Azeotropic and Extractive Distillation for Bio-Ethanol Dehydration: Process Design, Simulation, and Cost Analysis
Abstract
1. Introduction
2. Methodology
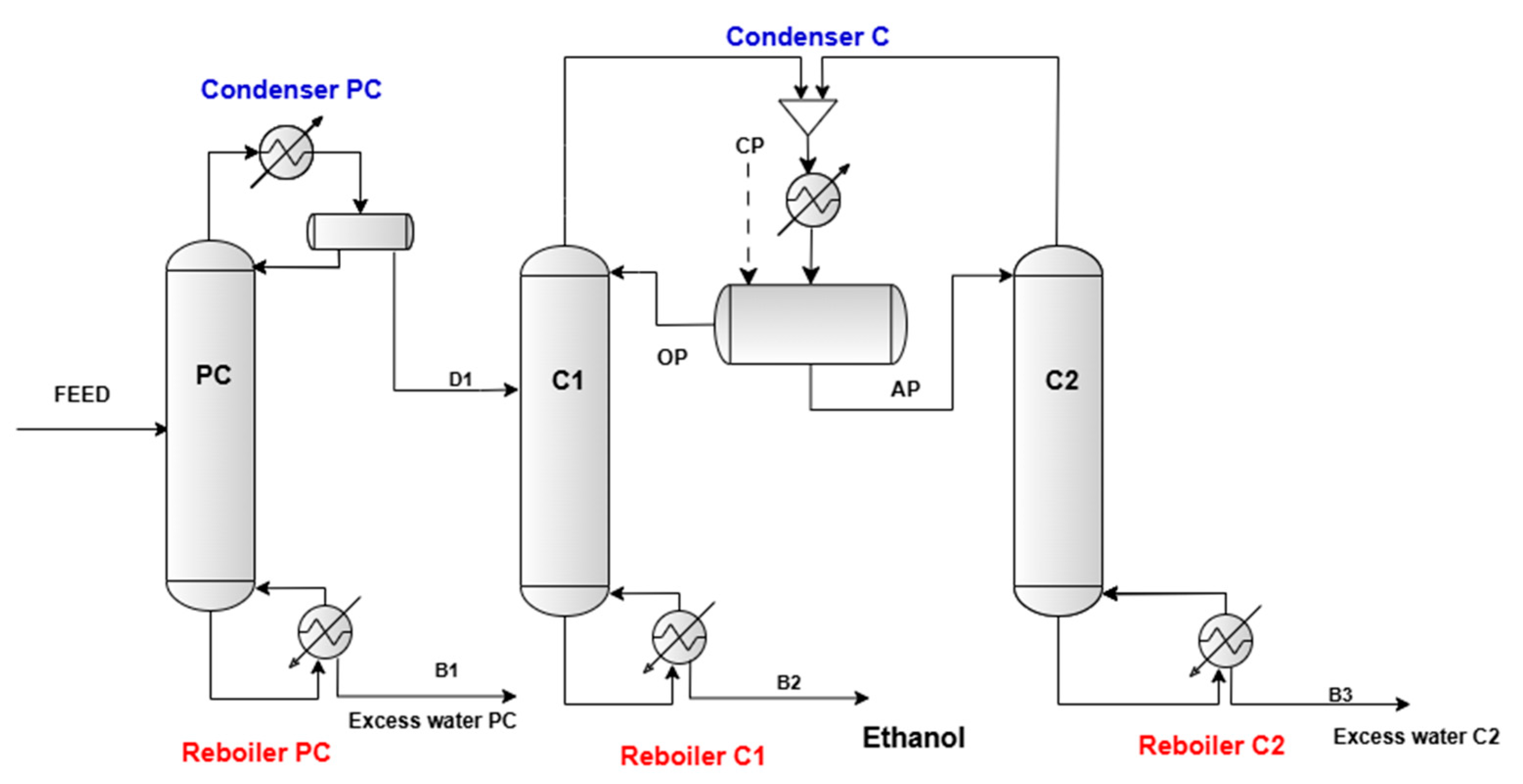
2.1. Proposed Flowsheet for Azeotropic Distillation Process
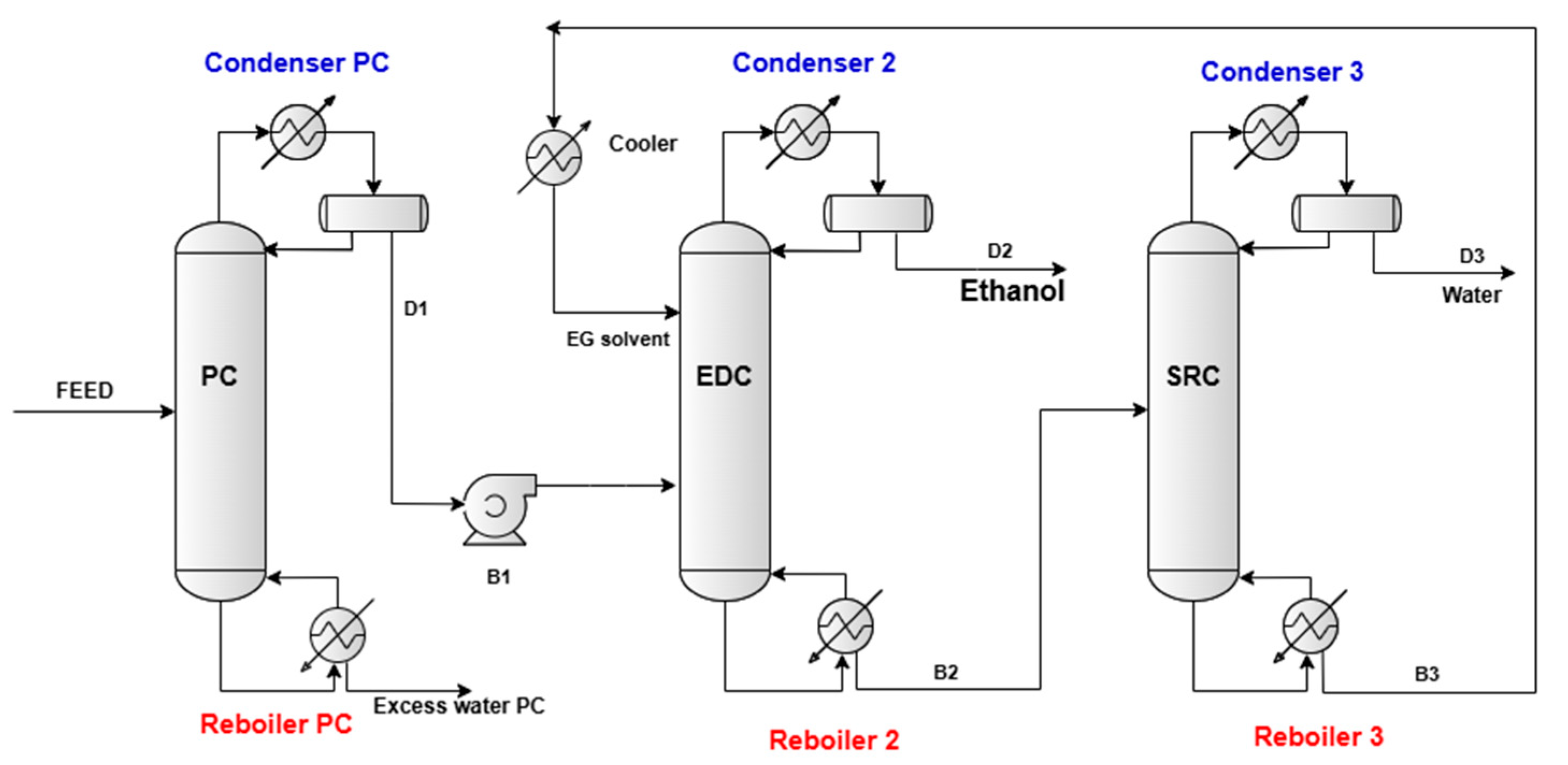
2.2. Proposed Flowsheet for Extractive Distillation Process
2.3. Economic Evaluation
3. Results and Discussion
3.1. Simulation Results and Discussion: Preconcentration Column
3.2. Simulation Results and Discussion: Azeotropic Distillation Dehydration Process
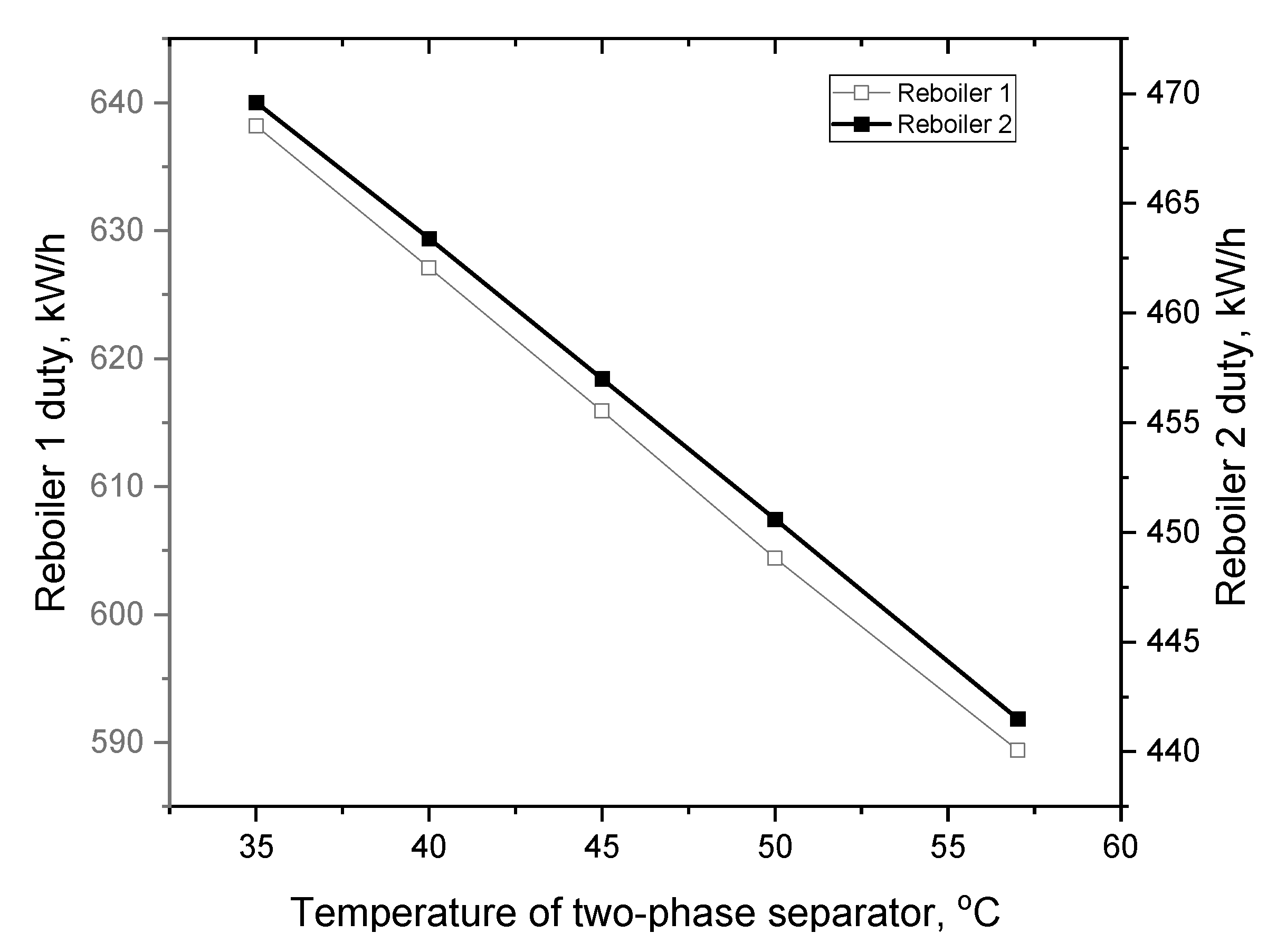
3.3. Simulation Results and Discussion: Extractive Distillation Dehydration Process
3.4. Results of Economic Evaluation and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| EG | ethylene glycol |
| ED | extractive distillation |
| LMTDs | log-mean temperature differences |
| NRTL | Non-Random Two Liquid |
| UNIFAC | Universal Functional-group Activity Coefficients |
| TACC | total annualized capital costs ($/year) |
| TAEC | total annual energy costs ($/year) |
| TAC | total annual cost ($/year) |
| Symbols | |
| PC | preconcentration column |
| C1 | ethanol dehydration column |
| C2 | water separation column |
| EDC | extractive distillation column |
| SRC | extractive-solvent recovery column |
| B n | bottom product for column n (kg/h) |
| D n | distillate product for column n (kg/h) |
| Reboiler n | reboiler of column n |
| Condenser n | condenser of column n |
| Condenser C | condenser of C1 and C2 column |
| Cooler | cooler |
References
- Lee, Y.-Y.; Srinivaas, M.; Li, I.-C.; Keharika, K.; Pothu, R.; Boddula, R.; Al-Qahtani, N.; Huang, B.-W.; Chang-Chien, G.-P. Enchanging Sustainable Energy Through Cutting-Edge Waste Biorefinery Technologies. Reactions 2024, 5, 1101–1147. [Google Scholar] [CrossRef]
- Areeya, S.; Pannakkal, E.J.; Kunmanee, P.; Tawai, A.; Amornraksa, S.; Sriarivanun, M. A Review of Sugarcane Biorefinery: From Waste to Value-Added Products. Appl. Sci. Eng. Prog. 2024, 17, 74022. [Google Scholar] [CrossRef]
