Fast Chromatographic Method for Explosive Profiling
Abstract
:1. Introduction
2. Experimental Section
2.1. Samples and VOC Collection
| N° | Commercial name | Detected active ingredients | Expected active ingredients | Explosive quantity (mg) |
|---|---|---|---|---|
| 1 | Prima Sheet | PETN | PETN | 12.4 |
| 2 | Detonation Cord | PETN | 10%–80% PETN | 23.7 |
| 3 | UEE Booster | PETN | PETN (10%–60%) TNT (>60%) | 18.6 |
| 4 | Anzomex | PETN and TNT | PETN & TNT | 25.0 |
| 5 | PPP Booster | TNT and RDX | PETN (0%–60%) RDX (0%–60%) TNT (35%–55%) | 25.8 |
2.2. Analyses
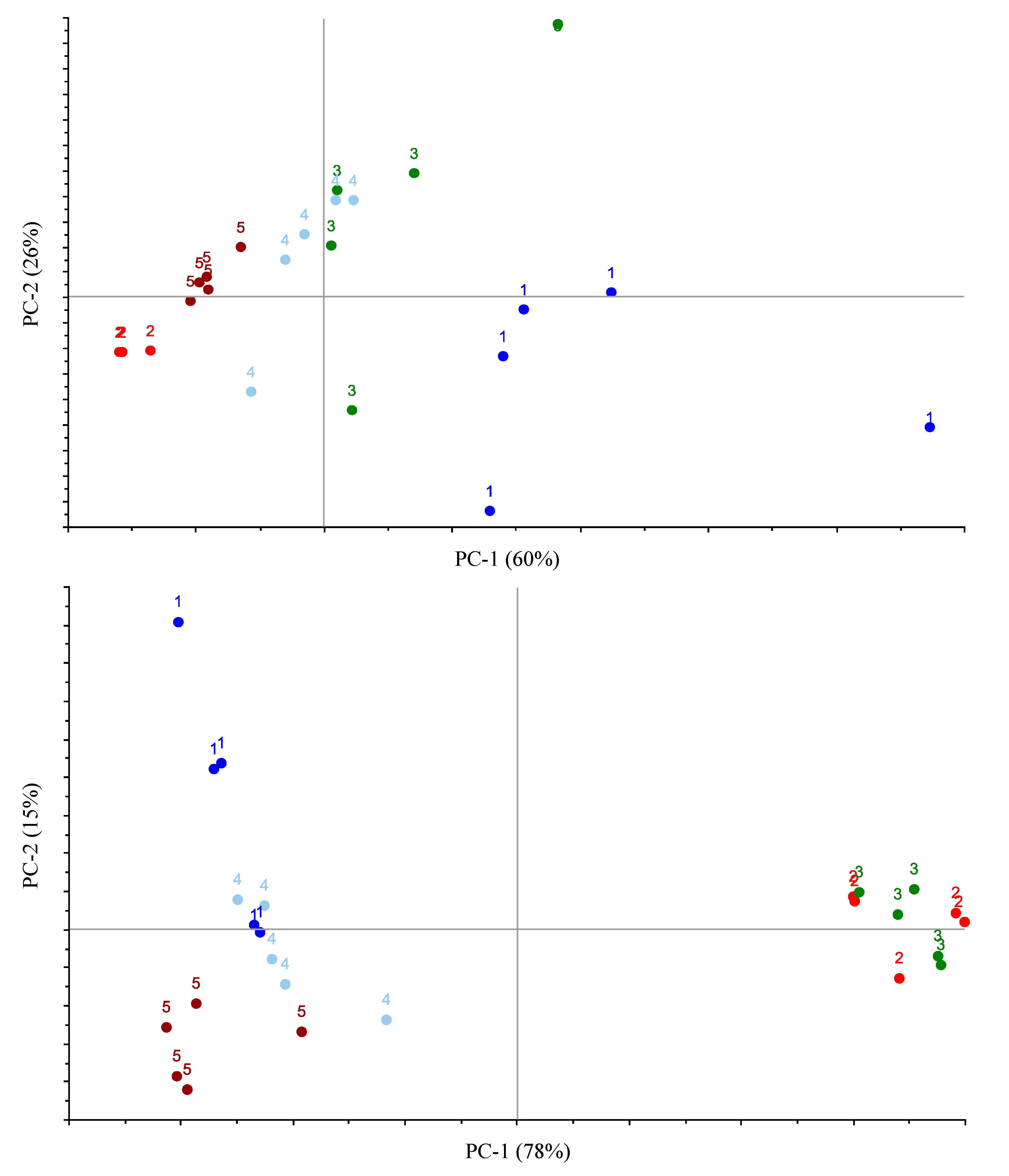
2.3. Data Processing and Statistical Analyses
3. Results and Discussion
3.1. Method Optimization
| SPME | |||||
|---|---|---|---|---|---|
| 1tR | 2tR | Match | Reverse | Probability | |
| PETN | 91 +/− 0 | 0.32 +/− 0.01 | 674 | 814 | 2877 |
| TNT | 181 +/− 0 | 0.35 +/− 0 | 852 | 858 | 9657 |
| RDX | 215.5 | 0.31 | 396 | 664 | 6060 |
| Liquid Injection | |||||
| 1tR | 2tR | Match | Reverse | Probability | |
| PETN | 91 +/− 0 | 0.31 +/− 0 | 887 | 835 | 3772 |
| TNT | 180 +/− 1 | 0.36 +/− 0.02 | 900 | 900 | 9886 |
| RDX | 212.5 | 0.31 | 887 | 890 | 9769 |
3.2. Fast GC×GC Explosive Detection
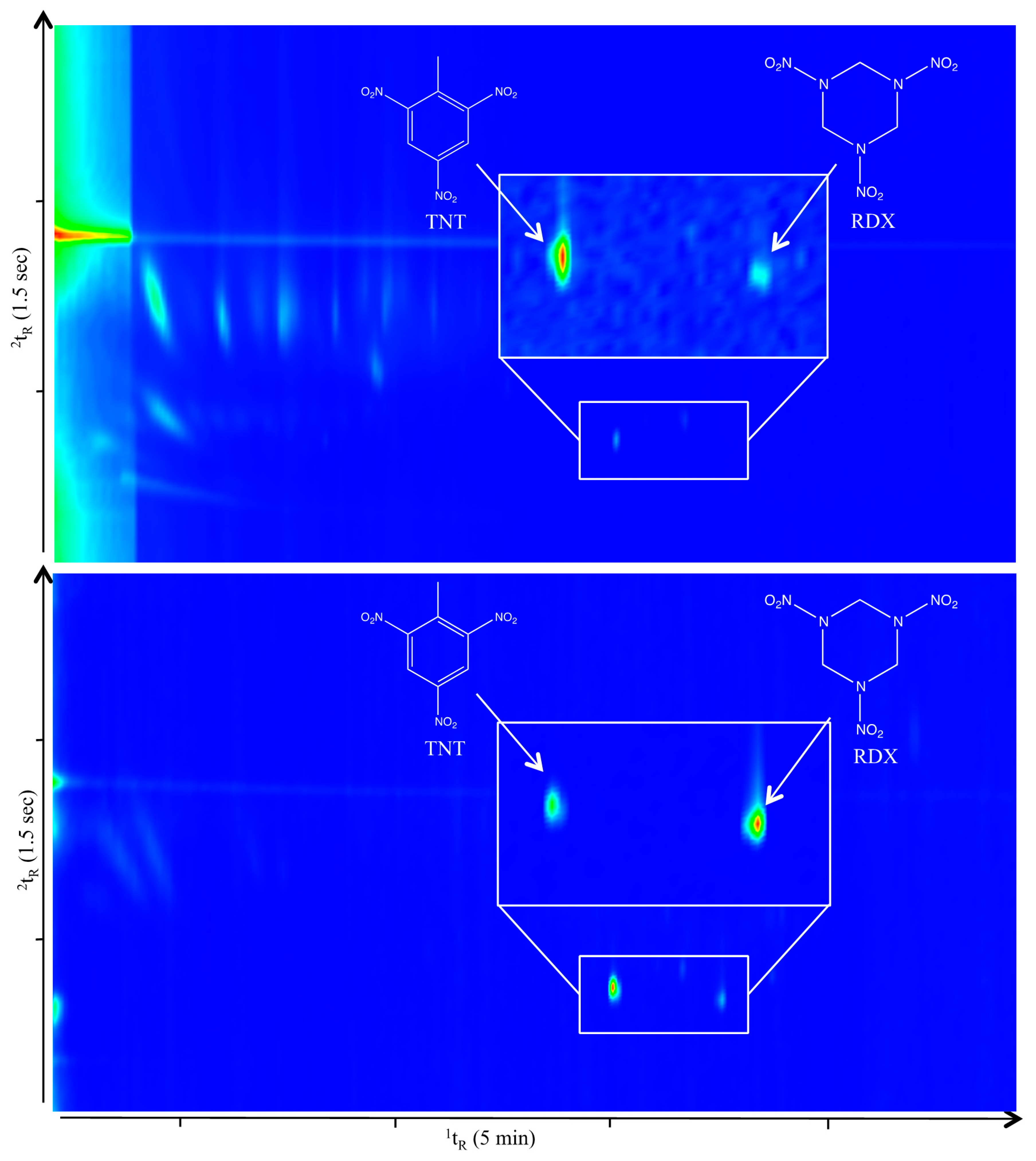
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lorenzo, N.; Wan, T.L.; Harper, R.J.; Hsu, Y.L.; Chow, M.; Rose, S.; Furton, K.G. Laboratory and field experiments used to identify Canis lupus var. Familiaris active odor signatures chemical from drugs, explosives, and humans. Anal. Bioanal. Chem. 2003, 376, 1212–1224. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.; Leung, A.; Magee, M.; Almirall, J.R. Identification of volatile chemical signatures from plastic explosives by SPME-GC/MS and detection by ion mobility spectrometry. Anal. Bioanal. Chem. 2010, 396, 2997–3007. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.; Guerra, P.; Joshi, M.; Almirall, J.R. Analysis of volatile components of drugs and explosives by solid phase microextraction-ion mobility spectrometry. J. Sep. Sci. 2008, 31, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Almirall, J.R. High-efficiency headspace sampling of volatile organic compounds in explosives using capillary microextraction of volatiles (CMV) coupled to gas chromatography-mass spectrometry (GC-MS). Anal. Bional. Chem. 2014, 406, 2189–2195. [Google Scholar] [CrossRef]
- Ewing, R.G.; Atkinson, D.A.; Eiceman, G.A.; Ewing, G.J. A critical review of ion mobility spectrometry for the detection of explosives and explosive related compounds. Talanta 2001, 54, 515–529. [Google Scholar] [CrossRef] [PubMed]
- Casetta, B.; Garofolo, F. Characterization of explosives by liquid chromatography/mass spectrometry and liquid chromatography/tandem mass spectrometry using electrospray ionization and parent-ion scanning techniques. J. Mass Spectrom. 2005, 29, 517–525. [Google Scholar]
- Ewing, R.G.; Waltman, M.J.; Atkinson, D.A.; Grate, J.W.; Hotchkiss, P.J. The vapor pressures of explosives. TrAC 2013, 42, 35–47. [Google Scholar]
- Grate, J.W.; Ewing, R.G.; Atkinson, D.A. Vapor-generation methods for explosives-detection research. TrAC 2012, 41, 1–13. [Google Scholar]
- Ewing, R.G.; Atkinson, D.A.; Clowers, B.H. Direct Real-Time Detection of RDX Vapors Under Ambient Conditions. Anal. Chem. 2013, 85, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Ewing, R.G.; Clowers, B.H.; Atkinson, D.A. Direct Real-Time Detection of Explosive Compounds. Anal. Chem. 2013, 85, 10977–10983. [Google Scholar] [CrossRef] [PubMed]
- Serrano, G.; Sukaew, T.; Edward, E.T. Hybrid preconcentrator/focuser module for determinations of explosive marker compounds with a micro-scale gas chromatograph. J. Chromatogr. A 2013, 1279, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Furton, K.G.; Almirall, J.R.; Bi, M.; Wang, J.; Wu, L. Application of solid phase microextraction to the recovery of explosives