Enhanced Anaerobic Mixed Culture Fermentation with Anion-Exchange Resin for Caproate Production
Abstract
:1. Introduction
2. Materials and Methods
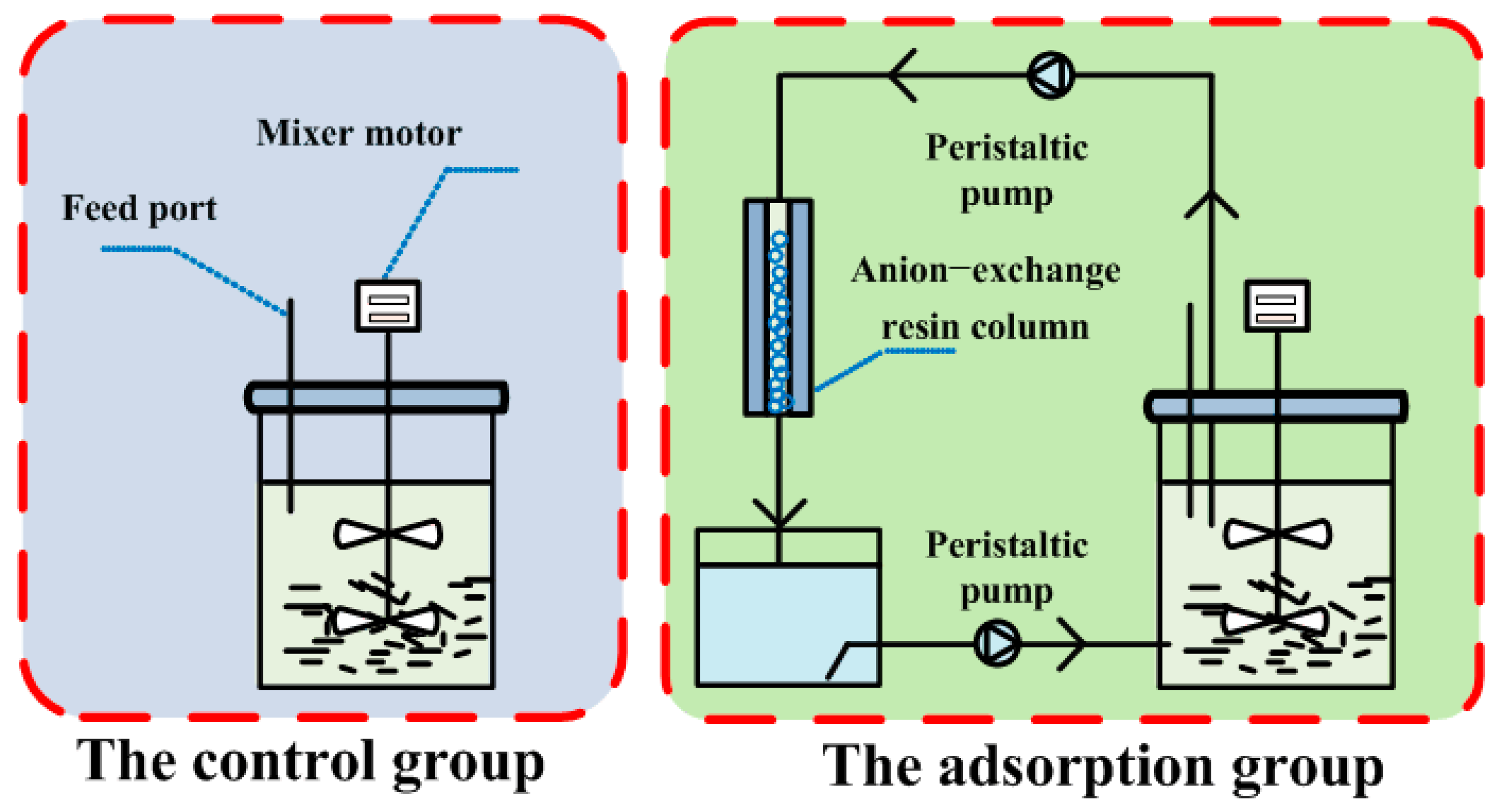
2.1. Experimental Materials for Resin Screening and Fermentation
2.2. Comparison and Selection of Optimal Resin
2.3. Enhancement of Caproate Fermentation with Anion-Exchange Resin Adsorption
2.4. Chemical Analyses
3. Results and Discussion
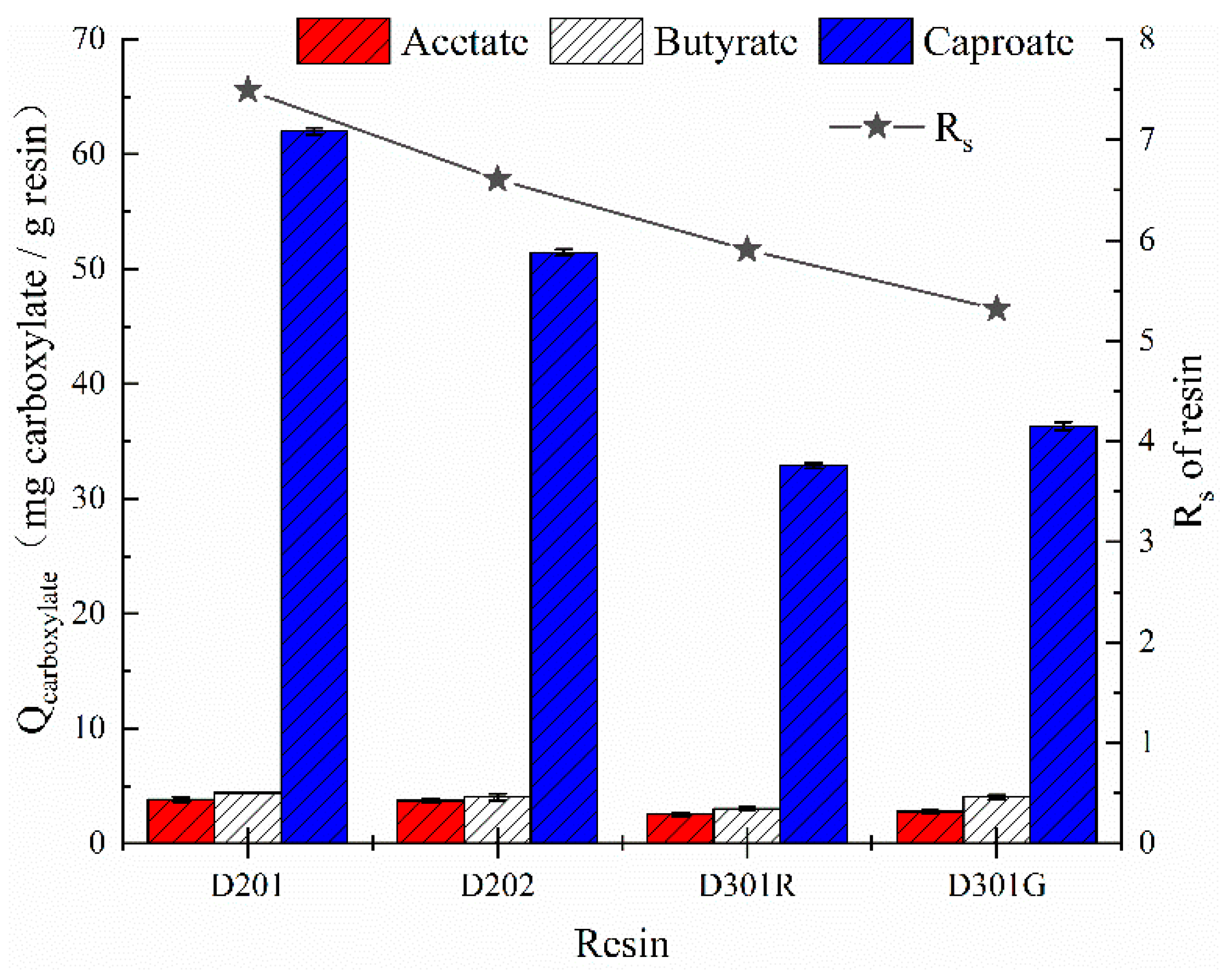
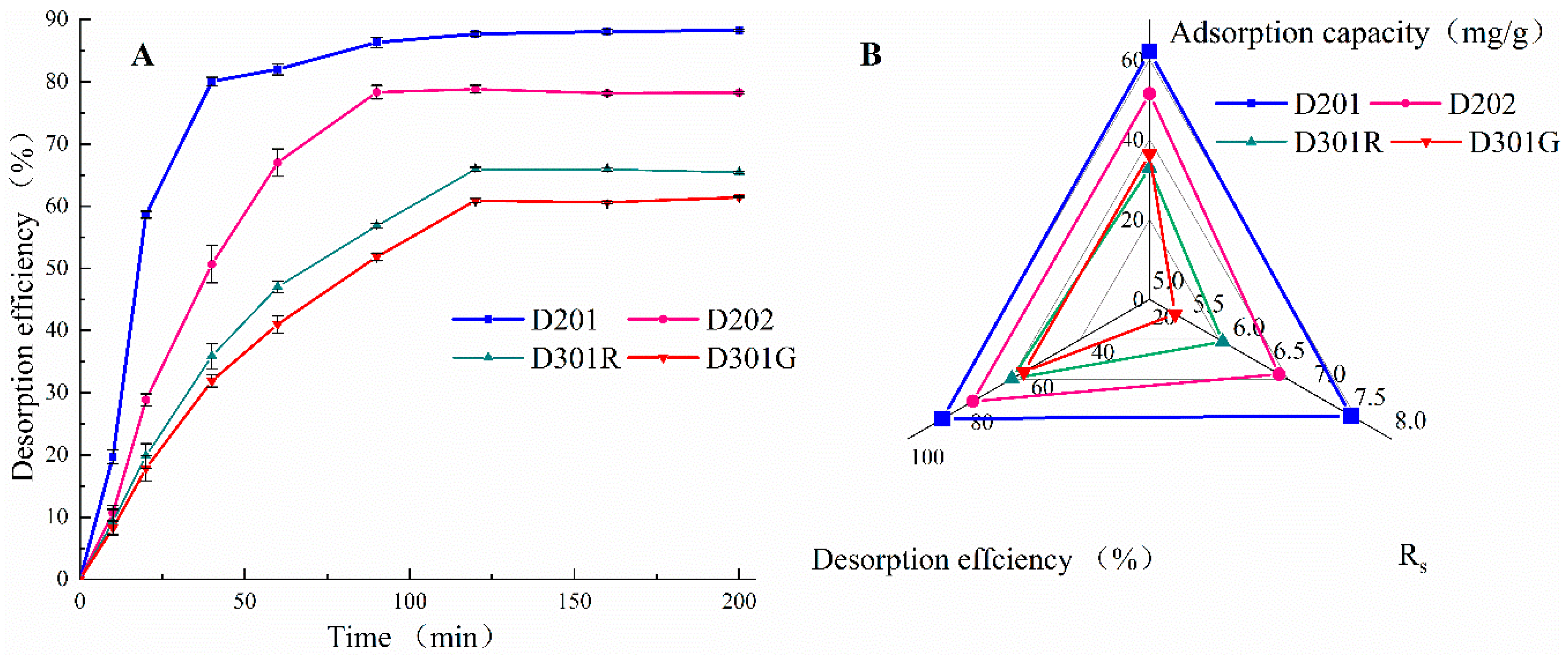
3.1. Optimal Resin for Enhancement of Caproate Production
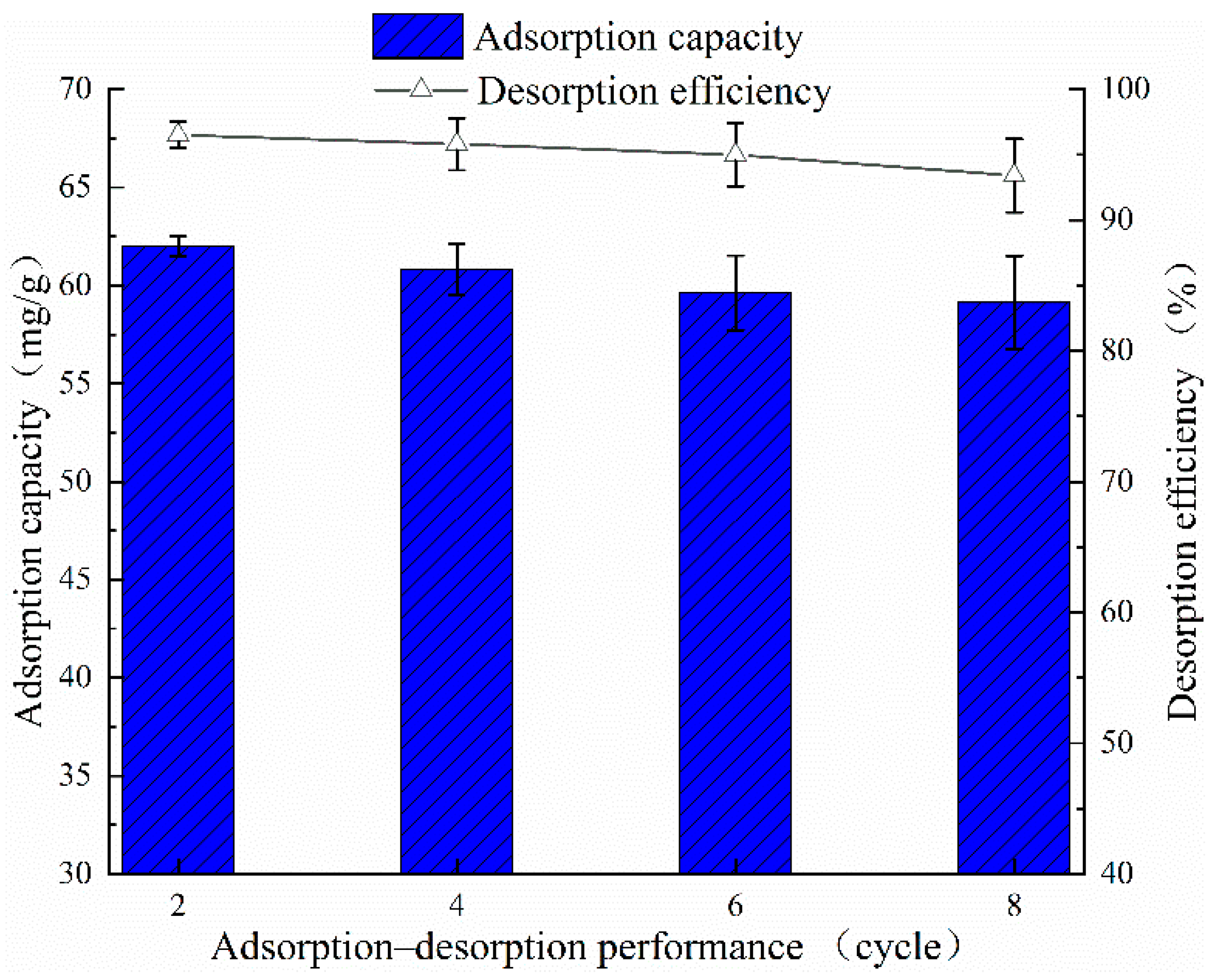
3.2. Optimal Desorption Condition and Reusability of D201
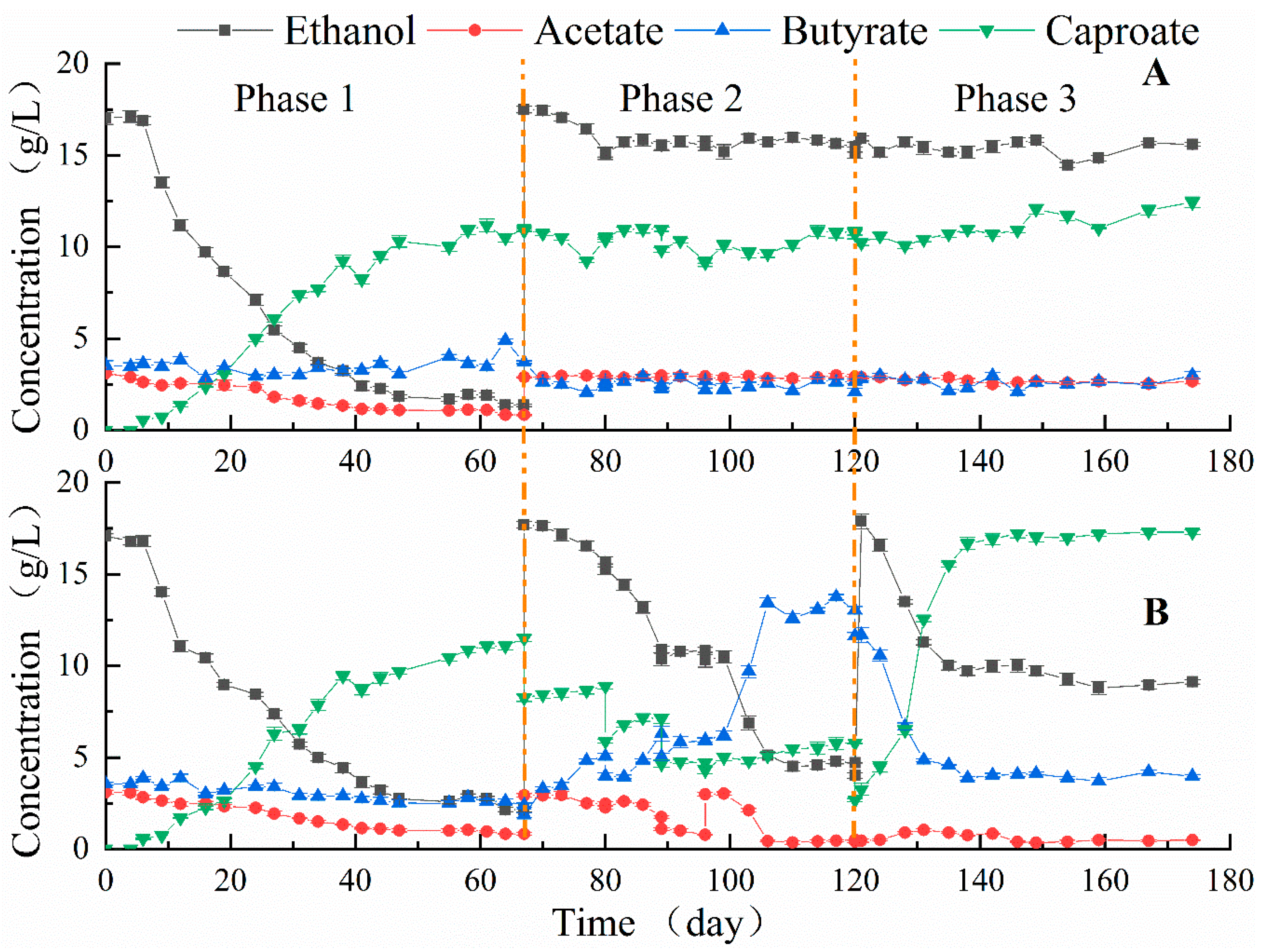
3.3. Enhanced Caproate Fermentation with Anion Exchange
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xu, J.; Guzman, J.J.L.; Andersen, S.J.; Korneel, R.; Angenent, L.T. In-line and selective phase separation of medium-chain carboxylic acids using membrane electrolysis. Chem. Commun. 2015, 51, 6847–6850. [Google Scholar] [CrossRef] [PubMed]
- Angenent, L.T.; Richter, H.; Buckel, W.; Spirito, C.M.; Steinbusch, K.J.; Plugge, C.M.; Strik, D.P.; Grootscholten, T.I.; Buisman, C.J.; Hamelers, H.V. Chain elongation with reactor microbiomes: Open-culture biotechnology to produce biochemicals. Environ. Sci. Technol. 2016, 50, 2796–2810. [Google Scholar] [CrossRef] [PubMed]
