Ultrasound-Assisted Microextraction for Food Chemical Contaminant Analysis: A Review
Abstract
1. Introduction
2. Principles of Ultrasound-Assisted Microextraction (UAME)
Types of UAME Techniques
3. Optimization Parameters Influencing UAME Performance
4. UAME in Food Chemical Contaminant Analysis
4.1. Pesticide Residues
4.2. Potentially Toxic Elements (PTEs)
| Analytes/Examples | Sample Matrix | UAME Approach | Recovery (%) | Detection | Reference |
|---|---|---|---|---|---|
| Potentially toxic elements | |||||
| Al, Ca, Cd, Cu, Mg, Mn, Ni, Ti, V, Zn | Edible oils | UA-EME | 92.9–109.1 | ICP-MS | [129] |
| As | Rice and flour | UA-EH-WPME * | 98–107 | ICP-MS | [132] |
| Se | Organic rice | UAME | 85.5–106.7 | ICP-MS | [133] |
| Hg (CH3Hg+ and Hg2+) | Fish | UA-CPE | 91.5–96.5 | UV-VIS | [105] |
| Zn, Ni, Co | Foods and vegetables | UA-CPE | >94 | FAAS | [104] |
| Cd and Pb | Edible vegetable oils | UA-SEME | 95.8–105.8 | GFAAS | [131] |
| Co, Ni, Pb, Hg, Cd | Rice | UA-EME | 88.6–107.0 | ICP-OES | [57] |
| Sb, Sn and Tl | Food and water | UA-CPE | 98–100 | ICP-OES | [130] |
| iAs (As3+ and As5+) | Food and water | UA-DµSPE | >95 | ICP-OES | [96] |
| Trace mercury (Hg2+) | Water | UA-MSPE | >90 | SVG-AFS | [110] |
| Sb3+ | Bottled beverages | UA-DµSPE | 96 | HGAAS | [65] |
| Al | Whey milk | UA-DLLME | >98 | ICP-OES | [62] |
| Total As | Food | UA-DµSPE | 96.0–98.5 | HG-AAS | [94] |
| Cu2+ | Beverages | UA-CPE | 94–103 | UV-VIS | [103] |
| Pb, Cd, Zn, Mn | Water and vegetables | UA-DLLME | 94.3–97.9 | FAAS | [87] |
4.3. Mycotoxins
4.4. Veterinary Drug Residues
| Analytes/Examples | Sample Matrix | UAME Approach | Recovery (%) | Detection | Reference |
|---|---|---|---|---|---|
| Veterinary drugs | |||||
| Oxytetracycline, tetracycline, epi-chlorotetracycline, chlorotetracycline and, doxycycline | Milk | UA- MSPD | 82–108 | HPLC–DAD | [100] |
| Tilmicosin and tylosin | Chicken fat | UA-EME | 73–117 | CE * | [89] |
| Chloramphenicol | Honey | UA-DLLME | / | UHPLC MS/MS | [85] |
| Sulfonamides: sulfapyridine, sulfamethazine, sulfadimethoxine | Fruit juices | UA-LLME | 88.09–97.84 | HPLC-UV | [138] |
| Quinolones: enrofloxacin, ciprofloxacin | Milk | UA-LLME | 84.4–95.4 | HPLC-UV | [139] |
| Sulfonamides: sulfacetamide, sulfamerazine, sulfanilamide pyridine, sulfadizine, sulfamonomethoxine, sulfamethoxazole, and sulfadimethoxine. | Water and seafood | UA-DLLME | 80.0–116.0 | HPLC-DAD | [140] |
| β-Lactam antibiotics: penicillin G, ampicillin, and amoxicillin | Egg, honey, and chicken muscle | UA-DLLME | >97 | HPLC–PDA | [121] |
| Sulfamethizole, sulfadiazine, sulphamethoxazole, sulfachloropyridazine, sulfisoxazole and sulfadimethoxin | Milk and egg | UA-DµSPE | 79.1–100.0 | HPLC-DAD | [95] |
| Tetracyclines, oxytetracycline, chlortetracycline, and doxycycline | Milk, egg, and honey | UA-DµSPE | 95.2–99.3 | HPLC–PDA | [93] |
4.5. Other Contaminants
| Analytes/Examples | Sample Matrix | UAME Approach | Recovery (%) | Detection | Reference |
|---|---|---|---|---|---|
| Bisphenol A | Beverage | UA-EME | ≥82 | GC-MS | [145] |
| PCBs | Tap waters | UA-EME | 87.29–92.83 | GC-MS | [146] |
| tert-butylhydroquinone (TBHQ) | Soybean oils | UA-LLME | 93.4–108.8 | HPLC-UV | [147] |
| Sudan dyes | Spice | UA-SLME | 85.55–99.29 | HPLC-UV | [90] |
| Non-steroidal anti-inflammatory drugs | Water and milk | UA-DLLME | 79.42–107.52 | HPLC-UV | [148] |
| PAHs | Soft drinks and non-alcoholic beers | UA-MSPE | 94.67–109.45 | GC-MS | [109] |
| Endocrine-disrupting phenols | Water, milk and beverage | UA-DLLME | 81.79–109.82 | HPLC-UV | [84] |
| Benzotriazole (BTRs) and benzothiazole (BTHs) derivatives | Tea beverages | UA-LPME | 65–107 | UHPLC | [143] |
| Parabens | Edible oil | UA-LLME | 85.1–106.8 | HPLC-UV | [144] |
| 5-hydroxymethylfurfural (5-HMF) | Honey | UA-DLLME | 92–103 | UV–VIS | [149] |
| PAHs | Tomato paste | UA-MSPE | 88.03–98.52 | GC-MS | [24] |
| Illegal colorants | Traditional Chinese medicines | UA-SLME | 94.2–103.1 | HPLC-DAD | [92] |
| Formaldehyde | Milk-based products | UA-CPE | 90.8–97.4 | UV-VIS | [103] |
| Bisphenol A | Milk | UA-MSPE | 89.1–99.4 | HPLC-UV | [150] |
5. Emerging Technologies and Future Perspectives
6. Limitations and Challenges
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Balkrishna, A.; Kumari, A.; Kumar, A.; Arya, V.; Chauhan, A.; Upadhyay, N.K.; Guleria, I.; Amarowicz, R.; Kumar, D.; Kuca, K. Biosensors for Detection of Pesticide Residue, Mycotoxins and Heavy Metals in Fruits and Vegetables: A Concise Review. Microchem. J. 2024, 205, 111292. [Google Scholar] [CrossRef]
- Fu, Y.; Yin, S.; Zhao, C.; Fan, L.; Hu, H. Combined Toxicity of Food-Borne Mycotoxins and Heavy Metals or Pesticides. Toxicon 2022, 217, 148–154. [Google Scholar] [CrossRef]
- Tian, M.; He, X.; Feng, Y.; Wang, W.; Chen, H.; Gong, M.; Liu, D.; Clarke, J.L.; Eerde, A. Van Pollution by Antibiotics and Antimicrobial Resistance in LiveStock and Poultry Manure in China, and Countermeasures. Antibiotics 2021, 10, 539. [Google Scholar] [CrossRef]
- Hasan, G.M.M.A.; Shaikh, M.A.A.; Satter, M.A.; Hossain, M.S. Detection of Indicator Polychlorinated Biphenyls (I-PCBs) and Polycyclic Aromatic Hydrocarbons (PAHs) in Cow Milk from Selected Areas of Dhaka, Bangladesh and Potential Human Health Risks Assessment. Toxicol. Rep. 2022, 9, 1514–1522. [Google Scholar] [CrossRef]
- Leong, W.H.; Teh, S.Y.; Hossain, M.M.; Nadarajaw, T.; Zabidi-Hussin, Z.; Chin, S.Y.; Lai, K.S.; Lim, S.H.E. Application, Monitoring and Adverse Effects in Pesticide Use: The Importance of Reinforcement of Good Agricultural Practices (GAPs). J. Environ. Manag. 2020, 260, 109987. [Google Scholar] [CrossRef] [PubMed]
- Targuma, S.; Njobeh, P.B.; Ndungu, P.G. Current Applications of Magnetic Nanomaterials for Extraction of Mycotoxins, Pesticides, and Pharmaceuticals in Food Commodities. Molecules 2021, 26, 4284. [Google Scholar] [CrossRef] [PubMed]
- Onjia, A.; Huang, X.; Trujillo González, J.M.; Egbueri, J.C. Chemometric Approach to Distribution, Source Apportionment, Ecological and Health Risk of Trace Pollutants. Front. Environ. Sci. 2022, 10, 1107465. [Google Scholar] [CrossRef]
- Oznur, F.; Eylem, A.; Nimo, O.; Yussuf, H.; Kabak, B. Co-Occurrence and Risk Assessment of Ochratoxin A and Deoxynivalenol in Tortillas. Mycotoxin Res. 2025, 41, 475–484. [Google Scholar] [CrossRef]
- Miletić, A.; Radomirović, M.; Đorđević, A.; Bogosavljević, J.; Lučić, M.; Onjia, A. Geospatial Mapping of Ecological Risk from Potentially Toxic Elements in Soil in the Pannonian-Carpathian Border Area South of the Danube. Carpathian J. Earth Environ. Sci. 2022, 17, 351–363. [Google Scholar] [CrossRef]
- Miletić, A.; Lučić, M.; Onjia, A. Exposure Factors in Health Risk Assessment of Heavy Metal(Loid)s in Soil and Sediment. Metals 2023, 13, 1266. [Google Scholar] [CrossRef]
- Savić, A.; Mutić, J.; Lučić, M.; Onjia, A. Dietary Intake of Minerals and Potential Human Exposure to Toxic Elements via Coffee Consumption. Biol. Trace Elem. Res. 2024, 203, 1817–1829. [Google Scholar] [CrossRef]
- EFSA. Science, Safe Food, Sustainability (EFSA). Available online: https://www.efsa.europa.eu/en (accessed on 27 June 2025).
- US EPA. U.S. Environmental Protection Agency (US EPA). Available online: https://www.epa.gov/ (accessed on 27 June 2025).
- FAO/WHO. CODEXALIMENTARIUS FAO-WHO. Available online: https://www.fao.org/fao-who-codexalimentarius/en/ (accessed on 27 June 2025).
