, 7, 11–19. [Google Scholar] [CrossRef]
- Shalabney, A.; Abdulhalim, I. Electromagnetic Fields Distribution in Multilayer Thin Film Structures and the Origin of Sensitivity Enhancement in Surface Plasmon Resonance Sensors. Sens. Actuators A Phys. 2010, 159, 24–32. [Google Scholar] [CrossRef]
- Carlisle, C.I.; King, D.A.; Bocquet, M.L.; Cerdá, J.; Sautet, P. Imaging the Surface and the Interface Atoms of an Oxide Film on Ag(111) by Scanning Tunneling Microscopy: Experiment and Theory. Phys. Rev. Lett. 2000, 84, 3899–3902. [Google Scholar] [CrossRef] [PubMed]
- Singh, Y.; Ansari, M.T.I.; Raghuwanshi, S.K. Design and Development of Titanium Dioxide (TiO)-Coated EFBG Sensor for the Detection of Petrochemicals Adulteration. IEEE Trans. Instrum. Meas. 2021, 70, 7002508. [Google Scholar] [CrossRef]
- Khodaie, A.; Heidarzadeh, H. Design and Analysis of a Multi-Modal Refractive Index Plasmonic Biosensor Based on Split Ring Resonator for Detection of the Various Cancer Cells. Opt. Quantum Electron. 2024, 56, 1439. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.