- Agler, M.T.; Spirito, C.M.; Usack, J.G.; Werner, J.J.; Angenent, L.T. Chain elongation with reactor microbiomes: Upgrading dilute ethanol to medium-chain carboxylates. Energy Environ. Sci. 2012, 5, 8189. [Google Scholar] [CrossRef]
- Chen, W.S.; Strik, D.; Buisman, C.J.N.; Kroeze, C. Production of caproic acid from mixed organic waste: An environmental life cycle perspective. Environ. Sci. Technol. 2017, 51, 7159–7168. [Google Scholar] [CrossRef] [PubMed]
- Grootscholten, T.I.; Steinbusch, K.J.; Hamelers, H.V.; Buisman, C.J. Chain elongation of acetate and ethanol in an upflow anaerobic filter for high rate MCFA production. Bioresour. Technol. 2013, 135, 440–445. [Google Scholar] [CrossRef]
- Grootscholten, T.I.M.; Strik, D.P.B.T.B.; Steinbusch, K.J.J.; Buisman, C.J.N.; Hamelers, H.V.M. Two-stage medium chain fatty acid (MCFA) production from municipal solid waste and ethanol. Appl. Energy 2014, 116, 223–229. [Google Scholar] [CrossRef]
- Steinbusch, K.J.J.; Hamelers, H.V.M.; Plugge, C.M.; Buisman, C.J.N. Biological formation of caproate and caprylate from acetate: Fuel and chemical production from low grade biomass. Energy Environ. Sci. 2011, 4, 216–224. [Google Scholar] [CrossRef]
- Steven, V.G.; Logan, B.E. Inhibition of biohydrogen production by undissociated acetic and butyric acids. Environ. Sci. Technol. 2005, 39, 9351. [Google Scholar]