- Wahab, S.; Muzammil, K.; Nasir, N.; Khan, M.S.; Ahmad, M.F.; Khalid, M.; Ahmad, W.; Dawria, A.; Reddy, L.K.V.; Busayli, A.M. Review Advancement and New Trends in Analysis of Pesticide Residues in Food: A Comprehensive Review. Plants 2022, 11, 1106. [Google Scholar] [CrossRef] [PubMed]
- El Hosry, L.; Sok, N.; Richa, R.; Al Mashtoub, L.; Cayot, P.; Bou-Maroun, E. Sample Preparation and Analytical Techniques in the Determination of Trace Elements in Food: A Review. Foods 2023, 12, 895. [Google Scholar] [CrossRef] [PubMed]
- López-Lorente, Á.I.; Pena-Pereira, F.; Pedersen-Bjergaard, S.; Zuin, V.G.; Ozkan, S.A.; Psillakis, E. The Ten Principles of Green Sample Preparation. TrAC Trends Anal. Chem. 2022, 148, 116530. [Google Scholar] [CrossRef]
- Wan, Y.C.; Kong, Z.L.; Wu, Y.H.S.; Huang, C.N.; Ogawa, T.; Lin, J.T.; Yang, D.J. Establishment of Appropriate Conditions for the Efficient Determination of Multiple Mycotoxins in Tea Samples and Assessment of Their Drinking Risks. Food Chem. 2025, 463, 141438. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; El-Nouby, M.A.M.; Kimani, P.K.; Lim, L.W.; Rabea, E.I. A Review of the Modern Principles and Applications of Solid-Phase Extraction Techniques in Chromatographic Analysis; Springer Nature: Singapore, 2022; Volume 38, ISBN 0123456789. [Google Scholar]
- Mogashane, T.M.; Mokoena, L.; Tshilongo, J. A Review on Recent Developments in the Extraction and Identification of Polycyclic Aromatic Hydrocarbons from Environmental Samples. Water 2024, 16, 2520. [Google Scholar] [CrossRef]
- Tolcha, T.; Gemechu, T.; Al-Hamimi, S.; Megersa, N.; Turner, C. Multivariate Optimization of a Combined Static and Dynamic Supercritical Fluid Extraction Method for Trace Analysis of Pesticides Pollutants in Organic Honey. J. Sep. Sci. 2021, 44, 1716–1726. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, D.; Zhang, L.; Zhou, Y.; Yang, M. Development and Optimization of a Method Based on QuEChERS-DSPE Followed by UPLC-MS/MS for the Simultaneous Determination of 21 Mycotoxins in Nutmeg and Related Products. Microchem. J. 2021, 168, 106499. [Google Scholar] [CrossRef]
- Narenderan, S.T.; Meyyanathan, S.N.; Babu, B. Review of Pesticide Residue Analysis in Fruits and Vegetables. Pre-Treatment, Extraction and Detection Techniques. Food Res. Int. 2020, 133, 109141. [Google Scholar] [CrossRef]
- Azari, A.; Kamani, H.; Sarkhosh, M.; Vatankhah, N.; Yousefi, M.; Mahmoudi-Moghaddam, H.; Razavinasab, S.A.; Masoudi, M.R.; Sadeghi, R.; Sharifi, N.; et al. Nectarine Core-Derived Magnetite Biochar for Ultrasound-Assisted Preconcentration of Polycyclic Aromatic Hydrocarbons (PAHs) in Tomato Paste: A Cost-Effective and Sustainable Approach. Food Chem. X 2024, 24, 101810. [Google Scholar] [CrossRef]
- Sajid, M.; Płotka-Wasylka, J. Green Analytical Chemistry Metrics: A Review. Talanta 2022, 238, 123046. [Google Scholar] [CrossRef] [PubMed]
- Kaya, S.I.; Cetinkaya, A.; Ozkan, S.A. Green Analytical Chemistry Approaches on Environmental Analysis. Trends Environ. Anal. Chem. 2022, 33, e00157. [Google Scholar] [CrossRef]
- Antos, J.; García-Cansino, L.; García, M.Á.; Ginter-Kramarczyk, D.; Marina, M.L.; Zembrzuska, J.; Câmara, J.S.; Pereira, J.A.M. Microextraction Techniques for Antibiotics Surveillance in the Food Chain and Environment. TrAC Trends Anal. Chem. 2024, 181, 118009. [Google Scholar] [CrossRef]
- Delić, M.; Ristić, M.; Đolić, M.; Perić-Grujić, A.; Onjia, A. Dispersive Liquid–Liquid Chelate Microextraction of Rare Earth Elements: Optimization and Greenness Evaluation. Metals 2025, 15, 52. [Google Scholar] [CrossRef]
- Slavković-Beškoski, L.; Ignjatović, L.; Bolognesi, G.; Maksin, D.; Savić, A.; Vladisavljević, G.; Onjia, A. Dispersive Solid–Liquid Microextraction Based on the Poly(HDDA)/Graphene Sorbent Followed by ICP-MS for the Determination of Rare Earth Elements in Coal Fly Ash Leachate. Metals 2022, 12, 791. [Google Scholar] [CrossRef]
- Ražić, S.; Bakić, T.; Topić, A.; Lukić, J.; Onjia, A. Deep Eutectic Solvent Based Reversed-Phase Dispersive Liquid–Liquid Microextraction and High-Performance Liquid Chromatography for the Determination of Free Tryptophan in Cold-Pressed Oils. Molecules 2023, 28, 2395. [Google Scholar] [CrossRef]
- Elahi, F.; Arain, M.B.; Ali Khan, W.; Ul Haq, H.; Khan, A.; Jan, F.; Castro-Muñoz, R.; Boczkaj, G. Ultrasound-Assisted Deep Eutectic Solvent-Based Liquid–Liquid Microextraction for Simultaneous Determination of Ni (II) and Zn (II) in Food Samples. Food Chem. 2022, 393, 133384. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, L.; Yan, Y.; Gong, B.; Chen, K.; Zhu, G.; Li, Z. Ultrasound Assisted Upper Critical Solution Temperature Type Switchable Deep Eutectic Solvent Based Liquid-Liquid Microextraction for the Determination of Triazole in Water. Anal. Chim. Acta 2024, 1328, 343172. [Google Scholar] [CrossRef]
- Bhargava, N.; Mor, R.S.; Kumar, K.; Sharanagat, V.S. Advances in Application of Ultrasound in Food Processing: A Review. Ultrason. Sonochem. 2021, 70, 105293. [Google Scholar] [CrossRef]
- Lučić, M.; Sredović Ignjatović, I.; Lević, S.; Pećinar, I.; Antić, M.; Đurđić, S.; Onjia, A. Ultrasound-Assisted Extraction of Essential and Toxic Elements from Pepper in Different Ripening Stages Using Box–Behnken Design. J. Food Process. Preserv. 2022, 46, e16493. [Google Scholar] [CrossRef]
- Lučić, M.; Miletić, A.; Savić, A.; Lević, S.; Sredović Ignjatović, I.; Onjia, A. Dietary Intake and Health Risk Assessment of Essential and Toxic Elements in Pepper (Capsicum Annuum). J. Food Compos. Anal. 2022, 111, 104598. [Google Scholar] [CrossRef]
- Savić, A.; Mutić, J.; Lučić, M.; Vesković, J.; Miletić, A.; Onjia, A. Ultrasound-Assisted Extraction Followed by Inductively Coupled Plasma Mass Spectrometry and Multivariate Profiling of Rare Earth Elements in Coffee. Foods 2025, 14, 275. [Google Scholar] [CrossRef]
- Kokosa, J.M. Dispersive Liquid-Liquid Microextraction. In Liquid-Phase Extraction; Elsevier: Amsterdam, The Netherlands, 2020; pp. 473–497. ISBN 9780128169117. [Google Scholar]
- Hussain, I.; Muhammad, N.; Yong-gang, Z.; Subhani, Q. In-Situ Generation of Novel Hydrophobic Dendritic Ionic Liquids from G 1. 0 PAMAM for Ultrasound-Assisted Liquid-Liquid Microextraction of Triazine Herbicides from Environmental Water and Fruit Juice Samples. Microchem. J. 2025, 218, 115242. [Google Scholar] [CrossRef]
- Alves, V.; de Andrade, J.K.; Felsner, M.L. Green and Fast Ultrasound-Assisted Extraction Procedures for Fe, Mn, Mg and Ca Analysis in Cane Syrups by FAAS. J. Food Compos. Anal. 2023, 123, 105495. [Google Scholar] [CrossRef]
- Zhou, F.; Deng, H.; Emiezi Agarry, I.; Hu, J.; Xu, D.; Feng, H.; Kan, J.; Cai, T.; Chen, K. Determination of Multiple Mycotoxins in Chili Powder Using Cold-Induced Liquid–Liquid Extraction and Fe3O4@MWCNTs-NH2 Coupled with UPLC-Q-TOF/MS. Food Chem. 2023, 423, 136291. [Google Scholar] [CrossRef] [PubMed]
- Pérez, R.A.; Albero, B. Ultrasound-Assisted Extraction Methods for the Determination of Organic Contaminants in Solid and Liquid Samples. TrAC Trends Anal. Chem. 2023, 166, 117204. [Google Scholar] [CrossRef]
- Albero, B.; Fernández-Cruz, M.L.; Pérez, R.A. Simultaneous Determination of 15 Mycotoxins in Aquaculture Feed by Liquid Chromatography–Tandem Mass Spectrometry. Toxins 2022, 14, 316. [Google Scholar] [CrossRef]
- García-Valcárcel, A.I.; Miguel, E.; Martín-Esteban, A. Natural Deep Eutectic Solvent-Based Matrix Solid-Phase Dispersion-Ultrasound Assisted Extraction of Pesticides in Pears and Their Determination by Liquid Chromatography-Tandem Mass Spectrometry. Adv. Sample Prep. 2025, 14, 100185. [Google Scholar] [CrossRef]
