Aqueous and Enzymatic Extraction of Oil and Protein from Almond Cake: A Comparative Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Almond Cake
2.2. Effects of Processing Variables on Oil and Protein Extractability from Almond Cake and Partitioning of Extracted Compounds
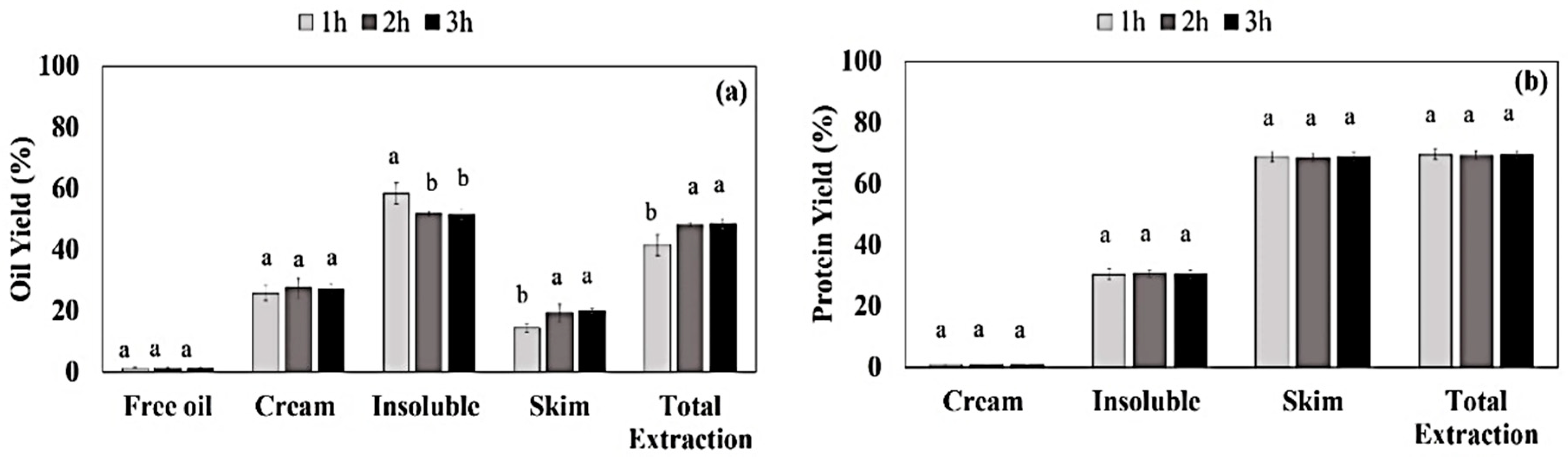
2.2.1. Aqueous Extraction Process (AEP) of Almond Cake: Processing Optimization and Validation
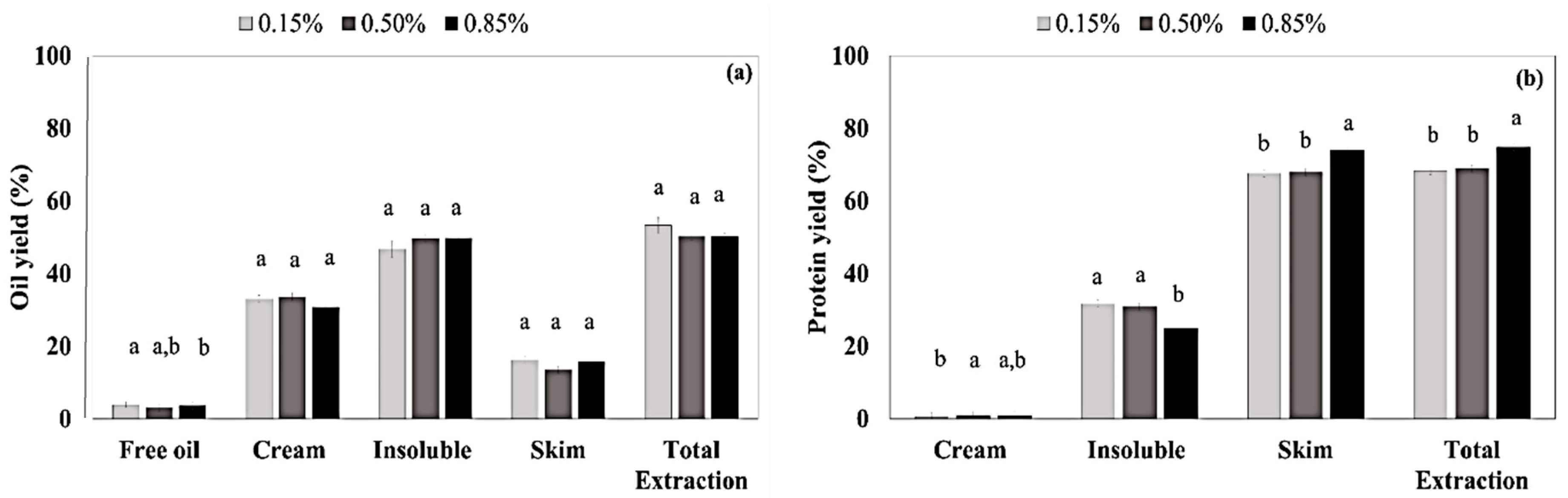
2.2.2. Enzyme-Assisted Aqueous Extraction Process (EAEP) of Almond Cake: Processing Optimization and Validation
2.3. Lipid, Protein, and Solids Recoveries
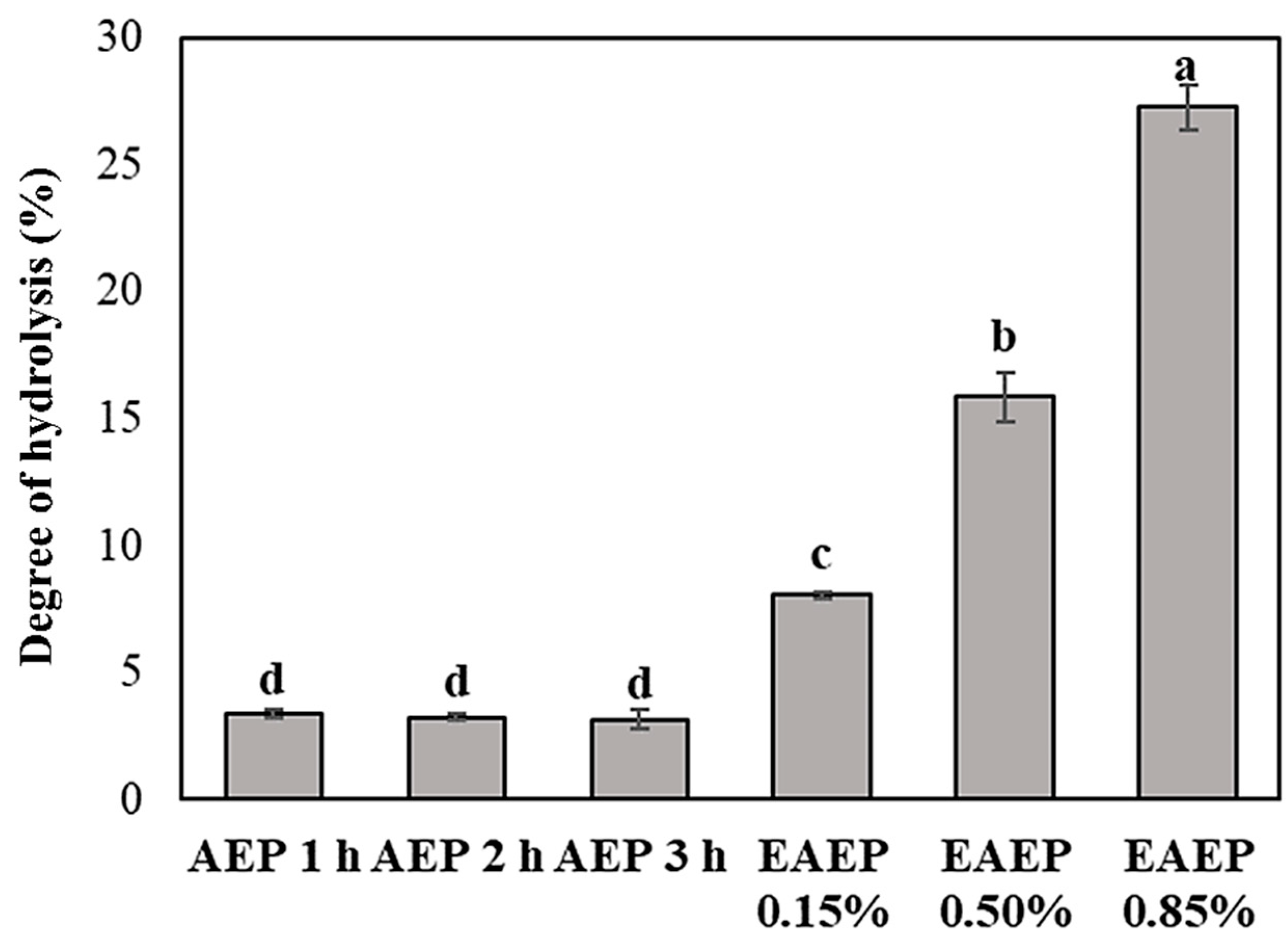
2.4. Degree of Hydrolysis
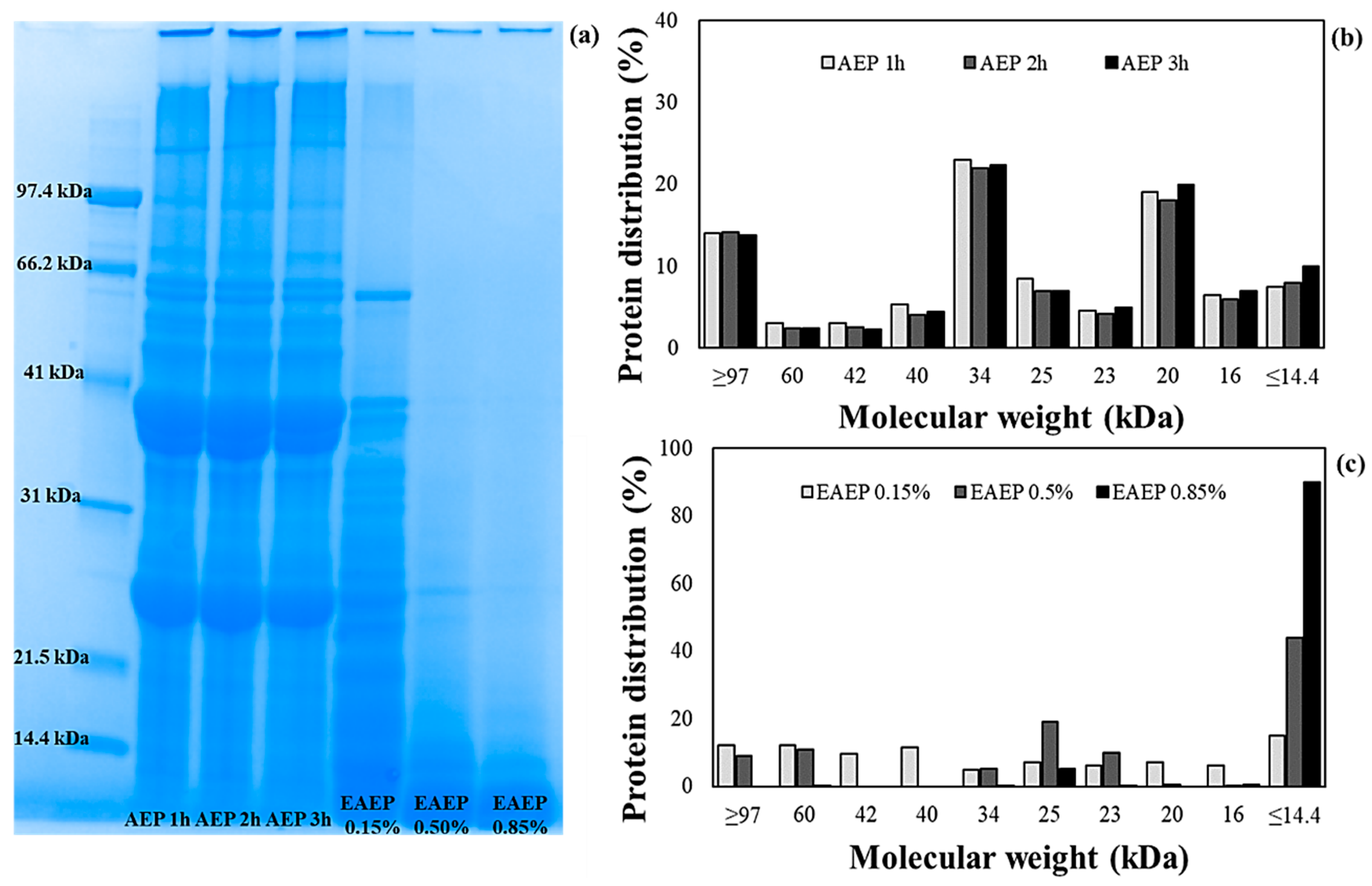
2.5. Low Molecular Weight (MW) Polypeptide Profile Characterization of AEP and EAEP Skim Proteins by SDS-PAGE
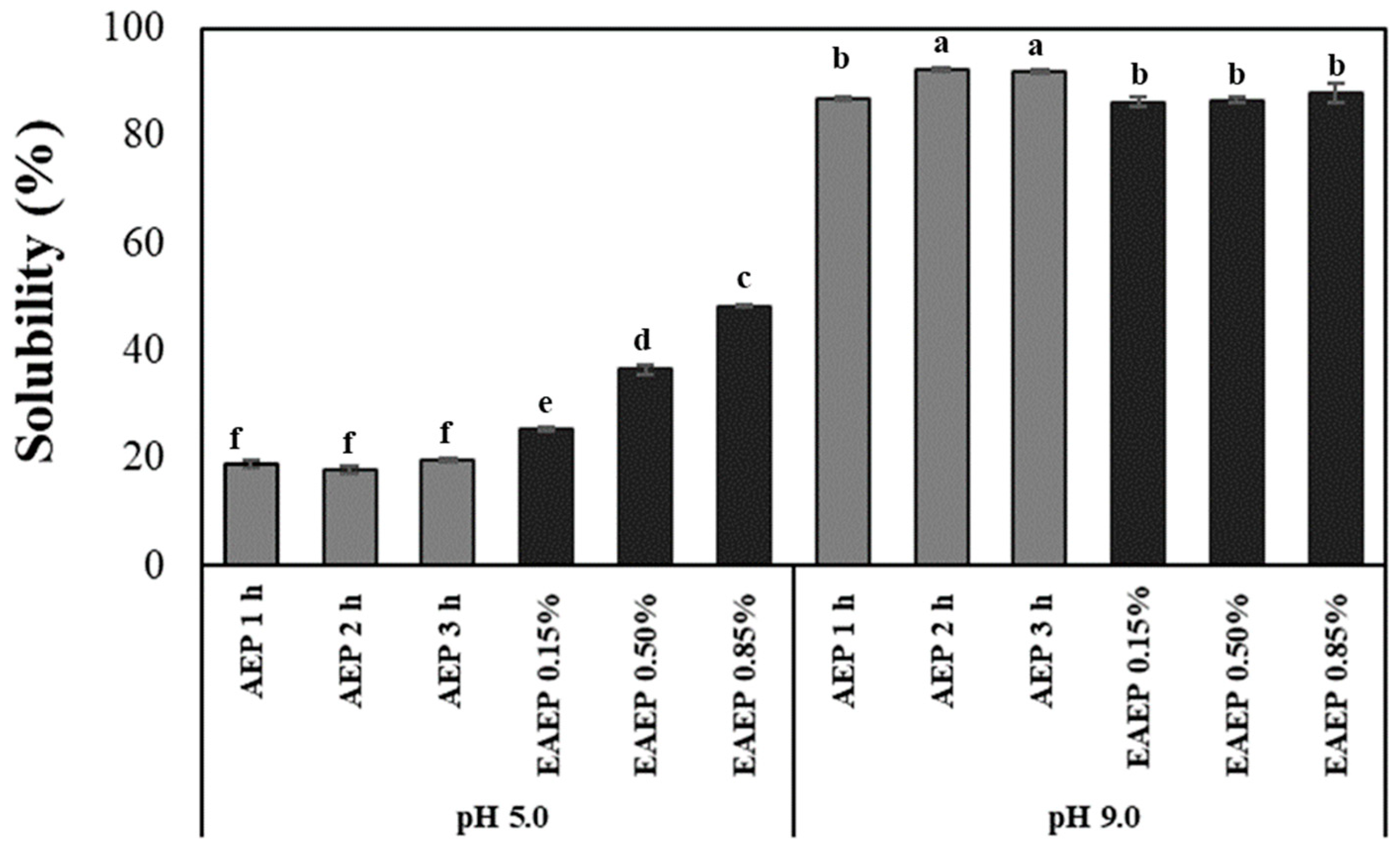
2.6. Effects of AEP and EAEP Processing Variables on Skim Protein Solubility
2.7. Statistical Analysis
3. Results and Discussion
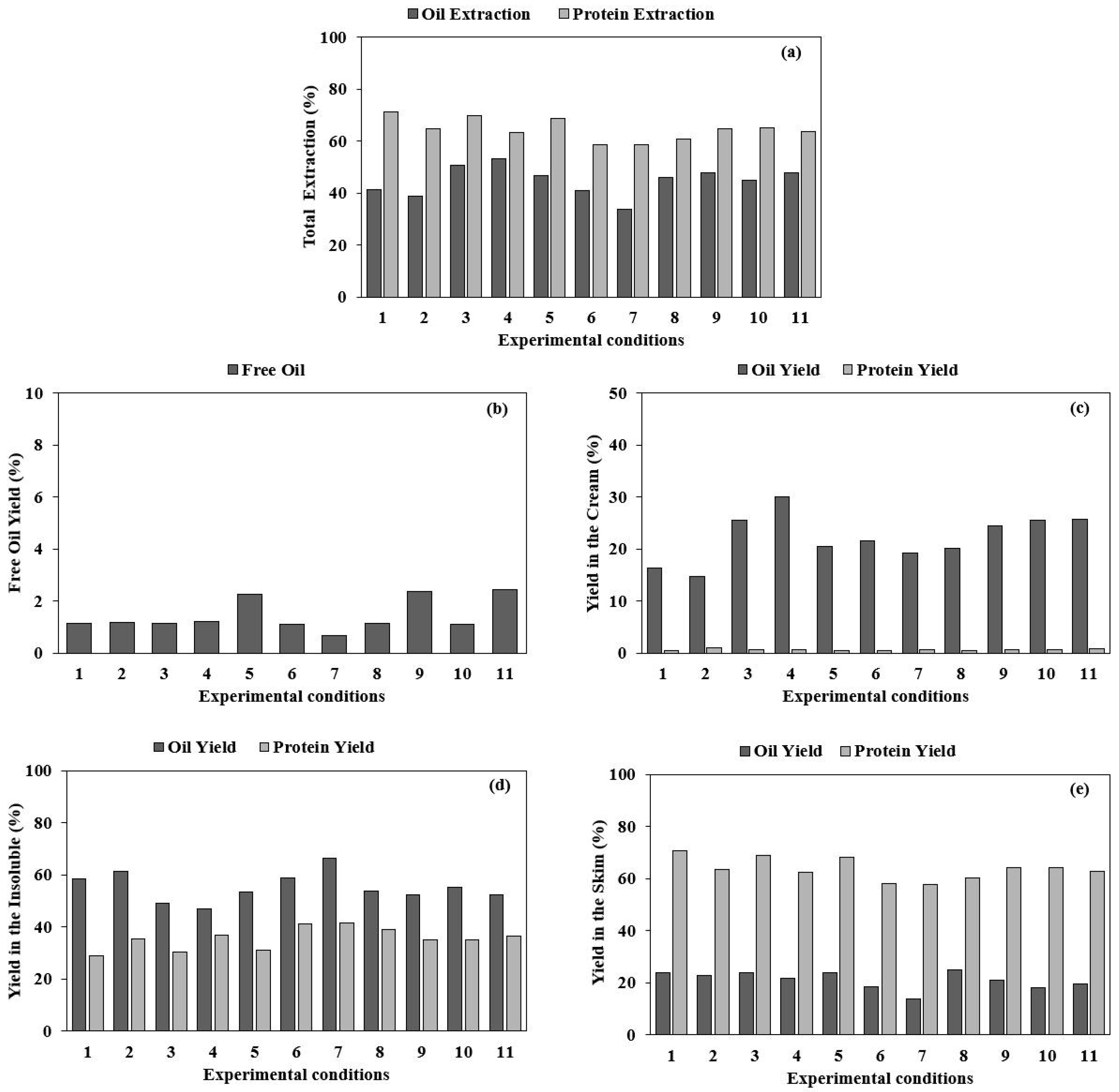
3.1. Aqueous Extraction Process of Almond Cake: Process Optimization and Validation
3.2. Enzyme-Assisted Aqueous Extraction Process of Almond Cake: Processing Optimization
3.3. Effects of Extraction Conditions on the MW Polypeptide Profile and Solubility of AEP and EAEP Skims
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nadathur, S.R.; Wanasundara, J.P.D.; Scanlin, L. Proteins in the diet. In Sustainable Protein Sources; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–19. ISBN 978-0-12-802778-3. [Google Scholar]
- Rodrigues, I.M.; Coelho, J.F.J.; Carvalho, M.G.V.S. Isolation and valorisation of vegetable proteins from oilseed plants: Methods, limitations and potential. J. Food Eng. 2012, 109, 337–346. [Google Scholar] [CrossRef]
- Sathe, S.K. Solubilization, electrophoretic characterization and in vitro digestibility of almond (Prunus amygdalus) proteins. J. Food Biochem. 1992, 16, 249–264. [Google Scholar] [CrossRef]
- Yada, S.; Lapsley, K.; Huang, G. A review of composition studies of cultivated almonds: Macronutrients and micronutrients. J. Food Compos. Anal. 2011, 24, 469–480. [Google Scholar] [CrossRef]