- Roghair, M.; Liu, Y.; Adiatma, J.C.; Weusthuis, R.A.; Bruins, M.E.; Buisman, C.J.N.; Strik, D. Effect of n-caproate concentration on chain elongation and competing processes. ACS Sustain. Chem. Eng. 2018, 6, 7499–7506. [Google Scholar] [CrossRef]
- Roddick, F.A.; Britz, M.L. Production of hexanoic acid by free and immobilised cells of megasphaera elsdenii: Influence of in-situ product removal using ion exchange resin. J. Chem. Technol. Biotechnol. 2015, 69, 383–391. [Google Scholar] [CrossRef]
- Liu, Y.; He, P.; Shao, L.; Zhang, H.; Lu, F. Significant enhancement by biochar of caproate production via chain elongation. Water Res. 2017, 119, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Roghair, M.; Strik, D.P.B.T.B.; Steinbusch, K.J.J.; Weusthuis, R.A.; Bruins, M.E.; Buisman, C.J.N. Granular sludge formation and characterization in a chain elongation process. Process Biochem. 2016, 51, 1594–1598. [Google Scholar] [CrossRef]
- Choi, K.; Jeon, B.S.; Kim, B.C.; Oh, M.K.; Um, Y.; Sang, B.I. In situ biphasic extractive fermentation for hexanoic acid production from sucrose by Megasphaera elsdenii NCIMB 702410. Appl. Biochem. Biotechnol. 2013, 171, 1094–1107. [Google Scholar] [CrossRef] [PubMed]