- Demesa, A.G.; Saavala, S.; Pöysä, M.; Koiranen, T. Overview and Toxicity Assessment of Ultrasound-Assisted Extraction of Natural Ingredients from Plants. Foods 2024, 13, 3066. [Google Scholar] [CrossRef]
- Zhao, J.; Meng, Z.; Zhao, Z.; Zhao, L. Ultrasound-Assisted Deep Eutectic Solvent as Green and Efficient Media Combined with Functionalized Magnetic Multi-Walled Carbon Nanotubes as Solid-Phase Extraction to Determine Pesticide Residues in Food Products. Food Chem. 2020, 310, 125863. [Google Scholar] [CrossRef]
- Khan, S.R.; Sharma, B.; Chawla, P.A.; Bhatia, R. Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES): A Powerful Analytical Technique for Elemental Analysis. Food Anal. Methods 2022, 15, 666–688. [Google Scholar] [CrossRef]
- Rezaee, M. Combination of Ultrasound-Assisted Extraction and Ultrasound-Assisted Emulsification Microextraction for Separation and Enrichment of Permethrin and Deltamethrin Residues in Spinach Samples. J. Anal. Chem. 2023, 78, 1406–1413. [Google Scholar] [CrossRef]
- Chaikhan, P.; Udnan, Y.; Ampiah-Bonney, R.J.; Chaiyasith, W.C. Deep Eutectic Solvent-Based Electromembrane Hollow Fiber Liquid Phase Microextraction for Determining Pb in Water and Food Samples. J. Food Compos. Anal. 2023, 118, 105214. [Google Scholar] [CrossRef]
- Gholizadeh, S.; Mirzaei, H.; Khandaghi, J.; Afshar Mogaddam, M.R.; Javadi, A. Ultrasound–Assisted Solvent Extraction Combined with Magnetic Ionic Liquid Based-Dispersive Liquid–Liquid Microextraction for the Extraction of Mycotoxins from Tea Samples. J. Food Compos. Anal. 2022, 114, 104831. [Google Scholar] [CrossRef]
- Yuvali, D.; Seyhaneyildizi, M.; Soylak, M.; Narin, İ.; Yilmaz, E. An Environment-Friendly and Rapid Liquid-Liquid Microextraction Based on New Synthesized Hydrophobic Deep Eutectic Solvent for Separation and Preconcentration of Erythrosine (E127) in Biological and Pharmaceutical Samples. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 244, 118842. [Google Scholar] [CrossRef]
- Azevedo Lemos, V.; Bastos Santos, L.; Santos Assis, R. Deep Eutectic Solvent in Ultrasound-Assisted Liquid-Phase Microextraction for Determination of Vanadium in Food and Environmental Waters. Microchem. J. 2022, 180, 107543. [Google Scholar] [CrossRef]
- Szreniawa-Sztajnert, A.; Zabiegała, B.; Namieśnik, J. Developments in Ultrasound-Assisted Microextraction Techniques for Isolation and Preconcentration of Organic Analytes from Aqueous Samples. TrAC Trends Anal. Chem. 2013, 49, 45–54. [Google Scholar] [CrossRef]
- Picó, Y. Ultrasound-Assisted Extraction for Food and Environmental Samples. TrAC Trends Anal. Chem. 2013, 43, 84–99. [Google Scholar] [CrossRef]
- Tiwari, B.K. Ultrasound: A Clean, Green Extraction Technology. TrAC Trends Anal. Chem. 2015, 71, 100–109. [Google Scholar] [CrossRef]
- Yuan, S.; Li, C.; Zhang, Y.; Yu, H.; Xie, Y.; Guo, Y.; Yao, W. Ultrasound as an Emerging Technology for the Elimination of Chemical Contaminants in Food: A Review. Trends Food Sci. Technol. 2021, 109, 374–385. [Google Scholar] [CrossRef]
- Khodadoust, S.; Ghaedi, M.; Hadjmohammadi, M.R. Dispersive Nano Solid Material-Ultrasound Assisted Microextraction as a Novel Method for Extraction and Determination of Bendiocarb and Promecarb: Response Surface Methodology. Talanta 2013, 116, 637–646. [Google Scholar] [CrossRef]
- Ebrahimi-Najafabadi, H.; Pasdaran, A.; Bezenjani, R.R.; Bozorgzadeh, E. Determination of Toxic Heavy Metals in Rice Samples Using Ultrasound Assisted Emulsification Microextraction Combined with Inductively Coupled Plasma Optical Emission Spectroscopy. Food Chem. 2019, 289, 26–32. [Google Scholar] [CrossRef]
- Ozcan, S.; Tor, A.; Aydin, M.E. Application of Ultrasound-Assisted Emulsification-Micro-Extraction for the Analysis of Organochlorine Pesticides in Waters. Water Res. 2009, 43, 4269–4277. [Google Scholar] [CrossRef]
- Khayatian, G.; Pourbahram, B. Ultrasound-Assisted Emulsification Microextraction and Preconcentration of Trace Amounts of Silver Ions as a Cyclam Complex. J. Anal. Sci. Technol. 2016, 7, 5. [Google Scholar] [CrossRef]
- Takahashi, F.; Kobayashi, K.; Jin, J. Development and Application of Ultrasound-Assisted Microextraction to Analysis of Fenitrothion in Environmental Samples. Anal. Bioanal. Chem. 2016, 408, 7473–7479. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi-Jouibari, T.; Shaahmadi, Z.; Moradi, M.; Fattahi, N. Extraction and Determination of Strobilurin Fungicides Residues in Apple Samples Using Ultrasound-Assisted Dispersive Liquid-Liquid Microextraction Based on a Novel Hydrophobic Deep Eutectic Solvent Followed by H.P.L.C-U.V. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2022, 39, 105–115. [Google Scholar] [CrossRef]
- Unar, A.A.; Kazi, T.G.; Afridi, H.I.; Baig, J.A.; Lashari, A.A. Evaluate the Aluminum Concentrations in Whey Milk Samples of Cows from Different Areas Using Deep Eutectic Solvent-Based Ultrasound-Assisted Dispersive Liquid-Liquid Microextraction Method. Talanta 2024, 273, 125847. [Google Scholar] [CrossRef] [PubMed]
- Jamil, L.A.; Sami, H.Z.; Aghaei, A.; Moinfar, S.; Ataei, S. Combination of Modified Ultrasound-Assisted Extraction with Continuous Sample Drop Flow Microextraction for Determination of Pesticides in Vegetables and Fruits. Microchem. J. 2021, 160, 105692. [Google Scholar] [CrossRef]
- Omena, E.; Oenning, A.L.; Merib, J.; Richter, P.; Rosero-Moreano, M.; Carasek, E. A Green and Simple Sample Preparation Method to Determine Pesticides in Rice Using a Combination of SPME and Rotating Disk Sorption Devices. Anal. Chim. Acta 2019, 1069, 57–65. [Google Scholar] [CrossRef]
- Altunay, N.; Hazer, B.; Farooque Lanjwani, M.; Tuzen, M.; Ul Haq, H.; Boczkaj, G. Ultrasound Assisted Dispersive Solid Phase Microextraction Using Polystyrene-Polyoleic Acid Graft Copolymer for Determination of Sb(III) in Various Bottled Beverages by HGAAS. Food Chem. 2023, 425, 136523. [Google Scholar] [CrossRef]
- Lukić, J.; Đurkić, T.; Onjia, A. Dispersive Liquid–Liquid Microextraction and Monte Carlo Simulation of Margin of Safety for Octocrylene, EHMC, 2ES, and Homosalate in Sunscreens. Biomed. Chromatogr. 2022, 37, e5590. [Google Scholar] [CrossRef] [PubMed]
- Gomez, N.A.; Lorenzetti, A.S.; Uriarte, D.A.; Acebal, C.; Padró, J.M.; Canals, A.; Garrido, M.; Domini, C.E. Revaluing Optical Techniques in the Light of Vortex- and Ultrasound-Assisted Microextraction. Adv. Sample Prep. 2025, 14, 100179. [Google Scholar] [CrossRef]
- Kazemi, M.; Niazi, A.; Yazdanipour, A. Extraction of Satureja Rechingeri Volatile Components through Ultrasound-Assisted and Microwave-Assisted Extractions and Comparison of the Chemical Composition with Headspace Solid-Phase Microextraction. J. Essent. Oil Res. 2022, 34, 21–35. [Google Scholar] [CrossRef]
- Drabińska, N.; Marcinkowska, M.A.; Wieczorek, M.N.; Jeleń, H.H. Application of Sorbent-Based Extraction Techniques in Food Analysis. Molecules 2023, 28, 7985. [Google Scholar] [CrossRef]
- Bian, Y.; Zhang, Y.; Zhou, Y.; Wei, B.; Feng, X. Recent Insights into Sample Pretreatment Methods for Mycotoxins in Different Food Matrices: A Critical Review on Novel Materials. Toxins 2023, 15, 215. [Google Scholar] [CrossRef]
- Merkle, S.; Kleeberg, K.; Fritsche, J. Recent Developments and Applications of Solid Phase Microextraction (SPME) in Food and Environmental Analysis—A Review. Chromatography 2015, 2, 293–381. [Google Scholar] [CrossRef]