- Wolf, W.J.; Sathe, S.K. Ultracentrifugal and polyacrylamide gel electrophoretic studies of extractability and stability of almond meal proteins. J. Sci. Food Agric. 1998, 78, 511–521. [Google Scholar] [CrossRef]
- Grundy, M.M.-L.; Lapsley, K.; Ellis, P.R. A review of the impact of processing on nutrient bioaccessibility and digestion of almonds. Int. J. Food Sci. Technol. 2016, 51, 1937–1946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandalari, G.; Rigby, N.M.; Bisignano, C.; Lo Curto, R.B.; Mulholland, F.; Su, M.; Venkatachalam, M.; Robotham, J.M.; Willison, L.N.; Lapsley, K. Effect of food matrix and processing on release of almond protein during simulated digestion. LWT Food Sci. Technol. 2014, 59, 439–447. [Google Scholar] [CrossRef]
- Sathe, S.K.; Wolf, W.J.; Roux, K.H.; Teuber, S.S.; Venkatachalam, M.; Sze-Tao, K.W.C. Biochemical Characterization of Amandin, the Major Storage Protein in Almond (Prunus dulcis L.). J. Agric. Food Chem. 2002, 50, 4333–4341. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, J.; Sheng, W.; Wang, S.; Fu, T.-J. Effects of heat and high-pressure treatments on the solubility and immunoreactivity of almond proteins. Food Chem. 2016, 199, 856–861. [Google Scholar] [CrossRef]
- Thompson, T.; Kane, R.R.; Hager, M.H. Food Allergen Labeling and Consumer Protection Act of 2004 in Effect. J. Am. Diet. Assoc. 2006, 106, 1742–1744. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Smith, K.H.; Stevens, G.W. The use of environmentally sustainable bio-derived solvents in solvent extraction applications—A review. Chin. J. Chem. Eng. 2016, 24, 215–220. [Google Scholar] [CrossRef]
- Moura, J.M.L.N.; Hernández-Ledesma, B.; de Almeida, N.M.; Hsieh, C.-C.; de Lumen, B.O.; Johnson, L.A. Lunasin and Bowman-Birk Protease Inhibitor Concentrations of Protein Extracts from Enzyme-Assisted Aqueous Extraction of Soybeans. J. Agric. Food Chem. 2011, 59, 6940–6946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Johnson, L.A.; Jung, S. Demulsification of oil-rich emulsion from enzyme-assisted aqueous extraction of extruded soybean flakes. Bioresour. Technol. 