- Patel, R.; Rajaraman, T.S.; Rana, H.P.; Ambegaonkar, J.N.; Patel, S. A review on techno-economic analysis of lignocellulosic biorefinery producing biofuels and high-value products. Results Chem. 2025, 13, 102052. [Google Scholar] [CrossRef]
- Rola, K.; Gruber, S.; Goričanec, D.; Urbancl, D. Waste Lignocellulosic Biomass as a Source for Bioethanol Production. Sustain. Chem. 2024, 5, 1–12. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, P.; Maity, K.S.; Agrawal, D.; Narisetty, V.; Jacob, S.; Kumar, G.; Bhatia, S.K.; Kumar, D.; Vivekanand, V. Recent advances in bio-based production of top platform chemical, succinic acid: An alternative to conventional chemistry. Biotechnol. Biofuels Bioprod. 2024, 17, 72. [Google Scholar] [CrossRef] [PubMed]
- Ioannidou, S.M.; Pateraki, C.; Ladakis, D.; Papapostolou, H.; Tsakona, M.; Vlysidis, A.; Kookos, I.K.; Koutinas, A. Sustainable production of bio-based chemicals and polymers via integrated biomass refining and bioprocessing in a circular bio-economy context. Bioresour. Tech. 2020, 307, 123093. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Y.; Chen, X.; Yu, X.; Li, W.; Zhang, S.; Meng, X.; Zhao, Z.-M.; Dong, T.; Anderson, A.; et al. Sustainable bioplastics derived from renewable natural resources for food packaging. Matter 2023, 6, 97–127. [Google Scholar] [CrossRef]
- Broda, M.; Yelle, J.D.; Serwańska, K. Bioethanol Production from Lignocellulosic Biomass—Challenges and Solutions. Molecules 2022, 27, 8717. [Google Scholar] [CrossRef]
- Raj, T.; Chandrasekhar, K.; Kumar, A.N.; Banu, J.R.; Yoon, J.-J.; Bhatia, S.K.; Yang, Y.-H.; Varjani, S.; Kim, S.-H. Recent advances in commercial biorefineries for lignocellulosic ethanol production: Current status, challenges and future perspectives. Bioresour. Technol. 2022, 344, 126292. [Google Scholar] [CrossRef]
- Kiss, A.A. Advanced Distillation Technologies: Design, Control and Applications; John Wiley & Sons Ltd.: Chichester, UK, 2013. [Google Scholar]
- Gil, I.D.; Gómez, J.M.; Rodríguez, G. Control of an extractive distillation process to dehydrate ethanol using glycerol as entrainer. Comput. Chem. Eng. 2012, 39, 129–142. [Google Scholar] [CrossRef]
