Hydrodynamic Cavitation-Assisted Hydrothermal Separation: A Pathway for Valorizing Lignocellulosic Biomass into Biopolymers and Extractives
Abstract
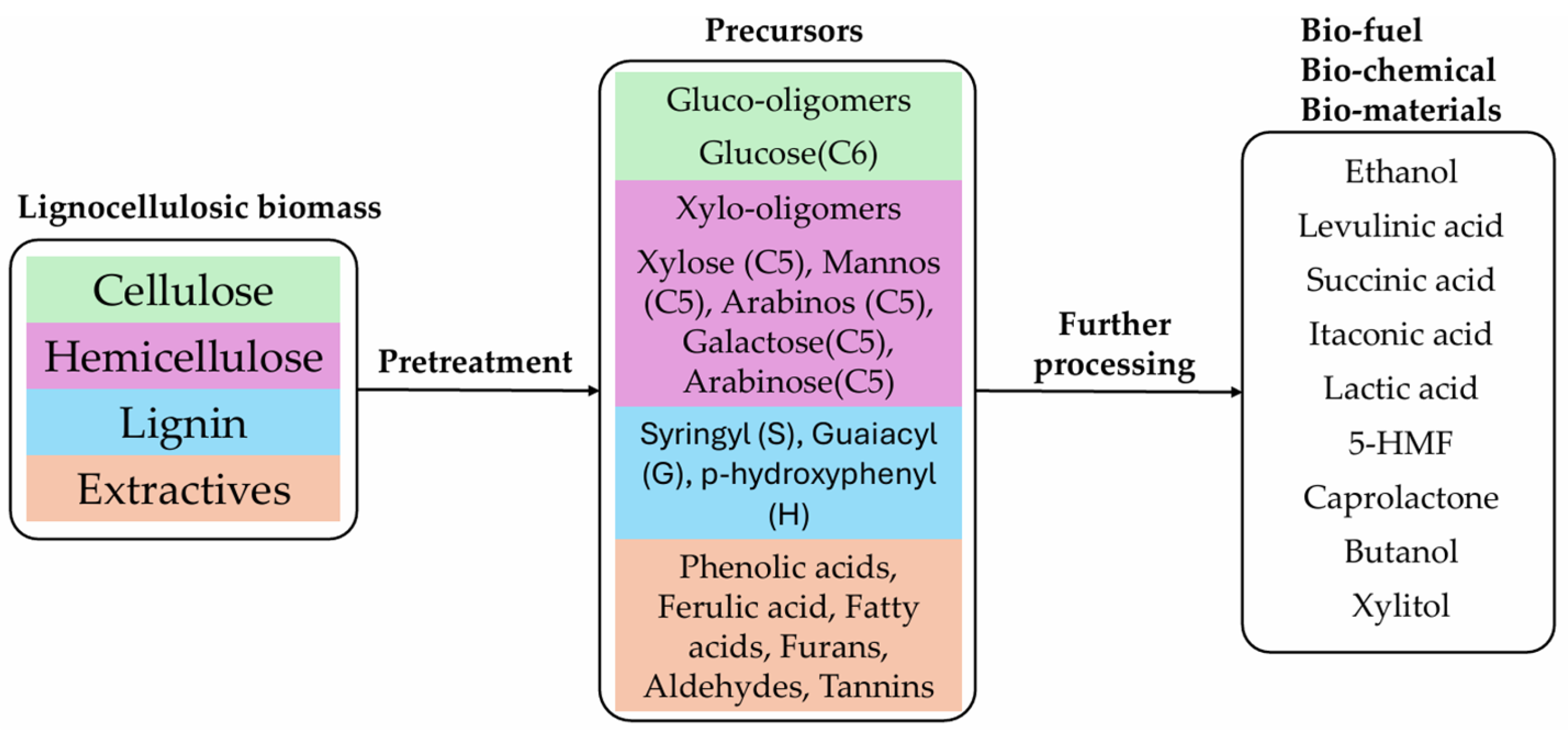
1. Introduction
2. Methods
2.1. Process Development and Calculation Methods
2.2. Lignocellulosic Biomass, Components Specification and Assumptions
- Steady-state process conditions.
- All the separators were ideal.
- No pressure drop occurred throughout the process.
- The hydrothermal separation reactor neglected the conversion of lignin and extractives from biomass.
- No chemical changes in water were considered during the process simulation.
- Ash was considered an inert component.