- de Cavalcante, W.A.; Leitão, R.C.; Gehring, T.A.; Angenent, L.T.; Santaella, S.T. Anaerobic fermentation for n-caproic acid production: A review. Process Biochem. 2017, 54, 106–119. [Google Scholar] [CrossRef]
- Huang, C.; Xu, T.; Zhang, Y.; Xue, Y.; Chen, G. Application of electrodialysis to the production of organic acids: State-of-the-art and recent developments. J. Membrane Sci. 2007, 288, 1–12. [Google Scholar] [CrossRef]
- da Silva, A.H.; Miranda, E.A. Adsorption/desorption of organic acids onto different adsorbents for their recovery from fermentation broths. J. Chem. Eng. Data 2013, 58, 1454–1463. [Google Scholar] [CrossRef]
- Jones, J.; Lee, C.H.; Wang, J.; Poenie, M. Use of anion exchange resins for one-step processing of algae from harvest to biofuel. Energies 2012, 5, 2608. [Google Scholar] [CrossRef]
- Pradhan, N.; Rene, E.R.; Lens, P.N.L.; Dipasquale, L.; D’Ippolito, G.; Fontana, A.; Panico, A.; Esposito, G. Adsorption behaviour of lactic acid on granular activated carbon and anionic resins: Thermodynamics, isotherms and kinetic studies. Energies 2017, 10, 665. [Google Scholar] [CrossRef]
- Ge, S.; Usack, J.G.; Spirito, C.M.; Angenent, L.T. Long-term n-caproic acid production from yeast-fermentation beer in an anaerobic bioreactor with continuous product extraction. Environ. Sci. Technol. 2015, 49, 8012–8021. [Google Scholar] [CrossRef]