- Sánchez-Correa, C.A.; Arturo-Calvache, J.E.; Gil-Chaves, I.D.; Rodríguez-Niño, G. Multiplicities in ethanol dehydration by extractive distillation processes with ethyleneglycol. Chem. Eng. Process. Process Intensif. 2022, 174, 108886. [Google Scholar] [CrossRef]
- Plesu Popescu, A.E.; Pellin, J.L.; Bonet, J.; Llorens, J. Bioethanol dehydration and mixing by heterogeneous azeotropic distillation. J. Clean. Prod. 2021, 320, 128810. [Google Scholar] [CrossRef]
- Mittal, N.; Bai, P.; Siepmann, J.I.; Daoutidis, P.; Tsapatsis, M. Bioethanol enrichment using zeolite membranes: Molecular modeling, conceptual process design and techno-economic analysis. J. Membr. Sci. 2017, 540, 464–476. [Google Scholar] [CrossRef]
- Karimi, S.; Yaraki, M.T.; Karri, R.R. A comprehensive review of the adsorption mechanisms and factors influencing the adsorption process from the perspective of bioethanol dehydration. Renew. Sustain. Energy Rev. 2019, 107, 535–553. [Google Scholar] [CrossRef]
- de Jesús Hernández-Hernández, E.; Cabrera-Ruiz, J.; Hernández-Escoto, H.; Gutiérrez-Antonio, C.; Hernández, S. Simulation study of the production of high purity ethanol using extractive distillation: Revisiting the use of inorganic salts. Chem. Eng. Process. Process Intensif. 2022, 170, 108670. [Google Scholar] [CrossRef]
- Kiss, A.A.; Suszwalak, D.J.P.C. Enhanced bioethanol dehydration by extractive and azeotropic distillation in dividing-wall columns. Sep. Purif. Technol. 2012, 86, 70–78. [Google Scholar] [CrossRef]
- Hernández-Escoto, H.; Zavala-Guzmán, A.M.; Maya-Yescas, R.; Zamudio-Lara, J.M.; Hernández, S. Operability assessment and systematic PI control of a class of Extractive Dividing Wall Distillation Columns: Case of ethanol dehydration. Chem. Eng. Res. Des. 2022, 187, 84–92. [Google Scholar] [CrossRef]
- Singh, A.; da Cunha, S.; Rangaiah, G.P. Heat-pump assisted distillation versus double-effect distillation for bioethanol recovery followed by pressure swing adsorption for bioethanol dehydration. Sep. Purif. Technol. 2019, 210, 574–586. [Google Scholar] [CrossRef]