2.3. Hydrodynamic Cavitation
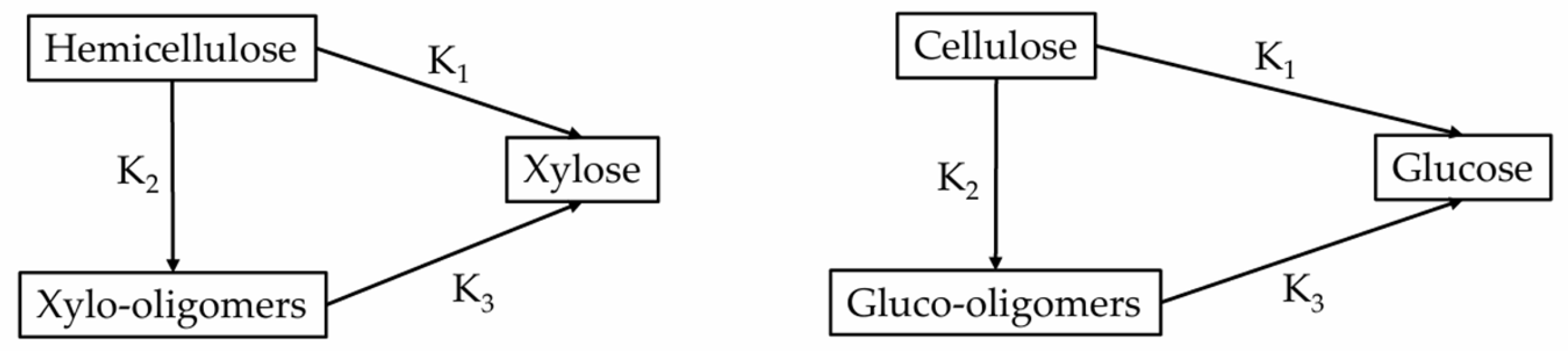
2.4. Hydrothermal Separation (HTS)
2.5. Aspen Plus Model Description
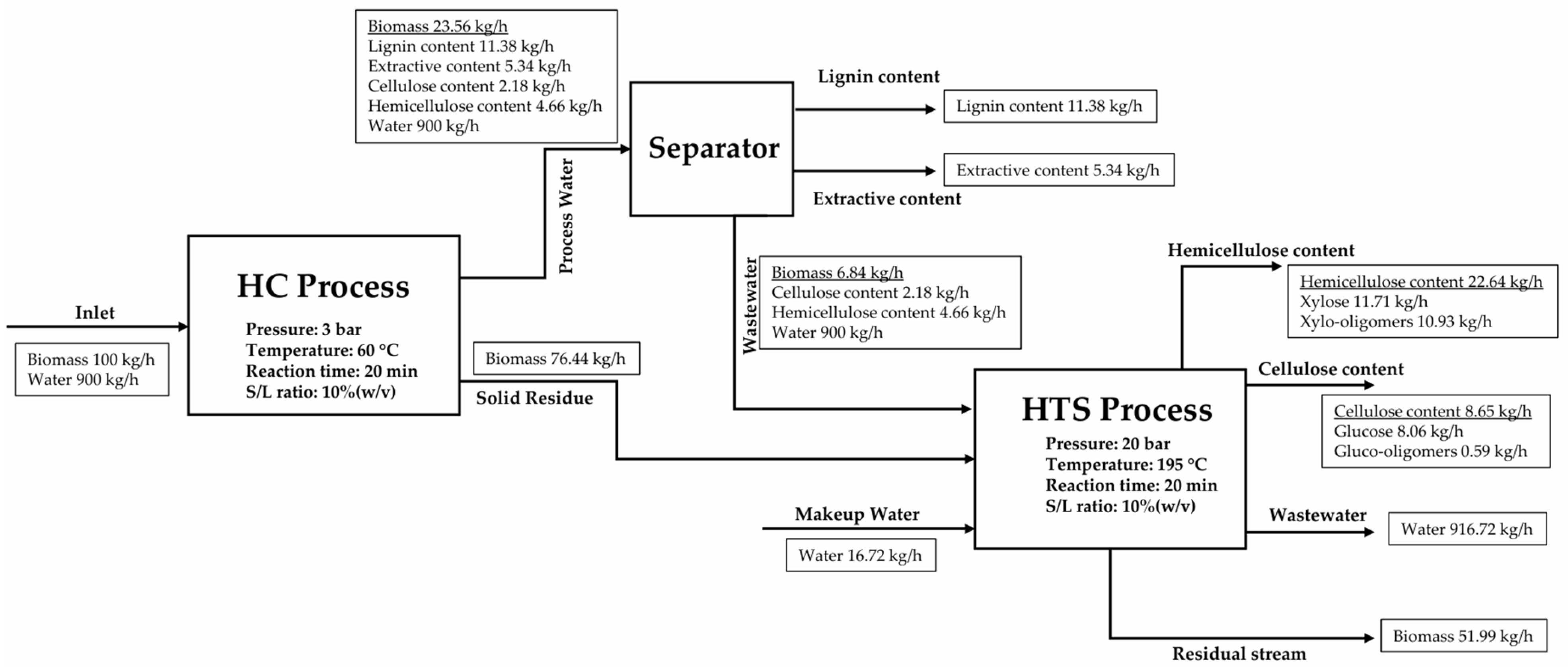
2.5.1. Model 1: Hydrodynamic Cavitation (HC) Process
2.5.2. Model 2: Hydrothermal Separation (HTS) Process
2.5.3. Model 3: Coupling of HC and HTS Process
2.6. Process Analysis
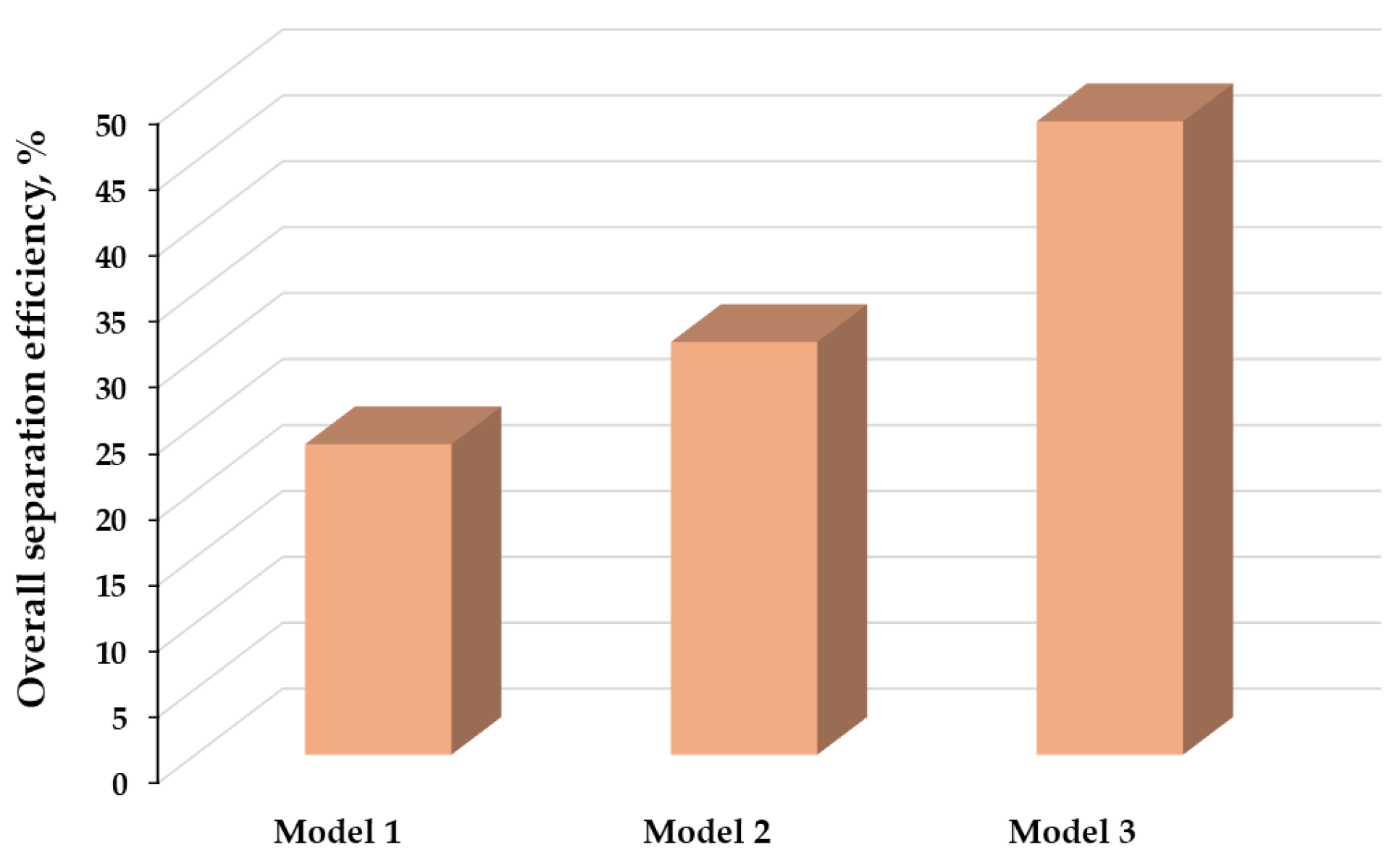
2.6.1. Analysis of Simulated Models
2.6.2. Sensitivity Analysis of the Coupled Process
2.6.3. Optimization of the Coupled Process
3. Results and Discussions
3.1. Model Validation
3.2. Mass Balance and Analysis of Simulation Models
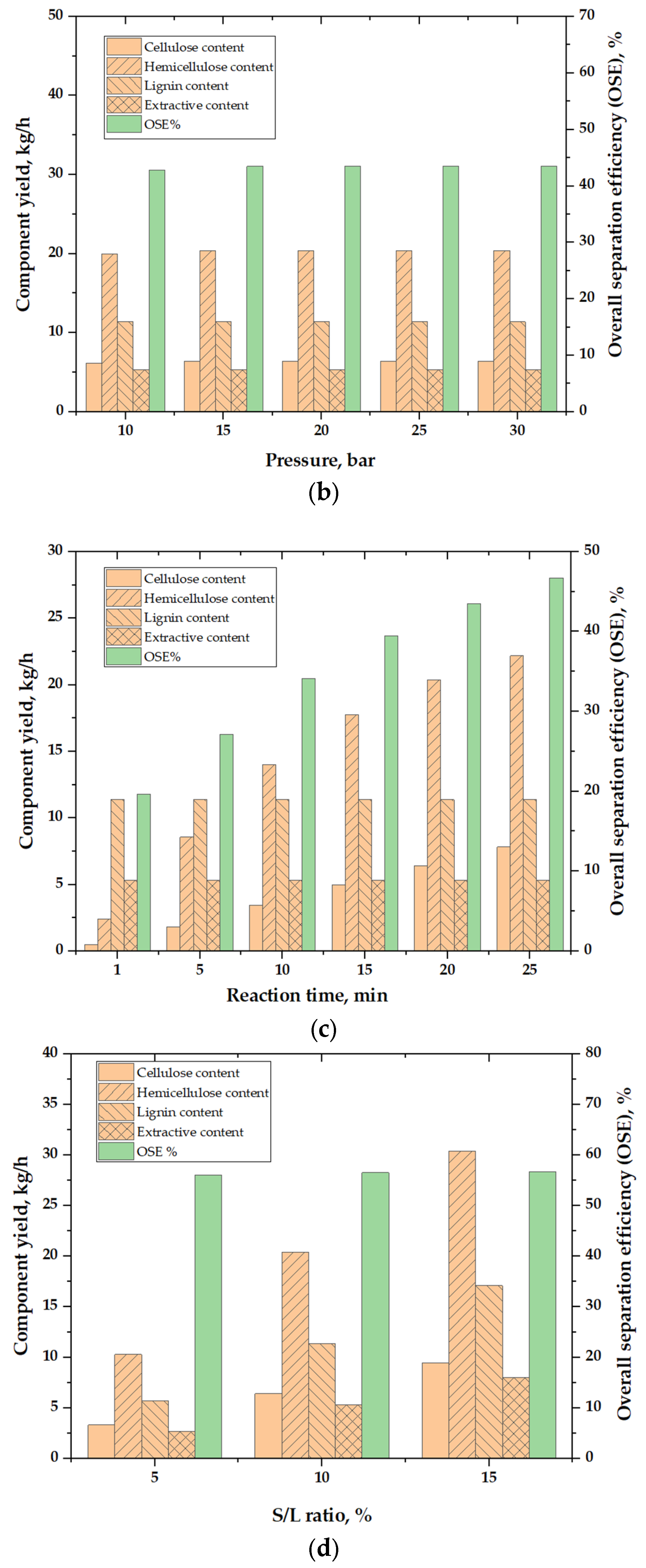
3.3. Sensitivity Analysis
3.3.1. Effects of HTS Temperature
3.3.2. Effects of HTS Pressure
3.3.3. Effects of HTS Reaction Time
3.3.4. Effects of Feedstock S/L Ratio
3.4. Process Optimization
3.4.1. Statistical Analysis and Response Model
3.4.2. Response Surface Plot
3.4.3. Optimization of Parameters for Maximum Overall Separation Efficiency
3.5. Limitations of the Study
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HC | Hydrodynamic Cavitation |
| HTS | Hydrothermal Separation |
| LCB | Lignocellulosic Biomass |
| RSM | Response Surface Methodology |
| CCD | Central Composite Design |
| OSE | Overall Separation Efficiency (%) |
| ṁfeed | Mass flow rate of feed biomass (kg/h) |
| ṁuntreated | Mass flow rate of residual untreated biomass after the process (kg/h) |
References
- Lu, Y.C.; Lu, Y.; Lu, Z.L.; Wei, X.Y. Detailed Componential Characterization of Extractable Species with Organic Solvents from Wheat Straw. Int. J. Anal. Chem. 2017, 2017, 7305682. [Google Scholar] [CrossRef] [PubMed]