- Xu, J.; Hao, J.; Guzman, J.J.L.; Spirito, C.M.; Harroff, L.A.; Angenent, L.T. Temperature-phased conversion of acid whey waste into medium-chain carboxylic acids via lactic acid: No external e-Donor. Joule 2018, 2, 280–295. [Google Scholar] [CrossRef]
- Moon, P.J.; Parulekar, S.J.; Tsai, S.P. Competitive anion transport in desalting of mixtures of organic acids by batch electrodialysis. J. Membrane Sci. 1998, 141, 75–89. [Google Scholar] [CrossRef]
- Arslan, D.; Zhang, Y.; Steinbusch, K.J.J.; Diels, L.; Hamelers, H.V.M.; Buisman, C.J.N.; De Wever, H. In-situ carboxylate recovery and simultaneous pH control with tailor-configured bipolar membrane electrodialysis during continuous mixed culture fermentation. Sep. Purif. Technol. 2017, 175, 27–35. [Google Scholar] [CrossRef]
- Kanazawa, N.; Urano, K.; Kokado, N.; Urushigawa, Y. Adsorption equilibrium equation of carboxylic acids on anion-exchange resins in water. J. Collid. Interf. Sci. 2001, 238, 196–202. [Google Scholar] [CrossRef]
- Kammerer, J.; Carle, R.; Kammerer, D.R. Adsorption and ion exchange: Basic principles and their application in food processing. J. Agric. Food Chem. 2011, 59, 22–42. [Google Scholar] [CrossRef] [PubMed]
- Rebecchi, S.; Pinelli, D.; Bertin, L.; Zama, F.; Fava, F.; Frascari, D. Volatile fatty acids recovery from the effluent of an acidogenic digestion process fed with grape pomace by adsorption on ion exchange resins. Chem. Eng. J. 2016, 306, 629–639. [Google Scholar] [CrossRef]