- Beluhan, S.; Mihajlovski, K.; Šantek, B.; Ivančić Šantek, M. The Production of Bioethanol from Lignocellulosic Biomass: Pretreatment Methods, Fermentation, and Downstream Processing. Energies 2023, 16, 7003. [Google Scholar] [CrossRef]
- Zhai, J.; Sun, X.; Huang, S.; Xie, H.; Chen, X. Economic, thermodynamic, environmental, and inherent safety investigation of heat pump-assisted extractive dividing wall column for separating binary azeotrope. Process Saf. Environ. Prot. 2023, 173, 202–214. [Google Scholar] [CrossRef]
- Zhao, G.; Gao, H.; Qu, Z.; Fan, H.; Meng, H. Anhydrous interfacial polymerization of sub-1 Å sieving polyamide membrane. Nat. Commun. 2023, 14, 7624. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wang, Z.; Xu, W.; Xin, L.; Hu, R.; Zhu, Z.; Wang, Y.; Cui, P. Innovative extractive distillation process combining preconcentration and solvent recovery functions for efficiently separating diisopropyl ether/isopropanol/water. Sep. Purif. Technol. 2025, 360, 131021. [Google Scholar] [CrossRef]
- Wang, Z.; Xin, L.; Wang, Y.; Wu, Q.; Xu, W.; Zhu, Z.; Wang, Y.; Cui, P. Optimization and dynamic control of green energy-saving process for azeotropes separation by mixed solvent extractive distillation. Renew. Sustain. Energy Rev. 2025, 218, 115813. [Google Scholar] [CrossRef]
- Duan, C.; Li, C. Energy-saving improvement of heat integration for separating dilute azeotropic components in extractive distillation: Part II—Process integration. Sep. Purif. Technol. 2025, 360, 131009. [Google Scholar] [CrossRef]
- Nicolae, M.; Neagu, M.; Cursaru, D.-L. Energy-Saving Extractive Distillation Process for Isopropanol Dehydration with Propylene Glycol as Novel Extractive Solvent. Appl. Sci. 2024, 14, 9420. [Google Scholar] [CrossRef]
- Luyben, W.L. Control of a multiunit heterogeneous azeotropic distillation process. AIChE J. 2006, 52, 623–637. [Google Scholar] [CrossRef]
- Gutiérrez-Antonio, C.; Jiménez-Gutiérrez, A. Design of side-stream azeotropic distillation columns. Chem. Eng. Res. Des. 2007, 85, 1384–1389. [Google Scholar] [CrossRef]
- Gomis, V.; Pedraza, R.; Saquete, M.D.; Font, A.; Garcia-Cano, J. Ethanol dehydration via azeotropic distillation with gasoline fractions as entrainers: A pilot-scale study of the manufacture of an ethanol–hydrocarbon fuel blend. Fuel 2015, 139, 568–574. [Google Scholar] [CrossRef]