- Tsegaye, B.; Balomajumder, C.; Roy, P. Microbial delignification and hydrolysis of lignocellulosic biomass to enhance biofuel production: An overview and future prospect. Bull. Natl. Res. Cent. 2019, 43, 51. [Google Scholar] [CrossRef]
- Das, N.; Jena, P.K.; Padhi, D.; Kumar Mohanty, M.; Sahoo, G. A comprehensive review of characterization, pretreatment and its applications on different lignocellulosic biomass for bioethanol production. Biomass Convers. Biorefinery 2023, 13, 1503–1527. [Google Scholar] [CrossRef]
- Ge, X.; Chang, C.; Zhang, L.; Cui, S.; Luo, X.; Hu, S.; Qin, Y.; Li, Y. Conversion of Lignocellulosic Biomass Into Platform Chemicals for Biobased Polyurethane Application, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; Volume 3. [Google Scholar] [CrossRef]
- Chen, H. Biotechnology of Lignocellulose; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Kim, I.; Lee, I.; Jeon, S.H.; Hwang, T.; Han, J.I. Hydrodynamic cavitation as a novel pretreatment approach for bioethanol production from reed. Bioresour. Technol. 2015, 192, 335–339. [Google Scholar] [CrossRef]
- Randhavane Shrikant, B.; Khambete, A.K. Hydrodynamic Cavitation: A Novel Treatment Approach. Mater. Today Proc. 2017, 4, 9680–9684. [Google Scholar] [CrossRef]
- Ge, M.; Sun, C.; Zhang, G.; Coutier-Delgosha, O.; Fan, D. Combined suppression effects on hydrodynamic cavitation performance in Venturi-type reactor for process intensification. Ultrason. Sonochem. 2022, 86, 106035. [Google Scholar] [CrossRef]
- Rudra, S.; Jayathilake, M. 5.08—Hydrothermal Liquefaction of Biomass for Biofuel Production, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2022; Volume 1–5. [Google Scholar] [CrossRef]