2009, 100, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Karaman, S.; Karasu, S.; Tornuk, F.; Toker, O.S.; Geçgel, Ü.; Sagdic, O.; Ozcan, N.; Gül, O. Recovery Potential of Cold Press Byproducts Obtained from the Edible Oil Industry: Physicochemical, Bioactive, and Antimicrobial Properties. J. Agric. Food Chem. 2015, 63, 2305–2313. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Pegg, R.B. Tree nut oils. In Specialty Oils Fats Food Nutrition; Elsevier: Amsterdam, The Netherlands, 2015; pp. 65–86. ISBN 978-1-78242-376-8. [Google Scholar]
- Pojić, M.; Mišan, A.; Tiwari, B. Eco-innovative technologies for extraction of proteins for human consumption from renewable protein sources of plant origin. Trends Food Sci. Technol. 2018, 75, 93–104. [Google Scholar] [CrossRef]
- Balvardi, M.; Rezaei, K.; Mendiola, J.A.; Ibáñez, E. Optimization of the Aqueous Enzymatic Extraction of Oil from Iranian Wild Almond. J. Am. Oil Chem. Soc. 2015, 92, 985–992. [Google Scholar] [CrossRef]
- De Moura, J.M.L.N.; Campbell, K.; de Almeida, N.M.; Glatz, C.E.; Johnson, L.A. Protein Extraction and Membrane Recovery in Enzyme-Assisted Aqueous Extraction Processing of Soybeans. J. Am. Oil Chem. Soc. 2011, 88, 877–889. [Google Scholar] [CrossRef]
- Rosenthal, A.; Pyle, D.L.; Niranjan, K. Aqueous and enzymatic processes for edible oil extraction. Enzym. Microb. Technol. 1996, 19, 402–420. [Google Scholar] [CrossRef]
- O’Brien, R.D.; Farr, W.E.; Wan, P.J. Introduction to Fats Oils Technology, 2nd ed.; AOCS Press: Champaign, IL, USA, 2000. [Google Scholar]
- Liu, J.; Gasmalla, M.A.A.; Li, P.; Yang, R. Enzyme-assisted extraction processing from oilseeds: Principle, processing and application. Innov. Food Sci. Emerg. Technol. 2016, 35, 184–193. [Google Scholar] [CrossRef]
- Campbell, K.A.; Glatz, C.E.; Johnson, L.A.; Jung, S.; de Moura, J.M.N.; Kapchie, V.; Murphy, P. Advances in Aqueous Extraction Processing of Soybeans. J. Am. Oil Chem. Soc. 2011, 88, 449–465. [Google Scholar] [CrossRef]