- Cane, C. Dehydration of Alcohols, Preferably Ethanol, Using a Cyclohexane Entrainer. EP0001681A1, 2 May 1979. [Google Scholar]
- Baiel, J.J.; Tsonopoulos, C. High-Pressure Azeotropic Distillation for the Manufacture of Anhydrous Alcohols. U.S. Patent 4161429A, 17 July 1979. [Google Scholar]
- Batista, F.R.M.; Follegatti-Romero, L.A.; Bessa, L.C.B.A.; Meirelles, A.J.A. Computational simulation applied to the investigation of industrial plants for bioethanol distillation. Comput. Chem. Eng. 2012, 46, 1–16. [Google Scholar] [CrossRef]
- de Figueiredo, M.F.; Brito, K.D.; Ramos, W.B.; Sales Vasconcelos, L.G.; Brito, R.P. Effect of Solvent Content on the Separation and the Energy Consumption of Extractive Distillation Columns. Chem. Eng. Commun. 2015, 202, 1191–1199. [Google Scholar] [CrossRef]
- Jaime, J.A.; Rodríguez, G.; Gil, I.D. Control of an Optimal Extractive Distillation Process with Mixed-Solvents as Separating Agent. Ind. Eng. Chem. Res. 2018, 57, 9615–9962. [Google Scholar] [CrossRef]
- Miranda, N.T.; Maciel Filho, R.; Wolf Maciel, M.R. Comparison of Complete Extractive and Azeotropic Distillation Processes for Anhydrous Ethanol Production Using Aspen Plus™ Simulator. Chem. Eng. Trans. 2020, 80, 43–48. [Google Scholar] [CrossRef]
- Fattahi, T.; Salehi, E.; Hosseini, Z. Intensification of Azeotropic Distillation for Ethanol Dehydration using Data-based Optimization, Steady-state Simulation and Sensitivity Analysis. Iran. J. Chem. Eng. IJCE 2023, 20, 15–32. [Google Scholar] [CrossRef]
- Nicolae, M.; Fendu, E.M. Experimental and Regression VLE Data for Ethanol + DPG Binary System: Ethanol Anhydrization Process Simulation Using DPG as Extractive Agent. Period. Polytech. Chem. Eng. 2024, 68, 454–469. [Google Scholar] [CrossRef]
- ANP 36; Establishing the Specification for Fuel Ethanol (Anhydrous and Hydrated) for Automotive Use Blended with Gasoline. National Petroleum, Natural Gas and Biofuels Agency: Brasília, Brazil, 2005.