- Ralph, J.; Lapierre, C.; Boerjan, W. Lignin structure and its engineering. Curr. Opin. Biotechnol. 2019, 56, 240–249. [Google Scholar] [CrossRef]
- Terán Hilares, R.; dos Santos, J.C.; Ahmed, M.A.; Jeon, S.H.; da Silva, S.S.; Han, J.I. Hydrodynamic cavitation-assisted alkaline pretreatment as a new approach for sugarcane bagasse biorefineries. Bioresour. Technol. 2016, 214, 609–614. [Google Scholar] [CrossRef]
- Martín, C.; Dixit, P.; Momayez, F.; Jönsson, L.J. Hydrothermal Pretreatment of Lignocellulosic Feedstocks to Facilitate Biochemical Conversion. Front. Bioeng. Biotechnol. 2022, 10, 846592. [Google Scholar] [CrossRef]
- Baxi, P.B.; Pandit, A.B. Using cavitation for delignification of wood. Bioresour. Technol. 2012, 110, 697–700. [Google Scholar] [CrossRef]
- Preece, K.E.; Hooshyar, N.; Krijgsman, A.J.; Fryer, P.J.; Zuidam, N.J. Intensification of protein extraction from soybean processing materials using hydrodynamic cavitation. Innov. Food Sci. Emerg. Technol. 2017, 41, 47–55. [Google Scholar] [CrossRef]
- Meneguzzo, F.; Brunetti, C.; Fidalgo, A.; Ciriminna, R.; Delisi, R.; Albanese, L.; Zabini, F.; Gori, A.; Nascimento, L.B.d.S.; De Carlo, A.; et al. Real-scale integral valorization of waste orange peel via hydrodynamic cavitation. Processes 2019, 7, 581. [Google Scholar] [CrossRef]
- Albanese, L.; Bonetti, A.; D’Acqui, L.P.; Meneguzzo, F.; Zabini, F. Affordable production of antioxidant aqueous solutions by hydrodynamic cavitation processing of silver fir (Abies alba Mill.) needles. Foods 2019, 8, 65. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, R.; Damizia, M.; De Filippis, P.; Patrizi, D.; Verdone, N.; Vilardi, G.; de Caprariis, B. Recent developments and future outlooks of hydrodynamic cavitation as an intensification technology for renewable biofuels production. J. Environ. Chem. Eng. 2023, 11, 110819. [Google Scholar] [CrossRef]