- Casas, M.P.; Domínguez González, H. Enzyme-assisted aqueous extraction processes. In Water Extraction of Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2017; pp. 333–368. ISBN 978-0-12-809380-1. [Google Scholar]
- Rosenthal, A.; Pyle, D.L.; Niranjan, K.; Gilmour, S.; Trinca, L. Combined effect of operational variables and enzyme activity on aqueous enzymatic extraction of oil and protein from soybean. Enzym. Microb. Technol. 2001, 28, 499–509. [Google Scholar] [CrossRef]
- Yoon, S.H.; Kim, I.H.; Kim, S.H.; Kwon, T.W. Effects of Enzyme Treatments and Ultrasonification on Extraction Yields of Lipids and Protein from Soybean by Aqueous Process. Korean J. Food Sci. Technol. 1991, 23, 673–676. [Google Scholar]
- De Moura, J.M.L.N.; de Almeida, N.M.; Johnson, L.A. Scale-up of Enzyme-Assisted Aqueous Extraction Processing of Soybeans. J. Am. Oil Chem. Soc. 2009, 86, 809–815. [Google Scholar] [CrossRef]
- Freitas, S.P.; Hartman, L.; Couri, S.; Jablonka, F.H.; de Carvalho, C.W.P. The combined application of extrusion and enzymatic technology for extraction of soybean oil. Fett Lipid 1997, 99, 333–337. [Google Scholar] [CrossRef]
- Jung, S. Aqueous extraction of oil and protein from soybean and lupin: A comparative study. J. Food Process. Preserv. 2009, 33, 547–559. [Google Scholar] [CrossRef]
- De Moura, J.M.L.N.; Johnson, L.A. Two-Stage Countercurrent Enzyme-Assisted Aqueous Extraction Processing of Oil and Protein from Soybeans. J. Am. Oil Chem. Soc. 2009, 86, 283–289. [Google Scholar] [CrossRef]
- Kapchie, V.N.; Wei, D.; Hauck, C.; Murphy, P.A. Enzyme-Assisted Aqueous Extraction of Oleosomes from Soybeans (Glycine max). J. Agric. Food Chem. 2008, 56, 1766–1771. [Google Scholar] [CrossRef]
- De Almeida, N.M.; de Moura Bell, J.M.L.N.; Johnson, L.A. Properties of Soy Protein Produced by Countercurrent, Two-Stage, Enzyme-Assisted Aqueous Extraction. J. Am. Oil Chem. Soc. 2014, 91, 1077–1085. [Google Scholar] [CrossRef]
- Jiang, L.; Hua, D.; Wang, Z.; Xu, S. Aqueous enzymatic extraction of peanut oil and protein hydrolysates. Food Bioprod. Process. 2010, 88, 233–238. [Google Scholar] [CrossRef]