- Fargues, C.; Lewandowski, R.; Lameloise, M.L. Evaluation of ion-exchange and adsorbent resins for the detoxification of beet distillery effluents. Ind. Eng. Chem. Res. 2010, 49, 377–379. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater; APHA: Washington, DC, USA, 2005; ISBN 9780875530475. [Google Scholar]
- Khor, W.C. Production of Lactic Acid and Derivatives from Grass Using Mixed Populations. Ph.D. Thesis, Ghent University, Ghent, Belgium, 2017. [Google Scholar]
- Lopez-Garzon, C.S.; Straathof, A.J. Recovery of carboxylic acids produced by fermentation. Biotechnol. Adv. 2014, 32, 873–904. [Google Scholar] [CrossRef] [PubMed]
- Chu, B.; Whitney, D.C.; Diamond, R.M. On anion-exchange resin selectivities. J. Inorg. Nucl. Chem. 1962, 24, 1405–1415. [Google Scholar] [CrossRef]
- Gluszcz, P.; Jamroz, T.; Sencio, B.; Ledakowicz, S. Equilibrium and dynamic investigations of organic acids adsorption onto ion-exchange resins. Bioprocess Biosyst. Eng. 2004, 26, 185–190. [Google Scholar]
- Lonkar, S.; Fu, Z.; Holtzapple, M. Optimum alcohol concentration for chain elongation in mixed-culture fermentation of cellulosic substrate. Biotechnol. Bioeng. 2016, 113, 2597–2604. [Google Scholar] [CrossRef]