- Rosendo, L.M.; Brinca, A.T.; Pires, B.; Catarro, G.; Rosado, T.; Guiné, R.P.F.; Araújo, A.R.T.S.; Anjos, O.; Gallardo, E. Miniaturized Solid Phase Extraction Techniques Applied to Natural Products. Processes 2023, 11, 243. [Google Scholar] [CrossRef]
- Shen, L.; Pang, S.; Zhong, M.; Sun, Y.; Qayum, A.; Liu, Y.; Rashid, A.; Xu, B.; Liang, Q.; Ma, H.; et al. A Comprehensive Review of Ultrasonic Assisted Extraction (UAE) for Bioactive Components: Principles, Advantages, Equipment, and Combined Technologies. Ultrason. Sonochem. 2023, 101, 106646. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Cai, S.; Hu, W.; Chen, H.; Liu, H. Ionic Liquid-Based Ultrasound-Assisted Dispersive Liquid-Liquid Microextraction Combined with Electrothermal Atomic Absorption Spectrometry for a Sensitive Determination of Cadmium in Water Samples. Spectrochim. Acta Part B At. Spectrosc. 2009, 64, 666–671. [Google Scholar] [CrossRef]
- Meng, L.; Zhang, W.; Meng, P.; Zhu, B.; Zheng, K. Comparison of Hollow Fiber Liquid-Phase Microextraction and Ultrasound-Assisted Low-Density Solvent Dispersive Liquid-Liquid Microextraction for the Determination of Drugs of Abuse in Biological Samples by Gas Chromatography-Mass Spectrometry. J. Chromatogr. B 2015, 989, 46–53. [Google Scholar] [CrossRef]
- Moret, S.; Hidalgo, M.; Sanchez, J.M. Hollow-Fiber Liquid-Phase Microextraction (HF-LPME) Coupled On-Line to Liquid Chromatography for the Determination of the Herbicides 2,4-Dichlorophenoxyacetic Acid and 2-Methyl-4-Chlorophenoxyacetic Acid and Their Main Metabolites in Soil Samples. Separations 2023, 10, 273. [Google Scholar] [CrossRef]
- Raoufi, A.; Raoufi, A.M.; Ismailzadeh, A.; Soleimani Rad, E.; Kiaeefar, A. Application of Hollow Fiber-Protected Liquid-Phase Microextraction Combined with GC-MS in Determining Endrin, Chlordane, and Dieldrin in Rice Samples. Environ. Geochem. Health 2023, 45, 5261–5277. [Google Scholar] [CrossRef]
- Jayasinghe, G.D.T.M.; Jinadasa, B.K.K.K.; Pohl, P.; Abdelkarim, A. Critical Review on Microextraction Techniques Used in Determination of Histamine in Food Samples. Discov. Food 2022, 2, 8. [Google Scholar] [CrossRef]
- Madikizela, L.M.; Pakade, V.E.; Ncube, S.; Tutu, H.; Chimuka, L. Application of Hollow Fibre-Liquid Phase Microextraction Technique for Isolation and Pre-Concentration of Pharmaceuticals in Water. Membranes 2020, 10, 311. [Google Scholar] [CrossRef]
- Câmara, J.S.; Perestrelo, R.; Berenguer, C.V.; Andrade, C.F.; Gomes, T.M.; Olayanju, B.; Kabir, A.; Rocha, C.M.R.; Teixeira, J.A.; Pereira, J.A.M. Green Extraction Techniques as Advanced Sample Preparation Approaches in Biological, Food, and Environmental Matrices: A Review. Molecules 2022, 27, 2953. [Google Scholar] [CrossRef]
- Kawaguchi, M.; Takatsu, A.; Ito, R.; Nakazawa, H. Applications of Stir-Bar Sorptive Extraction to Food Analysis. TrAC Trends Anal. Chem. 2013, 45, 280–293. [Google Scholar] [CrossRef]
- Heidari, H.; Ghanbari-Rad, S.; Habibi, E. Optimization Deep Eutectic Solvent-Based Ultrasound-Assisted Liquid-Liquid Microextraction by Using the Desirability Function Approach for Extraction and Preconcentration of Organophosphorus Pesticides from Fruit Juice Samples. J. Food Compos. Anal. 2020, 87, 103389. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, J.; Zhao, J.; Mao, L.; Zhao, S.; Wang, B.; Wei, X.; Shi, Q.; Chen, J.; Sun, J. Ultrasound-Enhanced Air-Assisted Liquid-Liquid Microextraction for the UPLC Determination of Organophosphorus Pesticides in River Water. Microchem. J. 2022, 183, 108046. [Google Scholar] [CrossRef]
- Qiao, L.; Sun, R.; Tao, Y.; Yan, Y. New Low Viscous Hydrophobic Deep Eutectic Solvents for the Ultrasound-Assisted Dispersive Liquid-Liquid Microextraction of Endocrine-Disrupting Phenols in Water, Milk and Beverage. J. Chromatogr. A 2022, 1662, 462728. [Google Scholar] [CrossRef]
- Campone, L.; Celano, R.; Piccinelli, A.L.; Pagano, I.; Cicero, N.; Di Sanzo, R.; Carabetta, S.; Russo, M.; Rastrelli, L. Ultrasound Assisted Dispersive Liquid-Liquid Microextraction for Fast and Accurate Analysis of Chloramphenicol in Honey. Food Res. Int. 2019, 115, 572–579. [Google Scholar] [CrossRef]
- Pour, P.H.; Daryanavard, S.M.; Memar, M.; Naccarato, A. Development of Ultrasound-Assisted Dispersive Liquid–Liquid Microextraction Based on Solidification of Floating Organic Droplets and Deep Eutectic Solvents for Multi-Class Pesticide Analysis in Agricultural Waters. Microchem. J. 2025, 212, 113404. [Google Scholar] [CrossRef]
- Bişgin, A.T.; Elik, A.; Altunay, N. Ultrasonic-Assisted Natural Deep Eutectic Solvent Based Dispersive Liquid-Liquid Microextraction of Toxic Heavy Metals in Various Water Matrices and Vegetable Samples. Microchem. J. 2025, 216, 114790. [Google Scholar] [CrossRef]
- Garcia-Jares, C.; Celeiro, M.; Lamas, J.P.; Iglesias, M.; Lores, M.; Llompart, M. Rapid Analysis of Fungicides in White Wines from Northwest Spain by Ultrasound-Assisted Emulsification-Microextraction and Gas Chromatography-Mass Spectrometry. Anal. Methods 2014, 6, 3108–3116. [Google Scholar] [CrossRef]
- Lorenzetti, A.S.; Lista, A.G.; Domini, C.E. Reverse Ultrasound-Assisted Emulsification-Microextraction of Macrolides from Chicken Fat Followed by Electrophoretic Determination. LWT 2019, 113, 108334. [Google Scholar] [CrossRef]
- Sivrikaya Ozak, S.; Yılmaz, Y. Ultrasound-Assisted Hydrophobic Deep Eutectic Solvent Based Solid-Liquid Microextraction of Sudan Dyes in Spice Samples. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 236, 118353. [Google Scholar] [CrossRef] [PubMed]
- Laurenčík, M.; Kirchner, M.; Tölgyessy, P.; Nagyová, S. Simultaneous Focused Ultrasound Solid–Liquid Extraction and Dispersive Solid-Phase Extraction Clean-up for Gas Chromatography–Tandem Mass Spectrometry Determination of Polycyclic Aromatic Hydrocarbons in Crustacean Gammarids Meeting the Requirements of Th. J. Chromatogr. A 2022, 1673, 463098. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Hassan, F.A.; Wu, J.; Xiong, C. Determination of Fifteen Illegal Colorants in Traditional Chinese Medicines by Two Hydrophobic DES-Based Microextraction Methods Coupled with an HPLC-DAD. Talanta 2024, 277, 126236. [Google Scholar] [CrossRef]
- Shirani, M.; Faraji, M.; Rashidi Nodeh, H.; Akbari-adergani, B.; Sepahi, S. An Efficient Deep Eutectic Magnetic Nano Gel for Rapid Ultrasound-Assisted Dispersive µ-Solid Phase Extraction of Residue of Tetracyclines in Food Samples. J. Food Sci. Technol. 2023, 60, 2802–2812. [Google Scholar] [CrossRef]
- Ali, J.; Tuzen, M.; Jatoi, W.B.; Hazer, B. A Novel Block Copolymer Containing Gadolinium Oxide Nanoparticles in Ultrasound Assisted-Dispersive Solid Phase Microextraction of Total Arsenic in Human Foodstuffs: A Multivariate Optimization Methodology. Food Chem. 2024, 437, 137908. [Google Scholar] [CrossRef]
- Pamık, D.T.; Seyhan Bozkurt, S.; Mumcu, T. Ultrasonic Assisted Dispersive Micro-Solid Phase Extraction of Some Sulfonamide Antibiotics from Milk and Egg Samples Using Polymeric Ionic Liquid-Based Chitosan before HPLC Analysis. Microchem. J. 2023, 191, 108876. [Google Scholar] [CrossRef]
- Ahmad, H.; Zhao, L.; Liu, C.; Cai, C.; Ma, F. Ultrasound Assisted Dispersive Solid Phase Microextraction of Inorganic Arsenic from Food and Water Samples Using CdS Nanoflowers Combined with ICP-OES Determination. Food Chem. 2021, 338, 128028. [Google Scholar] [CrossRef]
- Mihaljević Žulj, M.; Maslov, L.; Tomaz, I.; Jeromel, A. Determination of 2-Aminoacetophenone in White Wines Using Ultrasound Assisted SPME Coupled with GC-MS. J. Anal. Chem. 2015, 70, 814–818. [Google Scholar] [CrossRef]