and ignitable liquid residues from forensic specimens. J. Chromatogr. A 2000, 885, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Perr, J.M.; Furton, K.G.; Almirall, J.R. Solid phase microextraction ion mobility spectrometer interface for explosive taggant detection. J. Sep. Sci. 2005, 28, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Popiel, S.; Sankowska, M. Determination of chemical warfare agents and related compounds in environmental samples by solid-phase microextraction with gas chromatography. J. Chromatogr. A 2011, 1218, 8457–8579. [Google Scholar] [CrossRef] [PubMed]
- Perrault, K.A.; Stuart, B.H.; Forbes, S.L. A Longitudinal Study of Decomposition Odour in Soil Using Sorbent Tubes and Solid Phase Microextraction. Chromatography 2014, 1, 120–140. [Google Scholar] [CrossRef]
- Hallowell, S.F.; Fischer, D.S.; Brasher, J.D.; Malone, R.L.; Gresham, G.L.; Rae, G.C. Effectiveness of quality-control aids in verifying K-9-team explosive detection performance. P. Soc. Photo.-Opt. Ins. 1997, 227. [Google Scholar] [CrossRef]
- Williams, M.; Johnston, J.M.; Cicoria, M.; Paletz, E.; Waggoner, L.P.; Edge, C.C.; Hallowell, S.F. Canine detection odor signatures for explosives. Enforcem. Secur. Technol. 1998, 3575, 291–301. [Google Scholar]
- Mastovska, K.; Lehotay, S.J. Pratical approaches to fast gas chromatography-mass spectrometry. J. Chromatogr. A 2003, 1000, 153–180. [Google Scholar] [CrossRef] [PubMed]
- Ramos, L. Comprehensive Two Dimensional Gas Chromatography; Barcelo, D., Ed.; Elsevier: Amsterdam, Nederland, 2009; p. 55. [Google Scholar]
- Harynuk, J.; Marriott, P.J. Fast GC×GC with Short Primary Columns. Anal. Chem. 2006, 78, 2028–2034. [Google Scholar] [CrossRef] [PubMed]