- Weimer, P.J.; Stevenson, D.M. Isolation, characterization, and quantification of Clostridium kluyveri from the bovine rumen. Appl. Microbiol. Biotechnol. 2012, 94, 461–466. [Google Scholar] [CrossRef] [PubMed]
| Property | Resin | |||
|---|---|---|---|---|
| D201 | D202 | D301R | D301G | |
| Type | Strongly basic | Strongly basic II | Weakly basic | Weakly basic |
| Matrix | Styrene-divinylbenzene (DVB) | Styrene-DVB | Styrene-DVB | Styrene-DVB |
| Functional group | Quaternary ammonium | Dimethyl ethanolamine | Tertiary amine | Tertiary amine |
| Ion form as shipped | Cl− | Cl− | Cl− | Cl− |
| Grain diameter (mm) | 0.4–0.7 | 0.42–0.6 | 0.4–0.7 | 0.65–0.72 |
| Elution Factor | c Desorption Effeciency | |||
|---|---|---|---|---|
| a Temperature (°C) | 5 | 88.10 ± 0.36 | 81.66 ± 0.33 | 65.79 ± 0.49 |
| 20 | 96.90 ± 0.29 | 87.74 ± 0.21 | 89.04 ± 0.12 | |
| 30 | 95.05 ± 0.23 | 86.75 ± 0.29 | 88.25 ± 0.29 | |
| 40 | 95.17 ± 0.19 | 86.12 ± 0.22 | 88.18 ± 0.13 | |
| b NaOH concentration (M) | 0.5 | 85.20 ± 0.10 | 80.17 ± 0.09 | 78.65 ± 0.32 |
| 1.5 | 99.84 ± 0.16 | 94.75 ± 0.13 | 95.74 ± 0.12 | |
| 2.0 | 99.80 ± 0.26 | 95.01 ± 0.33 | 95.94 ± 0.21 | |
| 2.5 | 99.71 ± 0.35 | 97.30 ± 0.16 | 96.12 ± 0.20 | |
| Adsorption/Desorption Operation | a Qacetate (mg/g) | Qbutyrate (mg/g) | Qcaproate (mg/g) | Desorption Efficiency (%) |
|---|---|---|---|---|