- EN 15376; Automotive Fuels—Ethanol as a Blending Component for Petrol—Requirements and Test Methods. European Committee for Standardization (CEN)—CEN-CENELEC Management Centre: Brussels, Belgium, 2014. Available online: https://www.intertekinform.com/preview/98703763611.pdf (accessed on 20 September 2025).
- ASTM D 4806-03; Standard Specification for Denatured Fuel Ethanol for Blending with Gasolines for Use as Automotive Spark-Ignition Engine Fuel. ASTM International: West Conshohocken, PA, USA, 2003. Available online: https://kelid1.ir/FilesUp/ASTM_STANDARS_971222/D4806.PDF?utm (accessed on 20 September 2025).
- Council Directive 91/271/EEC of 21 May 1991 Concerning Urban Waste-Water Treatment. Available online: https://eur-lex.europa.eu/eli/dir/1991/271/oj/eng (accessed on 6 September 2025).
- Process Simulation of Aveva Software, version AVEVA PRO/II; AVEVA: Cambridge, UK, 2024.
- Lide, D.R.; Kehiaian, H.V. CRC Handbook of Thermophysical and Thermochemical Data; CRC Press: Boca Raton, FL, USA, 1994. [Google Scholar]
- Horsley, L.H. Azeotropic Data, III; American Chemical Society: Washington, DC, USA, 1973; Volume 17, p. 134. [Google Scholar]
- Howard, H.P.; Michalenko, M.E.; Meylan, M.W.; Basu, K.D.; Beauman, A.J.; Sage, W.G.; Jarvis, F.W.; Gray, D.A. Chapter: Cyclopentane. In Handbook of Environmental Fate and Exposure Data for Organic Chemicals, 1st ed.; Howard, P., Ed.; Imprint CRC Press: Boca Raton, FL, USA, 1993; Volume IV. [Google Scholar]
- Wu, J.; Gao, L.-G.; Ren, W.; Truhlar, D.G. Anharmonic kinetics of the cyclopentane reaction with hydroxyl radical. Chem. Sci. 2020, 11, 2511. [Google Scholar] [CrossRef]
- Jindamanee, K.; Keawboonchu, J.; Pinthong, N.; Meeyai, A.; Inchai, P.; Thepanondh, S. Environmental impacts and emission profiles of volatile organic compounds from petroleum refineries. Sci. Rep. 2025, 15, 15509. [Google Scholar] [CrossRef]
- Stokes, R.; Moosemiller, M. Distillation Improvement Opportunities, Part 6: Safety Implications from New Technologies. Chem. Eng. 2023, 989. Available online: https://www.thechemicalengineer.com/features/distillation-improvement-opportunities-part-6-safety-implications-from-new-technologies (accessed on 31 October 2025).