- de Melo Antipoff, V.V.; dos Santos, R.R.; Augusti, D.V.; de Lourdes Cardeal, Z.; Menezes, H.C. Determination of Polycyclic Aromatic Hydrocarbons in Eggs Exposed to Fire Using a Simple and Efficient Method. Food Anal. Methods 2021, 14, 1194–1201. [Google Scholar] [CrossRef]
- Manoochehri, M.; Asgharinezhad, A.A.; Safaei, M. Multivariate Optimisation of an Ultrasound Assisted-Matrix Solid-Phase Dispersion Method Combined with LC-Fluorescence Detection for Simultaneous Extraction and Determination of Aflatoxins in Pistachio Nut Samples. Food Addit. Contam. Part A 2013, 30, 1954–1962. [Google Scholar] [CrossRef]
- Karageorgou, E.; Armeni, M.; Moschou, I.; Samanidou, V. Ultrasound-Assisted Dispersive Extraction for the High Pressure Liquid Chromatographic Determination of Tetracyclines Residues in Milk with Diode Array Detection. Food Chem. 2014, 150, 328–334. [Google Scholar] [CrossRef]
- Manoochehri, M.; Asgharinezhad, A.A.; Safaei, M. Determination of Aflatoxins in Rice Samples by Ultrasound-Assisted Matrix Solid-Phase Dispersion. J. Chromatogr. Sci. 2015, 53, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Giannioti, Z.; Albero, B.; Hernando, M.D.; Bontempo, L.; Perez, R.A. Determination of Regulated and Emerging Mycotoxins in Organic and Conventional Gluten-Free Flours by LC-MS/MS. Toxins 2023, 15, 155. [Google Scholar] [CrossRef] [PubMed]
- Temel, N.K. Ultrasound Assisted-Cloud Point Extraction Coupled with Spectrophotometry for Determination of Low Levels of Formaldehyde from Milk-Based Products. J. Food Compos. Anal. 2024, 126, 105919. [Google Scholar] [CrossRef]
- Altunay, N.; Elik, A.; Bulutlu, C.; Gürkan, R. Application of Simple, Fast and Eco-Friendly Ultrasound-Assisted-Cloud Point Extraction for Pre-Concentration of Zinc, Nickel and Cobalt from Foods and Vegetables Prior to Their Flame Atomic Absorption Spectrometric Determinations. Int. J. Environ. Anal. Chem. 2018, 98, 655–675. [Google Scholar] [CrossRef]
- Altunay, N. Utility of Ultrasound Assisted-Cloud Point Extraction and Spectophotometry as a Preconcentration and Determination Tool for the Sensitive Quantification of Mercury Species in Fish Samples. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 189, 167–175. [Google Scholar] [CrossRef]
- You, X.; Wang, S.; Liu, F.; Shi, K. Ultrasound-Assisted Surfactant-Enhanced Emulsification Microextraction Based on the Solidification of a Floating Organic Droplet Used for the Simultaneous Determination of Six Fungicide Residues in Juices and Red Wine. J. Chromatogr. A 2013, 1300, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Lemos, V.A.; Oliveira, L.A. Ultrasound-Assisted Temperature-Controlled Ionic Liquid Microextraction for the Preconcentration and Determination of Cadmium Content in Mussel Samples. Food Control 2015, 50, 901–906. [Google Scholar] [CrossRef]
- Albero, B.; Tadeo, J.L.; Pérez, R.A. Ultrasound-Assisted Extraction of Organic Contaminants. TrAC Trends Anal. Chem. 2019, 118, 739–750. [Google Scholar] [CrossRef]
- Azari, A.; Abtahi, M.; Saeedi, R.; Yari, A.R.; Vaziri, M.H.; Mohammadi, G. Integrated Ultrasound-Assisted Magnetic Solid-Phase Extraction for Efficient Determination and Pre-Concentration of Polycyclic Aromatic Hydrocarbons from High-Consumption Soft Drinks and Non-Alcoholic Beers in Iran. J. Sep. Sci. 2022, 45, 3139–3149. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, X.-A.; Jin, C.-Z.; Zhang, W.-B. bing Ultrasonic Assisted Magnetic Solid Phase Extraction of Ultra-Trace Mercury with Ionic Liquid Functionalized Materials. Anal. Chim. Acta 2023, 1245, 340865. [Google Scholar] [CrossRef]
- Ghiasi, A.; Malekpour, A.; Mahpishanian, S. Metal-Organic Framework MIL101 (Cr)-NH2 Functionalized Magnetic Graphene Oxide for Ultrasonic-Assisted Magnetic Solid Phase Extraction of Neonicotinoid Insecticides from Fruit and Water Samples. Talanta 2020, 217, 121120. [Google Scholar] [CrossRef]
- Liu, C.; Ji, Y.; Jiang, X.; Yuan, X.; Zhang, X.; Zhao, L. The Determination of Pesticides in Tea Samples Followed by Magnetic Multiwalled Carbon Nanotube-Based Magnetic Solid-Phase Extraction and Ultra-High Performance Liquid Chromatography-Tandem Mass Spectrometry. New J. Chem. 2019, 43, 5395–5403. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound Assisted Extraction of Food and Natural Products. Mechanisms, Techniques, Combinations, Protocols and Applications. A Review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Gao, M.; Qu, J.; Chen, K.; Jin, L.; Dahlgren, R.A.; Wang, H.; Tan, C.; Wang, X. Salting-out-Enhanced Ionic Liquid Microextraction with a Dual-Role Solvent for Simultaneous Determination of Trace Pollutants with a Wide Polarity Range in Aqueous Samples. Anal. Bioanal. Chem. 2017, 409, 6287–6303. [Google Scholar] [CrossRef] [PubMed]
- Legesse, A.; Megersa, N.; Chandravanshi, B.S. Ultrasound-Assisted Emulsification Liquid-Liquid Microextraction Based on Deep Eutectic Solvents for Selective Enrichment and Detection of Triazine Herbicides and Their Degradation Products in Water, Fruit and Honey Samples by HPLC-DAD. Food Chem. 2025, 492, 145514. [Google Scholar] [CrossRef] [PubMed]
- Azhar, A.N.H.; Amran, N.A.; Yusup, S.; Mohd Yusoff, M.H. Ultrasonic Extraction of 2-Acetyl-1-Pyrroline (2AP) from Pandanus Amaryllifolius Roxb. Using Ethanol as Solvent. Molecules 2022, 27, 4906. [Google Scholar] [CrossRef] [PubMed]
- Carreira-Casais, A.; Carpena, M.; Pereira, A.G.; Chamorro, F.; Soria-Lopez, A.; Perez, P.G.; Otero, P.; Cao, H.; Xiao, J.; Simal-Gandara, J.; et al. Critical Variables Influencing the Ultrasound-Assisted Extraction of Bioactive Compounds—A Review. Chem. Proc. 2021, 5, 50. [Google Scholar] [CrossRef]
- Jaganmohanrao, L. Role of Deep Eutectic Solvents as Alternate Solvents in the Microextraction and Estimation of Pesticide, Insecticide, Fungicide Residues and Metal Contaminants in Tea (Camellia Sinensis). Microchem. J. 2025, 208, 112515. [Google Scholar] [CrossRef]
- Gürsoy, N.; Sırtbaşı, B.; Şimşek, S.; Elik, A.; Altunay, N. Optimization and Application of Ultrasound-Assisted Sugar Based Deep Eutectic Solvent Dispersive Liquid–Liquid Microextraction for the Determination and Extraction of Aflatoxin M1 in Milk Samples. Microchem. J. 2022, 172, 106974. [Google Scholar] [CrossRef]
- dos Santos, E.O.; Gonzales, J.O.; Ores, J.C.; Marube, L.C.; Caldas, S.S.; Furlong, E.B.; Primel, E.G. Sand as a Solid Support in Ultrasound-Assisted MSPD: A Simple, Green and Low-Cost Method for Multiresidue Pesticide Determination in Fruits and Vegetables. Food Chem. 2019, 297, 124926. [Google Scholar] [CrossRef]
- Shirani, M.; Akbari-adergani, B.; Shahdadi, F.; Faraji, M.; Akbari, A. A Hydrophobic Deep Eutectic Solvent-Based Ultrasound-Assisted Dispersive Liquid–Liquid Microextraction for Determination of β-Lactam Antibiotics Residues in Food Samples. Food Anal. Methods 2022, 15, 391–400. [Google Scholar] [CrossRef]
- Elik, A.; Ablak, Ö.; Haq, H.U.; Boczkaj, G.; Altunay, N. Combination of Homogeneous Liquid–Liquid Extraction and Vortex Assisted Dispersive Liquid–Liquid Microextraction for the Extraction and Analysis of Ochratoxin A in Dried Fruit Samples: Central Composite Design Optimization. J. Food Compos. Anal. 2023, 124, 105656. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, H.; Peng, B.; Li, S.; Zhou, Z. Comparison of the Performance of Conventional, Temperature-Controlled, and Ultrasound-Assisted Ionic Liquid Dispersive Liquid-Liquid Microextraction Combined with High-Performance Liquid Chromatography in Analyzing Pyrethroid Pesticides in Honey Samples. J. Chromatogr. A 2011, 1218, 6621–6629. [Google Scholar] [CrossRef]