| The first operation at the 67th day | 2.13 ± 0.02 | 10.87 ± 0.09 | 60.17 ± 0.17 | 93.4 ± 0.27 |
| The second operation at the 80th day | 4.13 ± 0.07 | 15.46 ± 0.10 | 59.36 ± 0.31 | 94.0 ± 0.31 |
| The third operation at the 89th day | 3.60 ± 0.03 | 19.24 ± 0.29 | 56.99 ± 0.29 | 95.1 ± 0.22 |
| The fourth operation at the 120th day | 0.74 ± 0.02 | 28.25 ± 0.23 | 55.13 ± 0.25 | 93.5 ± 0.13 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Liao, J.; Huang, Z.; Wu, P.; Zhao, M.; Liu, C.; Ruan, W. Enhanced Anaerobic Mixed Culture Fermentation with Anion-Exchange Resin for Caproate Production. Processes 2019, 7, 404. https://doi.org/10.3390/pr7070404
Yu J, Liao J, Huang Z, Wu P, Zhao M, Liu C, Ruan W. Enhanced Anaerobic Mixed Culture Fermentation with Anion-Exchange Resin for Caproate Production. Processes. 2019; 7(7):404. https://doi.org/10.3390/pr7070404
Chicago/Turabian StyleYu, Jiangnan, Jialin Liao, Zhenxing Huang, Peng Wu, Mingxing Zhao, Chunmei Liu, and Wenquan Ruan. 2019. "Enhanced Anaerobic Mixed Culture Fermentation with Anion-Exchange Resin for Caproate Production" Processes 7, no. 7: 404. https://doi.org/10.3390/pr7070404
APA StyleYu, J., Liao, J., Huang, Z., Wu, P., Zhao, M., Liu, C., & Ruan, W. (2019). Enhanced Anaerobic Mixed Culture Fermentation with Anion-Exchange Resin for Caproate Production. Processes, 7(7), 404. https://doi.org/10.3390/pr7070404