- Li, P.; Zhang, W.; Han, X.; Liu, J.; Liu, Y.; Gasmalla, M.A.A.; Yang, R. Demulsification of oil-rich emulsion and characterization of protein hydrolysates from peanut cream emulsion of aqueous extraction processing. J. Food Eng. 2017, 204, 64–72. [Google Scholar] [CrossRef]
- Sharma, A.; Khare, S.K.; Gupta, M.N. Enzyme-assisted aqueous extraction of peanut oil. J. Am. Oil Chem. Soc. 2002, 79, 215–218. [Google Scholar] [CrossRef]
- Aliakbarian, B.; De Faveri, D.; Converti, A.; Perego, P. Optimisation of olive oil extraction by means of enzyme processing aids using response surface methodology. Biochem. Eng. J. 2008, 42, 34–40. [Google Scholar] [CrossRef]
- Najafian, L.; Ghodsvali, A.; Haddad Khodaparast, M.H.; Diosady, L.L. Aqueous extraction of virgin olive oil using industrial enzymes. Food Res. Int. 2009, 42, 171–175. [Google Scholar] [CrossRef]
- Bisht, T.S.; Sharma, S.K.; Sati, R.C.; Rao, V.K.; Yadav, V.K.; Dixit, A.K.; Sharma, A.K.; Chopra, C.S. Improvement of efficiency of oil extraction from wild apricot kernels by using enzymes. J. Food Sci. Technol. 2015, 52, 1543–1551. [Google Scholar] [CrossRef] [PubMed]
- Moreau, R.A.; Dickey, L.C.; Johnston, D.B.; Hicks, K.B. A Process for the Aqueous Enzymatic Extraction of Corn Oil from Dry Milled Corn Germ and Enzymatic Wet Milled Corn Germ (E-Germ). J. Am. Oil Chem. Soc. 2009, 86, 469–474. [Google Scholar] [CrossRef]
- Moreau, R.A.; Johnston, D.B.; Powell, M.J.; Hicks, K.B. A comparison of commercial enzymes for the aqueous enzymatic extraction of corn oil from corn germ. J. Am. Oil Chem. Soc. 2004, 81, 1071–1075. [Google Scholar] [CrossRef]
- Ndlela, S.C.; de Moura, J.M.L.N.; Olson, N.K.; Johnson, L.A. Aqueous Extraction of Oil and Protein from Soybeans with Subcritical Water. J. Am. Oil Chem. Soc. 2012, 89, 1145–1153. [Google Scholar] [CrossRef]
- AOAC. Official Methods Analysis, 15th ed.; AOAC: Washington, DC, USA, 1990. [Google Scholar]
- Nielsen, P.M.; Petersen, D.; Dambmann, C. Improved Method for Determining Food Protein Degree of Hydrolysis. J. Food Sci. 2001, 66, 642–646. [Google Scholar] [CrossRef]