- Sharafi, K.; Fattahi, N.; Mahvi, A.H.; Pirsaheb, M.; Azizzadeh, N.; Noori, M. Trace Analysis of Some Organophosphorus Pesticides in Rice Samples Using Ultrasound-Assisted Dispersive Liquid-Liquid Microextraction and High-Performance Liquid Chromatography. J. Sep. Sci. 2015, 38, 1010–1016. [Google Scholar] [CrossRef]
- Chunhong, J.; Xiaodan, Z.; Li, C.; Min, H.; Pingzhong, Y.; Ercheng, Z. Extraction of Organophosphorus Pesticides in Water and Juice Using Ultrasound-Assisted Emulsification-Mixroextraction. J. Sep. Sci. 2010, 33, 244–250. [Google Scholar] [CrossRef]
- Moreno-González, D.; Huertas-Pérez, J.F.; García-Campaña, A.M.; Bosque-Sendra, J.M.; Gámiz-Gracia, L. Ultrasound-Assisted Surfactant-Enhanced Emulsification Microextraction for the Determination of Carbamates in Wines by Ultra-High Performance Liquid Chromatography-Tandem Mass Spectrometry. J. Chromatogr. A 2013, 1315, 1–7. [Google Scholar] [CrossRef]
- Rouhi Maleki, M.; Movassaghghazani, M.; Afshar Mogaddam, M.R. Development of Deep Eutectic Solvent-Based Ultrasonic Assisted Liquid–Liquid Microextraction Coupled with GC–MS.; Application in Analysis of Organochlorine Pesticides from Cheese Samples. Microchem. J. 2024, 206, 111565. [Google Scholar] [CrossRef]
- Du, Y.; Wang, Q.; Yang, G.; Han, F. Determination of 43 Pesticide Residues in Intact Grape Berries (Vitis vinifera L.) by Using an Ultrasound-Assisted Acetonitrile Extraction Method Followed by LC–MS/MS. Food Control 2022, 140, 109123. [Google Scholar] [CrossRef]
- Kara, D.; Fisher, A.; Hill, S. Extraction of Trace Elements by Ultrasound-Assisted Emulsification from Edible Oils Producing Detergentless Microemulsions. Food Chem. 2015, 188, 143–148. [Google Scholar] [CrossRef]
- Biata, N.R.; Mashile, G.P.; Ramontja, J.; Mketo, N.; Nomngongo, P.N. Application of Ultrasound-Assisted Cloud Point Extraction for Preconcentration of Antimony, Tin and Thallium in Food and Water Samples Prior to ICP-OES Determination. J. Food Compos. Anal. 2019, 76, 14–21. [Google Scholar] [CrossRef]
- Yao, L.; Liu, H.; Wang, X.; Xu, W.; Zhu, Y.; Wang, H.; Pang, L.; Lin, C. Ultrasound-Assisted Surfactant-Enhanced Emulsification Microextraction Using a Magnetic Ionic Liquid Coupled with Micro-Solid Phase Extraction for the Determination of Cadmium and Lead in Edible Vegetable Oils. Food Chem. 2018, 256, 212–218. [Google Scholar] [CrossRef]
- Yilmaz, E. Use of Hydrolytic Enzymes as Green and Effective Extraction Agents for Ultrasound Assisted-Enzyme Based Hydrolytic Water Phase Microextraction of Arsenic in Food Samples. Talanta 2018, 189, 302–307. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, B.; Liu, Y.; Sun, H.; Zhang, H.; Li, N.; Qing, Y.; Elango, J.; Zhao, D.; Wu, W. Ultrasound-Assisted Determination of Selenium in Organic Rice Using Deep Eutectic Solvents Coupled with Inductively Coupled Plasma Mass Spectrometry. Foods 2025, 14, 384. [Google Scholar] [CrossRef] [PubMed]
- Elik, A.; Altunay, N. Optimization of Deep Eutectic Solvent Based Ultrasonic Assisted Microextraction for Determination of Zearalenone Residues in Foods. J. Food Compos. Anal. 2024, 132, 106304. [Google Scholar] [CrossRef]
- Jayasinghe, G.D.T.M.; Domínguez-González, R.; Bermejo-Barrera, P.; Moreda-Piñeiro, A. Combining Ultrasound-Assisted Extraction and Vortex-Assisted Liquid–Liquid Microextraction for the Sensitive Assessment of Aflatoxins in Aquaculture Fish Species. J. Sep. Sci. 2020, 43, 1331–1338. [Google Scholar] [CrossRef]
- Pi, J.; Jin, P.; Zhou, S.; Wang, L.; Wang, H.; Huang, J.; Gan, L.; Yuan, T.; Fan, H. Combination of Ultrasonic-Assisted Aqueous Two-Phase Extraction with Solidifying Organic Drop-Dispersive Liquid–Liquid Microextraction for Simultaneous Determination of Nine Mycotoxins in Medicinal and Edible Foods by HPLC with In-Series DAD and FLD. Food Anal. Methods 2022, 15, 428–439. [Google Scholar] [CrossRef]
- Altunay, N.; Elik, A.; Gürkan, R. A Novel, Green and Safe Ultrasound-Assisted Emulsification Liquid Phase Microextraction Based on Alcohol-Based Deep Eutectic Solvent for Determination of Patulin in Fruit Juices by Spectrophotometry. J. Food Compos. Anal. 2019, 82, 103256. [Google Scholar] [CrossRef]
- Ji, Y.; Meng, Z.; Zhao, J.; Zhao, H.; Zhao, L. Eco-Friendly Ultrasonic Assisted Liquid–Liquid Microextraction Method Based on Hydrophobic Deep Eutectic Solvent for the Determination of Sulfonamides in Fruit Juices. J. Chromatogr. A 2020, 1609, 460520. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Ji, Y.; Zhao, J.; Zhao, L. Deep Eutectic Solvent Based-Ferrofluid Ultrasonic-Assisted Liquid–Liquid Microextraction for Determination of Quinolones in Milk Samples. Microchem. J. 2022, 179, 107664. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Ji, L.; Chen, L. Simultaneous Determination of Sulfonamides Antibiotics in Environmental Water and Seafood Samples Using Ultrasonic-Assisted Dispersive Liquid-Liquid Microextraction Coupled with High Performance Liquid Chromatography. Molecules 2022, 27, 2160. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Wang, C.; Lin, Y.; Jiang, T.F.; Lv, Z. Determination of Fluoroquinolones Illegally Added in Traditional Prostate Medicines by Ultrasonic-Assisted Dispersive Liquid Liquid Micro-Extraction Based on Deep Eutectic Solvent Combined with Quantitative 19F Nuclear Magnetic Resonance Method. Microchem. J. 2021, 170, 106725. [Google Scholar] [CrossRef]
- Dorival-García, N.; Junza, A.; Zafra-Gómez, A.; Barrón, D.; Navalón, A. Simultaneous Determination of Quinolone and β-Lactam Residues in Raw Cow Milk Samples Using Ultrasound-Assisted Extraction and Dispersive-SPE Prior to UHPLC-MS/MS Analysis. Food Control 2016, 60, 382–393. [Google Scholar] [CrossRef]
- Hsu, C.J.; Ding, W.H. Determination of Benzotriazole and Benzothiazole Derivatives in Tea Beverages by Deep Eutectic Solvent-Based Ultrasound-Assisted Liquid-Phase Microextraction and Ultrahigh-Performance Liquid Chromatography-High Resolution Mass Spectrometry. Food Chem. 2022, 368, 130798. [Google Scholar] [CrossRef]
- Cao, J.; Wang, C.; Shi, L.; Cheng, Y.; Hu, H.; Zeng, B.; Zhao, F. Water Based-Deep Eutectic Solvent for Ultrasound-Assisted Liquid–Liquid Microextraction of Parabens in Edible Oil. Food Chem. 2022, 383, 132586. [Google Scholar] [CrossRef] [PubMed]
- Fontana, A.R.; Muñoz De Toro, M.; Altamirano, J.C. One-Step Derivatization and Preconcentration Microextraction Technique for Determination of Bisphenol a in Beverage Samples by Gas Chromatography-Mass Spectrometry. J. Agric. Food Chem. 2011, 59, 3559–3565. [Google Scholar] [CrossRef]
- Yurdakok-Dikmen, B.; Kuzukiran, O.; Filazi, A.; Kara, E. Measurement of Selected Polychlorinated Biphenyls (PCBs) in Water via Ultrasound Assisted Emulsification-Microextraction (USAEME) Using Low-Density Organic Solvents. J. Water Health 2016, 14, 214–222. [Google Scholar] [CrossRef]
- Liu, W.; Zong, B.; Yu, J.; Bi, Y. Ultrasonic-Assisted Liquid-Liquid Microextraction Based on Natural Deep Eutectic Solvent for the HPLC-UV Determination of Tert-Butylhydroquinone from Soybean Oils. Food Anal. Methods 2018, 11, 1797–1803. [Google Scholar] [CrossRef]
- Qiao, L.; Sun, R.; Yu, C.; Tao, Y.; Yan, Y. Novel Hydrophobic Deep Eutectic Solvents for Ultrasound-Assisted Dispersive Liquid-Liquid Microextraction of Trace Non-Steroidal Anti-Inflammatory Drugs in Water and Milk Samples. Microchem. J. 2021, 170, 106686. [Google Scholar] [CrossRef]
- Altunay, N. Experimental Design of Magnetic Ionic Liquid Ultrasound-Assisted Dispersive Liquid-Liquid Microextraction for the Determination of 5-HMF in Honey Samples. J. Food Compos. Anal. 2022, 114, 104817. [Google Scholar] [CrossRef]