- Batista, G.; Souza, R.B.A.; Pratto, B.; dos Santos-Rocha, M.S.R.; Cruz, A.J.G. Effect of severity factor on the hydrothermal pretreatment of sugarcane straw. Bioresour. Technol. 2019, 275, 321–327. [Google Scholar] [CrossRef]
- Zhou, W.; Yang, M.; Wang, C.; Liu, J.; Xing, J. Changes in plant cell-wall structure of corn stover due to hot compressed water pretreatment and enhanced enzymatic hydrolysis. World J. Microbiol. Biotechnol. 2014, 30, 2325–2333. [Google Scholar] [CrossRef]
- dos Santos Rocha, M.S.R.; Pratto, B.; de Sousa, R.; Almeida, R.M.R.G.; Cruz, A.J.G. da A kinetic model for hydrothermal pretreatment of sugarcane straw. Bioresour. Technol. 2017, 228, 176–185. [Google Scholar] [CrossRef]
- Ghavami, N.; Özdenkçi, K.; Chianese, S.; Musmarra, D.; De Blasio, C. Process simulation of hydrothermal carbonization of digestate from energetic perspectives in Aspen Plus. Energy Convers. Manag. 2022, 270, 116215. [Google Scholar] [CrossRef]
- Parmar, P.; Mukherjee, S.; Kumar Singh, V.; Meikap, B.C. Thermo-kinetic analysis of sugarcane bagasse as a sustainable energy resource evaluation. Therm. Sci. Eng. Prog. 2024, 54, 102836. [Google Scholar] [CrossRef]
- Wooley, R.J.; Putsche, V. Development of an ASPEN PLUS Physical Property Database for Biofuels Components; National Renewable Energy Laboratory: Golden, CO, USA, 1996. Available online: https://scispace.com/pdf/development-of-an-aspen-plus-physical-property-database-for-4u1k1wcwxb.pdf (accessed on 19 June 2025).