- Liu, R.-L.; Ge, X.-L.; Gao, X.-Y.; Zhan, H.-Y.; Shi, T.; Su, N.; Zhang, Z.-Q. Two angiotensin-converting enzyme-inhibitory peptides from almond protein and the protective action on vascular endothelial function. Food Funct. 2016, 7, 3733–3739. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Rickert, D.A.; Johnson, L.A.; Murphy, P.A. Functional Properties of Improved Glycinin and β-Conglycinin Fractions. Food Chem. Toxicol. 2004, 69, 303–311. [Google Scholar]
- Morr, C.V.; German, B.; Kinsella, J.E.; Regenstein, J.M.; Buren, J.P.V.; Kilara, A.; Lewis, B.A.; Mangino, M.E. A Collaborative Study to Develop a Standardized Food Protein Solubility Procedure. J. Food Sci. 1985, 50, 1715–1718. [Google Scholar] [CrossRef]
- Johnson, L.A.; Lusas, E.W. Comparison of alternative solvents for oils extraction. J. Am. Oil Chem. Soc. 1983, 60, 229–242. [Google Scholar] [CrossRef]
- De Moura, J.M.L.N.; Maurer, D.; Jung, S.; Johnson, L.A. Integrated Countercurrent Two-Stage Extraction and Cream Demulsification in Enzyme-Assisted Aqueous Extraction of Soybeans. J. Am. Oil Chem. Soc. 2011, 88, 1045–1051. [Google Scholar] [CrossRef]
- Yao, L.; Jung, S. 31P NMR Phospholipid Profiling of Soybean Emulsion Recovered from Aqueous Extraction. J. Agric. Food Chem. 2010, 58, 4866–4872. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.A.; Glatz, C.E. Mechanisms of aqueous extraction of soybean oil. J. Agric. Food Chem. 2009, 57, 10904–10912. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, A.; Pyle, D.L.; Niranjan, K. Simultaneous Aqueous Extraction of Oil and Protein from Soybean: Mechanisms for Process Design. Food Bioprod. Process. 1998, 76, 224–230. [Google Scholar] [CrossRef]
- Esteban, R.M.; López-Andréu, F.J.; Carpena, O. Protein extractability of almond (Prunus amygdalus, batsch) seed. J. Sci. Food Agric. 1985, 36, 485–490. [Google Scholar] [CrossRef]
- Chodar Moghadas, H.; Rezaei, K. Laboratory-Scale Optimization of Roasting Conditions Followed by Aqueous Extraction of Oil from Wild Almond. J. Am. Oil Chem. Soc. 2017, 94, 867–876. [Google Scholar] [CrossRef]