- Filippou, O.; Deliyanni, E.A.; Samanidou, V.F. Fabrication and Evaluation of Magnetic Activated Carbon as Adsorbent for Ultrasonic Assisted Magnetic Solid Phase Dispersive Extraction of Bisphenol A from Milk Prior to High Performance Liquid Chromatographic Analysis with Ultraviolet Detection. J. Chromatogr. A 2017, 1479, 20–31. [Google Scholar] [CrossRef]
- Ma, S.; Jin, X.; Wei, H.; Liu, Y.; Guo, M. Hydrophobic Deep Eutectic Solvent-Based Ultrasonic-Assisted Liquid-Liquid Micro-Extraction Combined with HPLC-FLD for Diphenylamine Determination in Fruit. Food Addit. Contam. Part A 2021, 38, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gao, Y.; Zhang, N.; Xie, Y.; Xu, X.; Gup, H.; Bao, T.; Sun, M.; Wang, S. Stir-Bar Sorptive Extraction Based on Hydroxyl-Functionalized Zirconium-Metal-Organic Framework for the Detection Ofthree Quinolones in Actual Samples. J. Sep. Sci. 2023, 46, e2200833. [Google Scholar] [CrossRef] [PubMed]
- Arvand, M.; Bozorgzadeh, E.; Shariati, S. Two-Phase Hollow Fiber Liquid Phase Microextraction for Preconcentration of Pyrethroid Pesticides Residues in Some Fruits and Vegetable Juices Prior to Gas Chromatography/Mass Spectrometry. J. Food Compos. Anal. 2013, 31, 275–283. [Google Scholar] [CrossRef]
- Dominguez-Tello, A.; Dominguez-Alfaro, A.; Gómez-Ariza, J.L.; Arias-Borrego, A.; García-Barrera, T. Effervescence-Assisted Spiral Hollow-Fibre Liquid-Phase Microextraction of Trihalomethanes, Halonitromethanes, Haloacetonitriles, and Haloketones in Drinking Water. J. Hazard. Mater. 2020, 397, 122790. [Google Scholar] [CrossRef]
- Passos, P.; Petronilho, S.; Ser, F.; Neto, A.C.M.; Torres, D.; Rudnitskaya, A.; Ciesarov, Z.; Rocha, S.M.; Coimbra, M.A. HS-SPME Gas Chromatography Approach for Underivatized Acrylamide Determination in Biscuits. Foods 2021, 10, 2183. [Google Scholar] [CrossRef]
- Wang, J.; Feng, J.; Sun, M.; Lian, Y.; Wang, M.; Qiao, L. Sulfonic Acid-Functionalized Covalent Organic Frameworks as the Coating for Stir Bar Sorptive Extraction of Fluoroquinolones in Milk Samples. Microchim. Acta 2023, 190, 5. [Google Scholar] [CrossRef]
- Song, G.; Guo, X.; Li, Q.; Liao, J.; Wang, D.; Yuan, T. Simultaneous Determination of Various Heavy Metal and Arsenic Ions in Seafood Using Functionalized Fibrous Silica (KCC-1) Coated Stir Bar Sorptive Extraction Prior to Inductively Coupled Plasma Mass Spectrometry. Food Control 2023, 152, 109846. [Google Scholar] [CrossRef]
- Wen, H.; Nan, S.; Wu, D.; Sun, Q.; Tong, Y.; Zhang, J.; Jin, S.; Shen, W. A Systematic Review on Intensifications of Artificial Intelligence Assisted Green Solvent Development. Ind. Eng. Chem. Res. 2023, 62, 20473–20491. [Google Scholar] [CrossRef]
- Tadić, T.; Marković, B.; Radulović, J.; Lukić, J.; Suručić, L.; Nastasović, A.; Onjia, A. A Core-Shell Amino-Functionalized Magnetic Molecularly Imprinted Polymer Based on Glycidyl Methacrylate for Dispersive Solid-Phase Microextraction of Aniline. Sustainability 2022, 14, 9222. [Google Scholar] [CrossRef]
- Tadić, T.; Marković, B.; Bulatović, S.; Lukić, J.; Radulović, J.; Nastasović, A.; Onjia, A. Greenness of Dispersive Microextraction Using Molecularly Imprinted Polymers. Rev. Anal. Chem. 2024, 43, 20230070. [Google Scholar] [CrossRef]
- Shirani, M.; Akbari-adergani, B.; Jazi, M.B.; Akbari, A. Green Ultrasound Assisted Magnetic Nanofluid-Based Liquid Phase Microextraction Coupled with Gas Chromatography-Mass Spectrometry for Determination of Permethrin, Deltamethrin, and Cypermethrin Residues. Microchim. Acta 2019, 186, 674. [Google Scholar] [CrossRef]
- Jouyban, A.; Farajzadeh, M.A.; Afshar Mogaddam, M.R. In Matrix Formation of Deep Eutectic Solvent Used in Liquid Phase Extraction Coupled with Solidification of Organic Droplets Dispersive Liquid-Liquid Microextraction; Application in Determination of Some Pesticides in Milk Samples. Talanta 2020, 206, 120169. [Google Scholar] [CrossRef]
- Almeida, J.S.; Anunciação, T.A.; Brandão, G.C.; Dantas, A.F.; Lemos, V.A.; Teixeira, L.S.G. Ultrasound-Assisted Single-Drop Microextraction for the Determination of Cadmium in Vegetable Oils Using High-Resolution Continuum Source Electrothermal Atomic Absorption Spectrometry. Spectrochim. Acta Part B 2015, 107, 159–163. [Google Scholar] [CrossRef]
- Gomez, N.A.; Lorenzetti, A.S.; Camiña, J.; Garrido, M.; Domini, C.E. In-Syringe Ultrasound-Assisted Dispersive Liquid–Liquid Microextraction for the Fluorescent Determination of Aluminum in Water and Milk Samples. Microchem. J. 2022, 183, 108117. [Google Scholar] [CrossRef]
- Martins, R.O.; Borsatto, J.V.B.; Will, C.; Lanças, F.M. Advancements in Microextraction by Packed Sorbent: Insights into Sorbent Phases and Automation Strategies. Separations 2025, 12, 11. [Google Scholar] [CrossRef]
- Inês, A.; Cosme, F. Biosensors for Detecting Food Contaminants—An Overview. Processes 2025, 13, 380. [Google Scholar] [CrossRef]
- Ashley, J.; Shahbazi, M.A.; Kant, K.; Chidambara, V.A.; Wolff, A.; Bang, D.D.; Sun, Y. Molecularly Imprinted Polymers for Sample Preparation and Biosensing in Food Analysis: Progress and Perspectives. Biosens. Bioelectron. 2017, 91, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Sahlan, M.; Rosarina, D.; Farida, H.; Suminar, R.; Pohan, Y.D.; Hidayatullah, I.M.; Narawangsa, D.R.; Putri, D.N.; Sari, E.; Perdani, M.S.; et al. Microwave—Ultrasound-Assisted Extraction Coupled with Natural Deep Eutectic Solvent Enables High-Yield, Low-Solvent Recovery of Curcumin from Curcuma longa L. Pharmaceutics 2025, 17, 818. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Maese, R.; Rodríguez-Saldaña, V.; Leal, L.O. Automation Systems in Pb Analysis: A Review on Environmental Water and Biological Samples. Water 2025, 17, 565. [Google Scholar] [CrossRef]
- Motyka, K.; Onjia, A.; Mikuška, P.; Večera, Z. Flow-Injection Chemiluminescence Determination of Formaldehyde in Water. Talanta 2007, 71, 900–905. [Google Scholar] [CrossRef]
- Alloun, W.; Calvio, C. Bio-Driven Sustainable Extraction and AI-Optimized Recovery of Functional Compounds from Plant Waste: A Comprehensive Review. Fermentation 2024, 10, 126. [Google Scholar] [CrossRef]
- Lučić, M.; Onjia, A. Probabilistic Dietary Exposure and Risk Ranking of Pesticides in Peppers (Capsicum annuum): Regional and Consumer Group Variability. Food Chem. 2025, 492, 145355. [Google Scholar] [CrossRef]
- Radulović, J.; Lučić, M.; Onjia, A. GC-MS/MS and LC-MS/MS Analysis Followed by Risk Ranking of Mepiquat and Pyrethroids in Coffee. J. Food Compos. Anal. 2024, 129, 106100. [Google Scholar] [CrossRef]
- Radulović, J.; Lučić, M.; Nešić, A.; Onjia, A. Multivariate Assessment and Risk Ranking of Pesticide Residues in Citrus Fruits. Foods 2023, 12, 2454. [Google Scholar] [CrossRef]
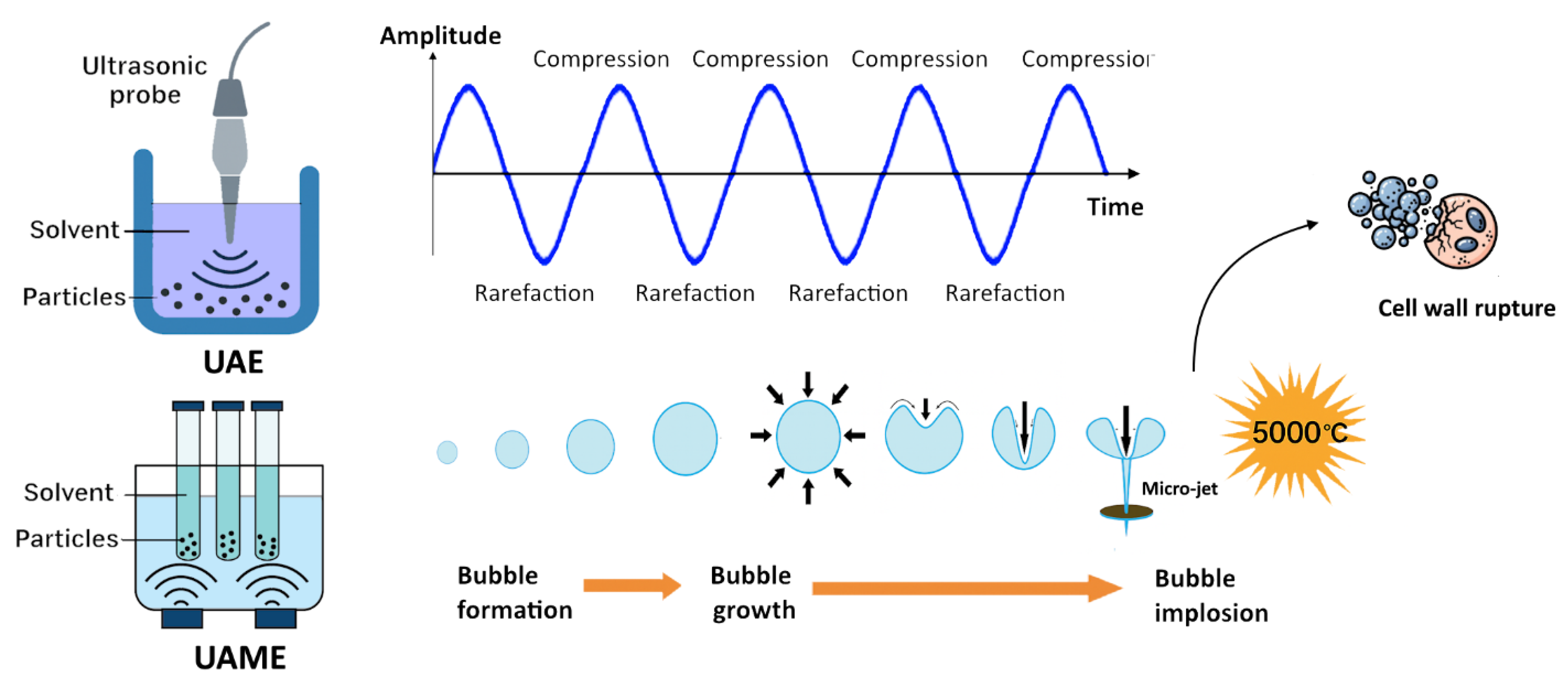
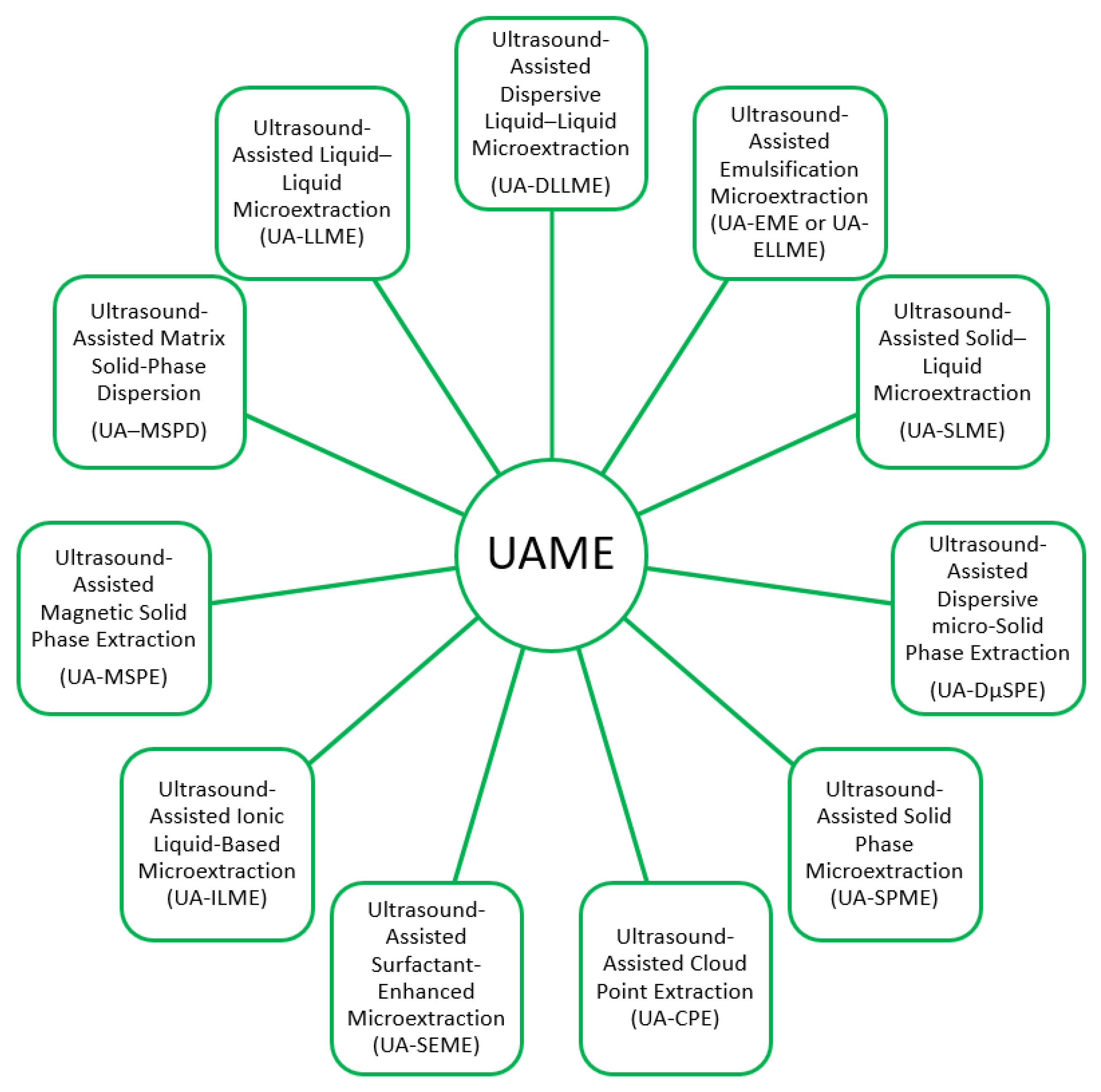

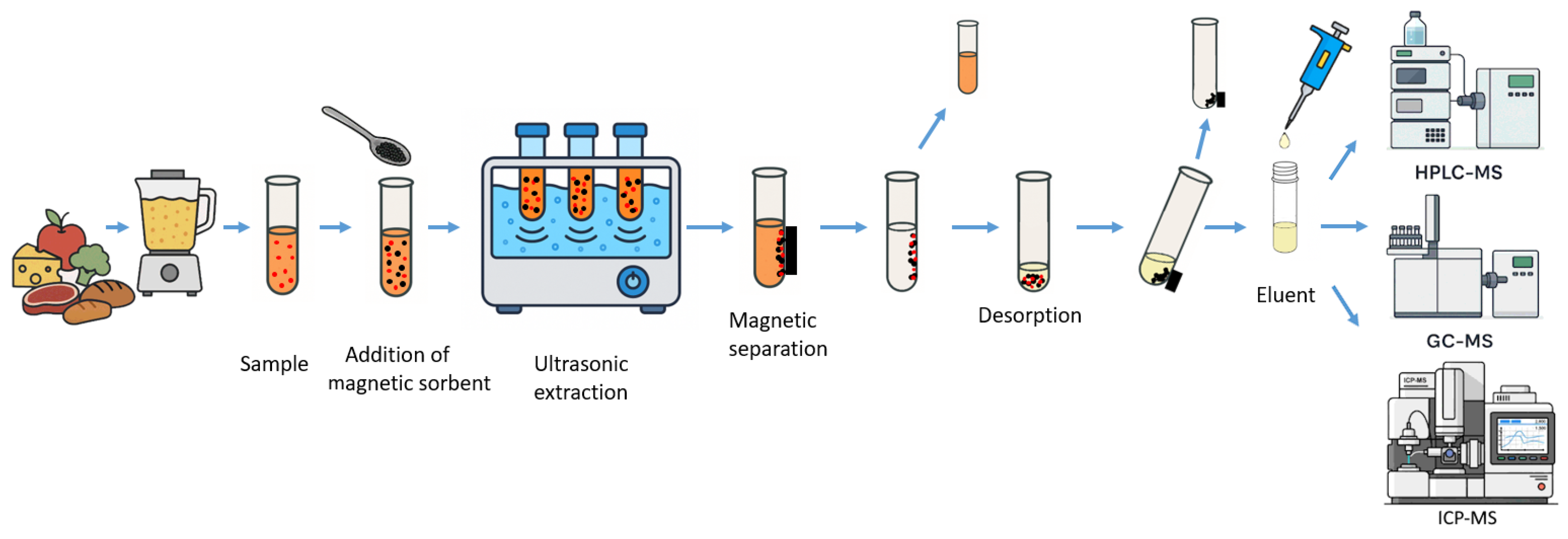
| Analytes/Examples | Sample Matrix | UAME Approach | Recovery (%) | Detection | Reference |
|---|---|---|---|---|---|
| Pesticides | |||||
| Organophosphorus pesticides: phorate, diazinon, parathion methyl, fenitrothion, and malathion, chlorpyrifos | Water and juice | UA-EMA | 80.0–110.0 | GC–FPD | [125] |
| Pyrethroid pesticides: ethofenprox, tetramethrin, meperfluthrin, and alpha-cypermethrin | Honey | UA-DLLME | 101.2–103.0 | HPLC-DAD | [123] |
| Fungicides: pyrimethanil, fludioxonil, procymidone, cyprodinil, kresoxim-methyl, pyraclostrobin | Fruit juices and red wine | UA-EME | 79.5–113.4 | HPLC-DAD | [106] |