- Valentini, F.; Vaccaro, L. Azeotropes as Powerful Tool for Waste Minimization in Industry and Chemical Processes. Molecules 2020, 25, 5264. [Google Scholar] [CrossRef] [PubMed]
- Luyben, W.L. Plantwide control of an isopropyl alcohol dehydration process. AIChE J. 2006, 52, 2290–2296. [Google Scholar] [CrossRef]
- Sinnott, R.K. Chemical Engineering Design, 4th ed.; Coulson & Richardson’s Chemical Engineering Series; Elsevier Butterworth-Heinemann: Oxford, UK, 2005; Volume 6. [Google Scholar]
- Cui, Y.; Zhang, Z.; Shi, X.; Guang, C.; Gao, J. Triple-column side-stream extractive distillation optimization via simulated annealing for the benzene/isopropanol/water separation. Sep. Purif. Technol. 2020, 236, 116303. [Google Scholar] [CrossRef]
- Prausnitz, J.; Lichtenthaler, R.; de Azevedo, E.G. Molecular Thermodynamics of Fluid-Phase Equilibria, 3rd ed.; Prentice Hall PTR: Upper Saddle River, NJ, USA, 1999. [Google Scholar]
- Sommer, S.; Melin, T. Design and Optimization of Hybrid Separation Processes for the Dehydration of 2-Propanol and Other Organics. Ind. Eng. Chem. Res. 2004, 43, 5248–5259. [Google Scholar] [CrossRef]
- Staples, C.A.; Williams, J.B.; Craig, G.R.; Roberts, K.M. Fate, effects and potential environmental risks of ethylene glycol: A review. Chemosphere 2001, 43, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Avilés-Martínez, A.; Medina-Herrera, N.; Jiménez-Gutiérrez, A.; Serna-González, M.; Castro-Montoya, A.J. Risk Analysis Applied to Bioethanol Dehydration Processes: Azeotropic Distillation versus Extractive Distillation. Comput. Aided Chem. Eng. 2015, 37, 1835–1840. [Google Scholar] [CrossRef]
| Parameters | Column PC | Column C1 | Column C2 |
|---|---|---|---|
| Number of trays | 17 1 | 24 1 | 19 1 |
| Feed tray number | 10 | 6 | 1 |
| Top pressure, bar | 1.6 | 1.6 | 1.6 |
| Bottom pressure, bar | 1.77 | 1.84 | 1.79 |
| Top temperature, °C | 86.55 | 57.47 | 81.16 |
| Bottom temperature, °C | 116.33 | 94.25 | 116.67 |
| Reflux ratio, molar 2 | 2.0 | - | - |
| Parameters | Column PC | Column EDC | Column SRC |
|---|---|---|---|
| Number of trays | 17 1 | 22 1 | 16 1 |
| Feed tray number | 10 | 18 | 7 |
| Feed solvent tray number | - | 5 | - |
| Top pressure, bar | 1.6 | 1.3 | 1.15 |
| Bottom pressure, bar | 1.77 | 1.52 | 1.22 |
| Top temperature, °C | 86.55 | 80.40 | 97.15 |
| Bottom temperature, °C | 116.33 | 184.21 | 203.87 |
| Reflux ratio, molar | 2.0 | 2.0 | 5.0 |
| Components | Distillate Flow (D1), kg/h | Mass Concentration in D1, wt.% | Bottom Flow (B1), kg/h | Mass Concentration in B1, wt.% |
|---|---|---|---|---|
| Ethanol | 991.0962 | 90.41 | 8.9038 | 0.100 |
| Water | 105.1584 | 9.59 | 8894.8416 | 99.90 |
| Total | 1096.2546 | 100.00 | 8903.7454 | 100.00 |
| Components | Organic Phase (OP) Flow, kg/h | Concentration in OP, wt.% | Aqueous Phase (AP) Flow, kg/h | Concentration in AP, wt.% | Bottom Flow (B2), kg/h | Concentration in B2, wt.% | Bottom Flow (B3), kg/h | Concentration in B3, wt.% |