- Ghorbanzadeh, R.; Rezaei, K. Optimization of an Aqueous Extraction Process for Pomegranate Seed Oil. J. Am. Oil Chem. Soc. 2017, 94, 1491–1501. [Google Scholar] [CrossRef]
- De Moura, J.M.L.N.; Campbell, K.; Mahfuz, A.; Jung, S.; Glatz, C.E.; Johnson, L. Enzyme-Assisted Aqueous Extraction of Oil and Protein from Soybeans and Cream De-emulsification. J. Am. Oil Chem. Soc. 2008, 85, 985–995. [Google Scholar] [CrossRef]
- Yusoff, M.M.; Gordon, M.H.; Niranjan, K. Aqueous enzyme assisted oil extraction from oilseeds and emulsion de-emulsifying methods: A review. Trends Food Sci. Technol. 2015, 41, 60–82. [Google Scholar] [CrossRef]
- De Moura Bell, J.M.L.N.; Maurer, D.; Yao, L.; Wang, T.; Jung, S.; Johnson, L.A. Characteristics of Oil and Skim in Enzyme-Assisted Aqueous Extraction of Soybeans. J. Am. Oil Chem. Soc. 2013, 90, 1079–1088. [Google Scholar] [CrossRef]
- Chandrasekaran, M. (Ed.) Enzymes Food Beverage Processing; CRC Press: Boca Raton, FL, USA, 2016; ISBN 978-1-4822-2128-2. [Google Scholar]
- Lamsal, B.P.; Johnson, L.A. Separating Oil from Aqueous Extraction Fractions of Soybean. J. Am. Oil Chem. Soc. 2007, 84, 785–792. [Google Scholar] [CrossRef]
- Sari, Y.W.; Bruins, M.E.; Sanders, J.P.M. Enzyme assisted protein extraction from rapeseed, soybean, and microalgae meals. Ind. Crops Prod. 2013, 43, 78–83. [Google Scholar] [CrossRef]
- Jamdar, S.N.; Rajalakshmi, V.; Pednekar, M.D.; Juan, F.; Yardi, V.; Sharma, A. Influence of degree of hydrolysis on functional properties, antioxidant activity and ACE inhibitory activity of peanut protein hydrolysate. Food Chem. 2010, 121, 178–184. [Google Scholar] [CrossRef]
- Derbyshire, E.; Wright, D.J.; Boulter, D. Legumin and vicilin, storage proteins of legume seeds. Phytochemistry 1976, 15, 3–24. [Google Scholar] [CrossRef]
- Garcia-Mas, J.; Messeguer, R.; Arús, P.; Puigdomènech, P. Molecular characterization of cDNAs corresponding to genes expressed during almond (Prunus amygdalus Batsch) seed development. Plant Mol. Biol. 1995, 27, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Wouters, A.G.B.; Rombouts, I.; Fierens, E.; Brijs, K.; Delcour, J.A. Relevance of the Functional Properties of Enzymatic Plant Protein Hydrolysates in Food Systems. Compr. Rev. Food Sci. Food Saf. 2016, 15, 786–800. [Google Scholar] [CrossRef]