- Stadler, S.; Stefanuto, P.-H.; Byer, J.D.; brokl, M.; Forbes, S.L.; Focant, J.-F. Analysis of synthetic canine training aids by comprehensive two-dimensional gas chromatography-time flight mass spectrometry. J. Chromatogr. A 2012, 1255, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Stadler, S.; Stefanuto, P.-H.; Brokl, M.; Forbes, S.L.; Focant, J.-F. Characterization of Volatile Organic Compounds from Human Analogue Decomposition Using Thermal Desorption Coupled to Comprehensive Two-Dimensionnal Gas Chromatography–Time–Flight Mass Spectrometry. Anal. Chem. 2013, 85, 998–1005. [Google Scholar] [CrossRef] [PubMed]
- Brokl, M.; Bishop, L.; Wright, C.G.; Liu, C.; McAdam, K.; Focant, J.-F. Multivariate analysis of mainstream tobacco smoke particulate phase by headspace solid-phase micro extraction coupled with comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry. J. Chromatogr. A 2014, 1370, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Stefanuto, P.-H.; Perrault, K.A.; Lloyd, R.M.; Stuart, B.; Rai, T.; Forbes, S.L.; Focant, J.-F. Exploring new dimensions in cadaveric decomposition odour analysis. Anal. Methods 2015. [Google Scholar] [CrossRef]
- Patterson, D.G., Jr.; Welch, S.M.; Turner, W.E.; Sjödin, A.; Focant, J.-F. Cryogenic zone compression for the measurement of dioxins in human serum by isotope dilution at the attogram level using modulated gas chromatography coupled to high resolution magnetic sector mass spectrometry. J. Chromatogr. A 2011, 1218, 3274–3281. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefanuto, P.-H.; Perrault, K.A.; Focant, J.-F.; Forbes, S.L. Fast Chromatographic Method for Explosive Profiling. Chromatography 2015, 2, 213-224. https://doi.org/10.3390/chromatography2020213
Stefanuto P-H, Perrault KA, Focant J-F, Forbes SL. Fast Chromatographic Method for Explosive Profiling. Chromatography. 2015; 2(2):213-224. https://doi.org/10.3390/chromatography2020213
Chicago/Turabian StyleStefanuto, Pierre-Hugues, Katelynn A. Perrault, Jean-François Focant, and Shari L. Forbes. 2015. "Fast Chromatographic Method for Explosive Profiling" Chromatography 2, no. 2: 213-224. https://doi.org/10.3390/chromatography2020213