|---|---|---|---|---|---|---|---|---|
| Ethanol | 12.01 | 0.286 | 902.39 | 43.01 | 664.73 | 99.94 | 0.0569 | 0.100 |
| Water | 1.37 | 0.0326 | 203.55 | 9.70 | 0.002 | 0.0003 | 56.8322 | 99.90 |
| Cyclopentane | 4184.43 | 99.68 | 992.04 | 47.29 | 0.418 | 0.0630 | 0.00 | 0.00 |
| Total | 4197.81 | 100.00 | 2097.98 | 100.00 | 665.15 | 100.00 | 56.8890 | 100.00 |
| Components | Distillate Flow (D2), kg/h | Concentration in D2, wt.% | Distillate Flow (D3), kg/h | Concentration in D3, wt.% | Bottom Flow (B2), kg/h | Concentration in B2, wt.% | Bottom Flow (B3), kg/h | Concentration in B3, wt.% |
|---|---|---|---|---|---|---|---|---|
| Ethanol | 991.02 | 99.94 | 0.0514 | 0.0492 | 0.0514 | 0.00263 | 0.0 | 0.00 |
| Water | 0.595 | 0.06 | 104.54 | 99.95 | 104.56 | 5.37 | 0.0184 | 0.001 |
| Ethylene glycol | 0.009 | 0.0 | 0.0002 | 0.0 | 1844.28 | 94.63 | 1844.28 | 100.00 |
| Total | 991.62 | 100.00 | 104.59 | 100.00 | 1948.89 | 100.00 | 1844.30 | 100.00 |
| Parameter, u.m | PC | Azeotropic Distillation Process | Extractive Distillation Process | ||
|---|---|---|---|---|---|
| C1 | C2 | EDC | SRC | ||
| Diameter, m | 0.762 | 0.762 | 0.61 | 0.61 | 0.381 1/0.61 2 |
| Height, m | 31.6 | 69.54 | 57.0 | 36.85 | 44.1 |
| Reboiler duty, kW/h | 1721.0 | 589.4 | 441.5 | 609.6 | 177.7 |
| Reboiler area, m2 | 70.5 | 15.96 | 18.05 | 26.32 | 6.22 |
| Steam consumption, kg/h | 2971.0 | 1017.0 | 762.0 | 1120.0 | 377.0 |
| Condenser duty, kW/h | 886.8 | 916.4 | 463.7 | 131.8 | |
| Condenser area, m2 | 22.1 | 54.43 | 13.2 | 2.58 | |
| Water consumption, kg/h | 42,362.306 | 43,776.022 | 22,152.801 | 6296.986 | |
| Cooler duty, kW/h | - | - | - | 195.4 | |
| Cooler area, m2 | - | - | - | 2.6 | |
| Water consumption, kg/h | - | - | - | 9334.344 | |
| Azeotropic Distillation Process | Extractive Distillation Process | |
|---|---|---|
| Capital cost (TACC) (a), $ | 4,615,995 | 2,929,493 |
| Utilities cost (TAEC), $/year | 634,986 | 640,647 |
| TAC (a), $/year | 2,173,651 | 1,617,144 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neagu, M.; Pricop-Nicolae, M. Azeotropic and Extractive Distillation for Bio-Ethanol Dehydration: Process Design, Simulation, and Cost Analysis. Processes 2025, 13, 3634. https://doi.org/10.3390/pr13113634
Neagu M, Pricop-Nicolae M. Azeotropic and Extractive Distillation for Bio-Ethanol Dehydration: Process Design, Simulation, and Cost Analysis. Processes. 2025; 13(11):3634. https://doi.org/10.3390/pr13113634
Chicago/Turabian StyleNeagu, Mihaela, and Marilena Pricop-Nicolae. 2025. "Azeotropic and Extractive Distillation for Bio-Ethanol Dehydration: Process Design, Simulation, and Cost Analysis" Processes 13, no. 11: 3634. https://doi.org/10.3390/pr13113634
APA StyleNeagu, M., & Pricop-Nicolae, M. (2025). Azeotropic and Extractive Distillation for Bio-Ethanol Dehydration: Process Design, Simulation, and Cost Analysis. Processes, 13(11), 3634. https://doi.org/10.3390/pr13113634