- Amirshaghaghi, Z.; Rezaei, K.; Habibi Rezaei, M. Characterization and functional properties of protein isolates from wild almond. J. Food Meas. Charact. 2017, 11, 1725–1733. [Google Scholar] [CrossRef]

| Experiment | Solids-to-Liquid Ratio (X1) | Reaction Time (h) (X2) | ||
|---|---|---|---|---|
| Coded Value | Real Value | Coded Value | Real Value | |
| 1 | −1 | 1:12 | −1 | 0.75 |
| 2 | +1 | 1:8 | −1 | 0.75 |
| 3 | −1 | 1:12 | 1 | 3.00 |
| 4 | 1 | 1:8 | 1 | 3.00 |
| 5 | −1.41 | 1:12.82 | 0 | 1.88 |
| 6 | 1.41 | 1:7.18 | 0 | 1.88 |
| 7 | 0 | 1:10 | −1.41 | 0.29 |
| 8 | 0 | 1:10 | +1.41 | 3.46 |
| 9 | 0 | 1:10 | 0 | 1.88 |
| 10 | 0 | 1:10 | 0 | 1.88 |
| 11 | 0 | 1:10 | 0 | 1.88 |
| Experiment | Solids-to-Liquid Ratio (X1) | Enzyme (%) (wt/wt *) (X2) | ||
|---|---|---|---|---|
| Coded Value | Real Value | Coded Value | Real Value | |
| 1 | −1 | 1:12 | −1 | 0.25 |
| 2 | +1 | 1:8 | −1 | 0.25 |
| 3 | −1 | 1:12 | 1 | 0.75 |
| 4 | 1 | 1:8 | 1 | 0.75 |
| 5 | −1.41 | 1:12.82 | 0 | 0.50 |
| 6 | 1.41 | 1:7.18 | 0 | 0.50 |
| 7 | 0 | 1:10 | −1.41 | 0.15 |
| 8 | 0 | 1:10 | +1.41 | 0.85 |
| 9 | 0 | 1:10 | 0 | 0.50 |
| 10 | 0 | 1:10 | 0 | 0.50 |
| 11 | 0 | 1:10 | 0 | 0.50 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza, T.S.P.; Dias, F.F.G.; Koblitz, M.G.B.; M. L. N. de M. Bell, J. Aqueous and Enzymatic Extraction of Oil and Protein from Almond Cake: A Comparative Study. Processes 2019, 7, 472. https://doi.org/10.3390/pr7070472
Souza TSP, Dias FFG, Koblitz MGB, M. L. N. de M. Bell J. Aqueous and Enzymatic Extraction of Oil and Protein from Almond Cake: A Comparative Study. Processes. 2019; 7(7):472. https://doi.org/10.3390/pr7070472
Chicago/Turabian StyleSouza, Thaiza S. P., Fernanda F. G. Dias, Maria G. B. Koblitz, and Juliana M. L. N. de M. Bell. 2019. "Aqueous and Enzymatic Extraction of Oil and Protein from Almond Cake: A Comparative Study" Processes 7, no. 7: 472. https://doi.org/10.3390/pr7070472