| Carbamates: asulam, aldicarb-sulfoxide, aldicarb-sulfone, oxamyl, methomyl, ethiofencarb-sulfone, pirimica—rb-desmethyl, ethiofencarb-sulfoxide, methio-carbsulfoxide, carbofuran-3-hidroxy, cymoxanil, aldicarb, metolcarb, propoxur, carbofuran, carbaryl, ethiofencarb, thiodicarb, isoprocarb, fenobucarb, diethofencarb, methiocarb, promecarb, napropamid and benthiocarb | Wines | UA-EME | 74–102 | UHPLC–MS/MS | [126] |
| Organophosphorus pesticides: diazinon, chlorpyrifos | Rice | UA-DLLME | 58.0–66.0 | HPLC-UV | [124] |
| Organophosphorus pesticides: phosalone, chlorpyrifos | Fruit juice | DES-UA-LLME | 87.3–116.7 | HPLC-UV | [82] |
| Organophosphorus pesticides: malathion, fenthion, dimethoate, imidan, phosphamidon, fenitrothion, and isocarbophos | Water | UE-AA-LLME | 75.4–112.6 | UPLC | [83] |
| Strobilurin fungicides: azoxystrobin, pyrimethanil and kresoxim-methyl | Apple | UA-DLLME | 76–92 | HPLC-UV | [61] |
| Organochlorine pesticides: endosulfan, aldrin, dieldrin, dichlorodiphenyldi- chloroethylene (DDE), and dichlorodiphenyltrichloroethane (DDT) | Cheese | UA-LLME | 57–79 | GC–MS | [127] |
| Thiamethoxam, thiacloprid, pirimicarb, acetamiprid, tebuconazole | Pears | UA–MSPD | 78.5–120 | LC-MS/MS | [43] |
| Triazine herbicides: atrazine, simazine, deisopro- pylatrazine, deethylatrazine, propazine, prometryn and terbutryn | Water, fruit and honey | UA-EME | 71.90–119.25 | HPLC-DAD | [115] |
| Ovex, oxidiazon, tetrasul, buprofezin, sulprophos, tebufenpyrad, cis-permethrin | Agricultural waters | UA-DLLME | 83–115 | GC–MS | [86] |
| Metalaxyl, napropamide and epoxiconazol | Tea | UA-MSPE | 75.1–101.2 | UHPLC-MS/MS | [112] |
| Acetamiprid and Imidacloprid | Fruit and water | UA-MSPE | 82.13–102.27 | [111] | |
| Analytes/Examples | Sample Matrix | UAME Approach | Recovery (%) | Detection | Reference |
|---|---|---|---|---|---|
| Mycotoxins | |||||
| Aflatoxins (B1, B2, G1 and G2) | Pistachio nut | UA-MSPD | 74–78 | LC-FLD | [99] |
| Aflatoxins (B1, B2, G1 and G2) | Rice | UA-MSPD | 78–83 | HPLC-FLD | [101] |
| Patulin | Fruit juices | UA-EMA | 90.2–106.9 | UV–VIS | [137] |
| Aflatoxins B1, B2, G1, and G2 and ochratoxin A | Tea | Ultrasound extraction + MIL-based DLLME * | 76–88 | LC-FLD | [49] |
| Aflatoxin M1 | Milk | UA-DLLME | 91.4 | UV-VIS | [119] |
| Aflatoxins B1, B2, G1, G2, and M1, ochratoxin A, zearalenone, deoxynivalenol and patulin | Medicinal and edible foods | UA Organic Drop- DLLME ** | 82.77–103.2 | HPLC–DAD-FLD | [136] |
| Aflatoxins G1, G2, B1, and B2, citrinin (CIT), HT-2, roquefortin C (ROQ C), T-2, ohratoxin A (OTA), sterigmatocistin (ST) | Chili powder | CI-LLE-MSPE *** | 70.6–111.7 | UPLC-Q-TOF/MS | [40] |
| Beauvericin and enniatins A1, B, and B1, aflatoxin B1, zearalenone, deoxynivalenol | Gluten-free flours | UA-MSPD | 84.8–121.7 | LC-MS/MS | [102] |
| Zearalenone | Food | DES-UA-ME | 96–98 | UV-VIS | [134] |
| Technique | Food Matrix | Target Contaminants | LOD | Enrichment Factor | Extraction Time | Solvent Consumption | Reference |
|---|---|---|---|---|---|---|---|
| UA-LLME | Fruit juices | Diphenylamine | 0.05 μg/L | / | 11 min | 500 µL of HP-DES | [151] |
| UA-EME | Water and juice | Organophosphorus pesticides | 5.3–10.0 ng/L | 241–311 | 10 min | 50 µL chlorobenzene | [125] |
| UA-LLME | Oils, fish, milk | Ni(II), Zn(II) | 0.029 μg/kg (Ni); 1.5 μg/kg (Zn) | / | 5 min | 8 mL of DES | [31] |
| UA-DSPME | Bottled beverages | Sb(III) | 1.5 ng/L | 90 | 15–20 min | 6 mL, 3.0 mol/L HNO3 and 4 mL, 2.0 mol/L H2O2 | [65] |
| UA-MSPD | Gluten-Free Flours | Mycotoxins | 1–100 µg/kg * | / | 20 min | 3.5 mL of ACN:H2O:acetic acid (79:20:1, v/v/v) in | [102] |
| UA-CPE | Milk-based products | Formaldehyde | 0.501 μg/L | 55.6 | 17 min | Microliter-scale extractant after preconcentration | [103] |
| USA-DLLME | Egg, honey, and chicken muscle | β-Lactam Antibiotics Residues | In µg/kg | 29.1–74.6 | 7 min | 50 µL HP-DES and 150 µL acetonitrile | [121] |
| SBSE | Fish | Quinolone antibiotics | 0.48–0.8 ng ng/mL | / | >1 h | Solvent-free (thermal desorption) | [152] |
| HF-LPME | Fruits and vegetable juices | Pyrethroid pesticides | 0.02–0.07 ng/mL | 519–528 | 41 min optimum | 24 µL organic phase in fiber | [153] |
| HF-LPME | Drinking water | Disinfection by-products | 10–220 ng/L | 13.1–140.1 | 30 min | 20 μL of octanol | [154] |
| HS-SPME | Biscuits | Acrylamide | 27.4 µg/kg | / | 45 min | 30 mL of propanol | [155] |
| SBSE | Milk samples | Fluoroquinolones | 1.20–2.62 μg/L | 56.2–61.5 | 60 min | 1 mL of organic solvent for elution | [156] |
| SBSE | Seafood | Heavy metal and arsenic ions | <0.08 μg/kg | / | 30 min | 10 mL 1 M HNO3 | [157] |
| *LOQ |
| Extraction Method | Advantages | Disadvantages |
|---|---|---|
| UA-LLME | Rapid mass transfer Minimal solvent High enrichment Simple setup | Emulsion separation can be difficult Heat can degrade analytes Choice of solvent is limited |
| UA-DLLME | Fast and efficient Uses little sample/solvent High enrichment | Requires phase separation Emulsions may trap analytes Risk of analyte loss with over-sonication |
| UA-EME | Fine emulsions efficiently extract hydrophobic/volatile compounds Extremely rapid and simple Low solvent uses | Less effective for highly polar analytes Phase separation can be slow Limited solvent compatibility |
| UA-SLME | Speeds extraction of solids Shorter time and less solvent Suitable for complex matrices | Needs post-extraction solid removal Dependent on particle size and mixing May miss strongly bound analytes |
| UA-DμSPE | High efficiency Little sorbent/solvent needed Fast enrichment of trace analytes | Requires sorbent separation Limited sorbent capacity Optimization needed for each matrix |
| UA-SPME | Greatly reduces SPME time Solvent-free High sensitivity for volatiles/pollutants | Requires specialized fibers and desorption Fibers are fragile Single-sample processing |
| UA-CPE | Uses mild surfactants High enrichment Fast cloud formation | Requires heating and centrifugation Surfactant cleanup needed Risk of micelle disruption |
| UA-SEME | Safe surfactants Works for polar and non-polar analytes Quick dispersion | Surfactant removal needed Optimization can be time-consuming Matrix effects possible |
| UA-ILME | Tunable selectivity Non-volatile and reusable solvents Broad polarity range | Ionic liquids can be expensive/toxic High viscosity slows extraction Requires solvent to remove IL phase |
| UA-MSPE | Magnetic separation simplifies cleanup High recovery and sensitivity Good for trace contaminants | Magnetic sorbents can be complex/costly Limited to analytes that bind sorbent Extra desorption step required |
| UA-MSPD | Integrates grinding, extraction, and cleanup Minimal solvent Rapid analyte release | Labor-intensive homogenization Reproducibility depends on uniform blending Requires matrix-specific optimization |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lučić, M.; Onjia, A. Ultrasound-Assisted Microextraction for Food Chemical Contaminant Analysis: A Review. Processes 2025, 13, 3677. https://doi.org/10.3390/pr13113677
Lučić M, Onjia A. Ultrasound-Assisted Microextraction for Food Chemical Contaminant Analysis: A Review. Processes. 2025; 13(11):3677. https://doi.org/10.3390/pr13113677
Chicago/Turabian StyleLučić, Milica, and Antonije Onjia. 2025. "Ultrasound-Assisted Microextraction for Food Chemical Contaminant Analysis: A Review" Processes 13, no. 11: 3677. https://doi.org/10.3390/pr13113677
APA StyleLučić, M., & Onjia, A. (2025). Ultrasound-Assisted Microextraction for Food Chemical Contaminant Analysis: A Review. Processes, 13(11), 3677. https://doi.org/10.3390/pr13113677