- Ranzi, E.; Debiagi, P.E.A.; Frassoldati, A. Mathematical Modeling of Fast Biomass Pyrolysis and Bio-Oil Formation. Note I: Kinetic Mechanism of Biomass Pyrolysis. ACS Sustain. Chem. Eng. 2017, 5, 2867–2881. [Google Scholar] [CrossRef]
- Cecílio, D.M.; Gonçalves, J.R.M.; Correia, M.J.N.; Mateus, M.M. Aspen Plus® Modeling and Simulation of an Industrial Biomass Direct Liquefaction Process. Fuels 2023, 4, 221–242. [Google Scholar] [CrossRef]
- Gorensek, M.B.; Shukre, R.; Chen, C.C. Development of a Thermophysical Properties Model for Flowsheet Simulation of Biomass Pyrolysis Processes. ACS Sustain. Chem. Eng. 2019, 7, 9017–9027. [Google Scholar] [CrossRef]
- Badve, M.; Gogate, P.; Pandit, A.; Csoka, L. Hydrodynamic cavitation as a novel approach for wastewater treatment in wood finishing industry. Sep. Purif. Technol. 2013, 106, 15–21. [Google Scholar] [CrossRef]
- Terán Hilares, R.; Ienny, J.V.; Marcelino, P.F.; Ahmed, M.A.; Antunes, F.A.F.; da Silva, S.S.; Santos, J.C. dos Ethanol production in a simultaneous saccharification and fermentation process with interconnected reactors employing hydrodynamic cavitation-pretreated sugarcane bagasse as raw material. Bioresour. Technol. 2017, 243, 652–659. [Google Scholar] [CrossRef]
- Sun, R.C.; Salisbury, D.; Tomkinson, J. Chemical composition of lipophilic extractives released during the hot water treatment of wheat straw. Bioresour. Technol. 2003, 88, 95–101. [Google Scholar] [CrossRef]
- Liu, Z.H.; Ragauskas, A. Emerging Technologies for Biorefineries, Biofuels, and Value-Added Commodities; Springer Nature: Berlin/Heidelberg, Germany, 2021. [Google Scholar] [CrossRef]
- Jaroenkhasemmeesuk, C.; Tippayawong, N.; Shimpalee, S.; Ingham, D.B.; Pourkashanian, M. Improved simulation of lignocellulosic biomass pyrolysis plant using chemical kinetics in Aspen Plus® and comparison with experiments. Alex. Eng. J. 2023, 63, 199–209. [Google Scholar] [CrossRef]
- Terán Hilares, R.; de Almeida, G.F.; Ahmed, M.A.; Antunes, F.A.F.; da Silva, S.S.; Han, J.I.; Santos, J.C. dos Hydrodynamic cavitation as an efficient pretreatment method for lignocellulosic biomassA parametric study. Bioresour. Technol. 2017, 235, 301–308. [Google Scholar] [CrossRef]
- Thangavelu, K.; Desikan, R.; Taran, O.P.; Uthandi, S. Delignification of corncob via combined hydrodynamic cavitation and enzymatic pretreatment: Process optimization by response surface methodology. Biotechnol. Biofuels 2018, 11, 203. [Google Scholar] [CrossRef]
- Pronyk, C.; Mazza, G. Kinetic modeling of hemicellulose hydrolysis from triticale straw in a pressurized low polarity water flow-through reactor. Ind. Eng. Chem. Res. 2010, 49, 6367–6375. [Google Scholar] [CrossRef]
- Qiao, D.; Hu, B.; Gan, D.; Sun, Y.; Ye, H.; Zeng, X. Extraction optimized by using response surface methodology, purification and preliminary characterization of polysaccharides from Hyriopsis cumingii. Carbohydr. Polym. 2009, 76, 422–429. [Google Scholar] [CrossRef]
- Terán Hilares, R.; Kamoei, D.V.; Ahmed, M.A.; da Silva, S.S.; Han, J.I.; Santos, J.C. dos A new approach for bioethanol production from sugarcane bagasse using hydrodynamic cavitation assisted-pretreatment and column reactors. Ultrason. Sonochem. 2018, 43, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, R.H.; Dey, A. Pre-Treatment and Characterization of Water Hyacinth Biomass (WHB) for Enhanced Xylose Production Using Dilute Alkali Treatment Method. Water 2025, 17, 301. [Google Scholar] [CrossRef]
- Young, F.R. Hydrodynamic Cavitation. Cavitation 1999, 187–317. [Google Scholar] [CrossRef]




| Proximate Analysis | wt% | Ultimate Analysis | wt% |
|---|---|---|---|
| Moisture | 7.59 | C | 43.38 |
| Volatile matter | 87.37 | H | 6.43 |
| Fixed carbon | 3.69 | N | 0.39 |
| Ash | 1.00 | S | 0.058 |
| O | 49.74 | ||
| HHV, MJ/kg | 19.68 |
| Kinetic Rate Constant | Hemicellulose | Cellulose | ||
|---|---|---|---|---|
| ln(A) | Ea, kJ/mol | ln(A) | Ea, kJ/mol | |
| K1 | 10.90 | 62.68 | 22.40 | 105.14 |
| K2 | 25.75 | 109.49 | 49.67 | 216.44 |
| K3 | 53.66 | 220.23 | 25.32 | 105.60 |
| Process Parameters | Unit | Value |
|---|---|---|
| Mass Flow Rate | kg/h | 1000 |
| Solid-to-Liquid (S/L) ratio | % | 10 |
| HC Reactor | ||
| Reactor Inlet Pressure a | bar | 3 |
| Reactor Temperature a | °C | 60 |
| Reaction Time a | min | 20 |
| HTS Reactor | ||
| Reactor Pressure | bar | 20 |
| Reactor Temperature | °C | 195 |
| Reaction Time | min | 20 |
| HC Process | HTS Process | |||||||
|---|---|---|---|---|---|---|---|---|
| [6] | [32] | [33] | This Study: Model 1 | [19] | [20] | [18] | This Study: Model 2 | |
| Biomass | Reed | Sugarcane bagasse | Corncob | Sugarcane bagasse | Corn Strover | Sugarcane bagasse | Sugarcane straw | Sugarcane Bagasse |
| Temperature, °C | 77 | 55 | 30 | 60 | 190 | 195 | 195 | 195 |
| Pressure, bar | 5 | 3 | 0.5 | 3 | - | - | - | 20 |
| Reaction time, min | 41.1 | 30 | 60 | 20 | - | - | 10 | 20 |
| Biomass loading | 11.8% (w/v) | - | 5% (w/v) | 10% (w/v) | 10% (w/v) | 1:10 (w/v) | 1:10 (w/v) | 10% (w/v) |
| Reactor type | Orifice | Orifice | Orifice | RYield | Tube reactor | High-pressure reactor | High-pressure reactor | RBatch |
| Overall separation efficiency, % | 23.6 | 22.81 | - | 23.56 | 28.88 a | - | 26.53 a | 31.31 |
| Lignin removal, % | 42.3 | 41.83 | 47.44 | 48.31 | - | - | - | - |
| Cellulose removal, % | - | - | - | - | 12.19 | 21 | 9.80 | 22.4 |
| Hemicellulose removal, % | - | - | - | - | 83.7 | 85 | 85.45 | 86.03 |
| Run no. | Temperature, °C | Reaction Time, min | Pressure, bar | S/L Ratio, % | Overall Separation Efficiency (OSE), % | |
|---|---|---|---|---|---|---|
| Numerical Model | Quadratic Model | |||||
| 1 | 207.5 | 10 | 25 | 12.5 | 48.8733 | 48.8909 |
| 2 | 207.5 | 10 | 25 | 7.5 | 46.4591 | 47.0059 |
| 3 | 195.0 | 15 | 20 | 10.0 | 43.7905 | 43.7905 |
| 4 | 182.5 | 20 | 25 | 12.5 | 37.0453 | 37.1190 |
| 5 | 207.5 | 20 | 15 | 7.5 | 55.5814 | 56.9240 |
| 6 | 182.5 | 20 | 15 | 7.5 | 35.1894 | 36.0625 |
| 7 | 207.5 | 20 | 25 | 12.5 | 60.0262 | 60.5084 |
| 8 | 170.0 | 15 | 20 | 10.0 | 26.2098 | 26.1680 |
| 9 | 195.0 | 15 | 20 | 15.0 | 43.4494 | 44.3352 |
| 10 | 182.5 | 10 | 15 | 12.5 | 29.2374 | 28.6709 |
| 11 | 195.0 | 15 | 20 | 5.0 | 43.7905 | 41.2448 |
| 12 | 207.5 | 20 | 15 | 12.5 | 59.6850 | 60.0897 |
| 13 | 207.5 | 10 | 15 | 12.5 | 48.5026 | 48.3304 |
| 14 | 207.5 | 20 | 25 | 7.5 | 56.1323 | 57.5896 |
| 15 | 195.0 | 15 | 10 | 10.0 | 43.0214 | 42.2474 |
| 16 | 182.5 | 20 | 25 | 7.5 | 35.2191 | 36.1605 |
| 17 | 195.0 | 15 | 20 | 10.0 | 43.7905 | 43.7905 |
| 18 | 182.5 | 10 | 25 | 12.5 | 29.2372 | 28.6638 |
| 19 | 207.5 | 10 | 15 | 7.5 | 45.3813 | 46.1984 |
| 20 | 195.0 | 5 | 20 | 10.0 | 29.6019 | 30.0107 |
| 21 | 195.0 | 25 | 20 | 10.0 | 51.2602 | 49.1915 |
| 22 | 182.5 | 10 | 15 | 7.5 | 28.2123 | 28.4992 |
| 23 | 182.5 | 10 | 25 | 7.5 | 28.2529 | 28.7390 |
| 24 | 220.0 | 15 | 20 | 10.0 | 68.8747 | 67.2566 |
| 25 | 182.5 | 20 | 15 | 12.5 | 37.0455 | 37.2678 |
| 26 | 195.0 | 15 | 30 | 10.0 | 43.7919 | 42.9059 |
| Source | DF | Adj SS | Adj MS | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 14 | 3169.73 | 226.41 | 152.80 | 0.000 | |
| Linear | 4 | 3099.24 | 774.81 | 522.91 | 0.000 | |
| X1 | 1 | 2532.42 | 2532.42 | 1709.10 | 0.000 | Significant |
| X2 | 1 | 551.85 | 551.85 | 372.44 | 0.000 | Significant |
| X3 | 1 | 0.65 | 0.65 | 0.44 | 0.517 | |
| X4 | 1 | 14.33 | 14.33 | 9.67 | 0.007 | Significant |
| Square | 4 | 55.17 | 13.79 | 9.31 | 0.000 | |
| X12 | 1 | 15.26 | 15.26 | 10.30 | 0.005 | Significant |
| X22 | 1 | 31.37 | 31.37 | 21.17 | 0.000 | Significant |
| X32 | 1 | 2.63 | 2.63 | 1.78 | 0.201 | |
| X42 | 1 | 1.79 | 1.79 | 1.21 | 0.288 | |
| 2-Way Interaction | 6 | 15.31 | 2.55 | 1.72 | 0.180 | |
| X1X2 | 1 | 10.00 | 10.00 | 6.75 | 0.019 | Significant |
| X1X3 | 1 | 0.32 | 0.32 | 0.22 | 0.647 | |
| X1X4 | 1 | 3.84 | 3.84 | 2.59 | 0.127 | |
| X2X3 | 1 | 0.02 | 0.02 | 0.01 | 0.909 | |
| X2X4 | 1 | 1.07 | 1.07 | 0.72 | 0.408 | |
| X3X4 | 1 | 0.06 | 0.06 | 0.04 | 0.842 | |
| Error | 16 | 23.71 | 1.48 | |||
| Lack-of-Fit | 10 | 23.71 | 2.37 | |||
| Pure Error | 6 | 0.00 | 0.00 | |||
| Total | 30 | 3193.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, M.B.; Rudra, S. Hydrodynamic Cavitation-Assisted Hydrothermal Separation: A Pathway for Valorizing Lignocellulosic Biomass into Biopolymers and Extractives. Processes 2025, 13, 2041. https://doi.org/10.3390/pr13072041
Ahmed MB, Rudra S. Hydrodynamic Cavitation-Assisted Hydrothermal Separation: A Pathway for Valorizing Lignocellulosic Biomass into Biopolymers and Extractives. Processes. 2025; 13(7):2041. https://doi.org/10.3390/pr13072041
Chicago/Turabian StyleAhmed, Md. Bayazid, and Souman Rudra. 2025. "Hydrodynamic Cavitation-Assisted Hydrothermal Separation: A Pathway for Valorizing Lignocellulosic Biomass into Biopolymers and Extractives" Processes 13, no. 7: 2041. https://doi.org/10.3390/pr13072041
APA StyleAhmed, M. B., & Rudra, S. (2025). Hydrodynamic Cavitation-Assisted Hydrothermal Separation: A Pathway for Valorizing Lignocellulosic Biomass into Biopolymers and Extractives. Processes, 13(7), 2041. https://doi.org/10.3390/pr13072041








