Phenol-Enriched Virgin Olive Oil Promotes Macrophage-Specific Reverse Cholesterol Transport In Vivo
Abstract
:1. Introduction
2. Experimental Section
2.1. Mice and Treatment
2.2. Lipid and Apolipoprotein Analyses
2.3. Enzyme Activities
2.4. In Vivo Macrophage-to-Feces RevCT
2.5. Intestinal Cholesterol Absorption
2.6. Ex Vivo Cholesterol Efflux Capacity
2.7. Bile Acid Analyses
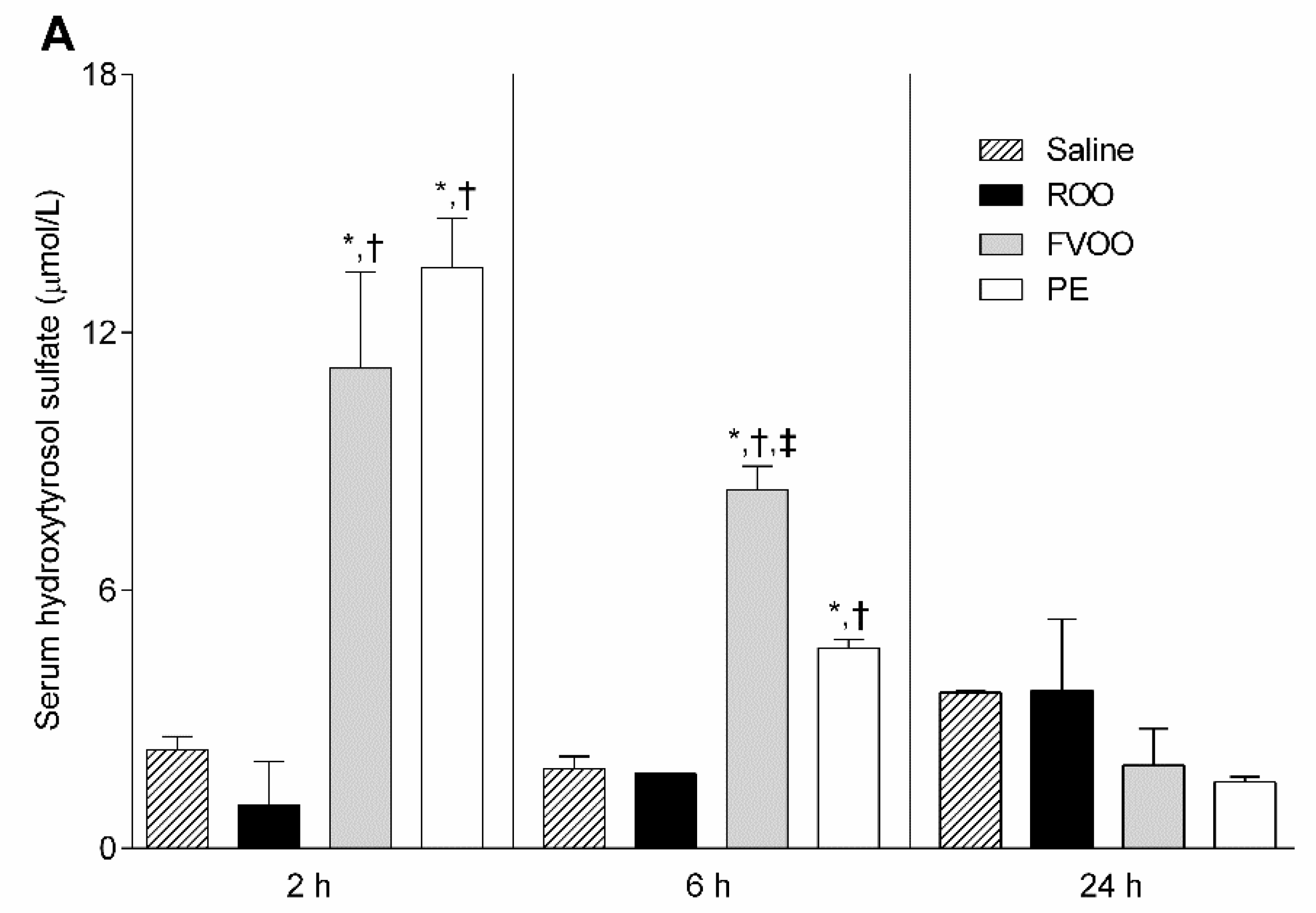
2.8. Phenolic Biological Metabolites Determination
2.9. Cell Culture and Treatment
2.10. Quantitative RT-PCR Analyses
2.11. Statistical Methods
3. Results
3.1. FVOO Increases HDL Cholesterol, APOA1, and the Formation of Nascent preβ-HDL Particles
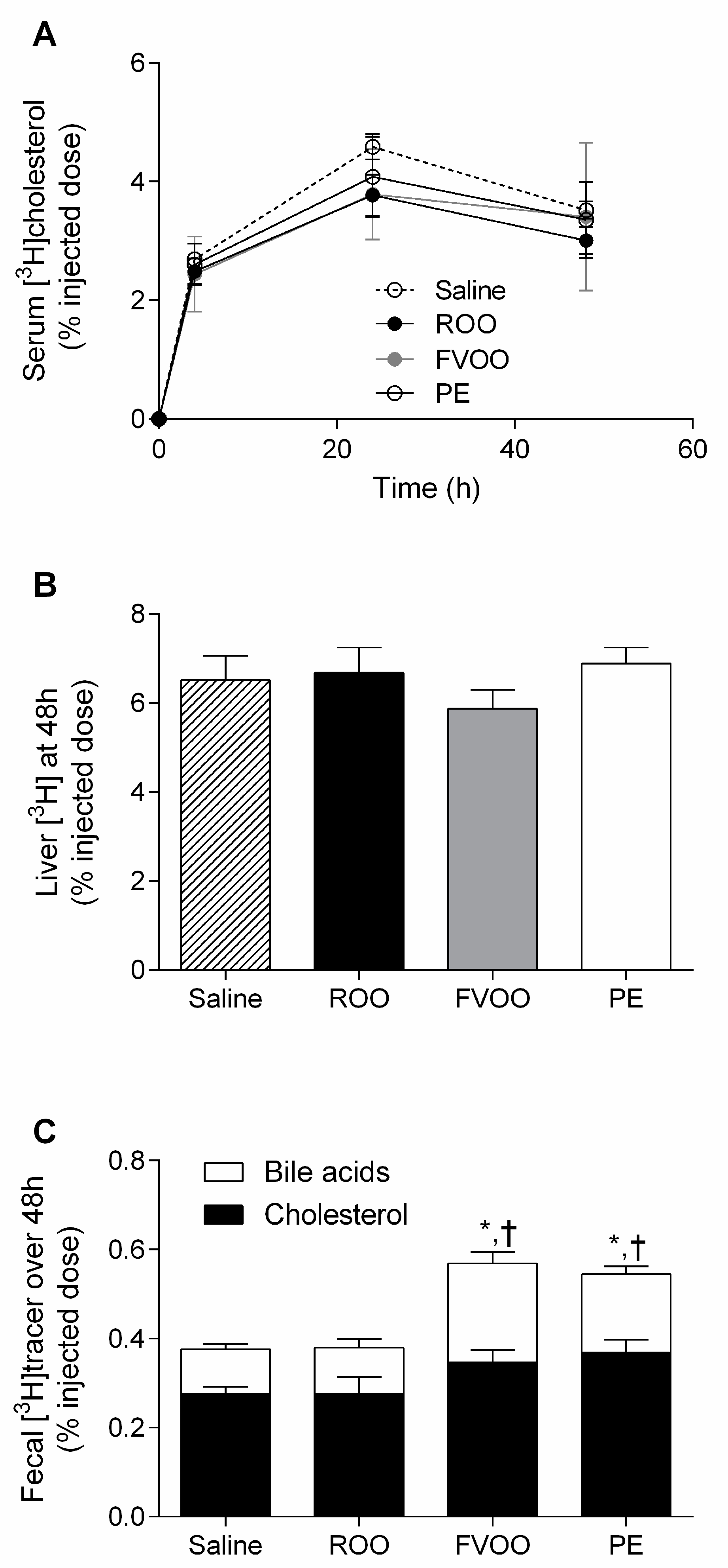
3.2. FVOO and Its Phenolic Compounds Promote Macrophage-to-Feces Reverse Cholesterol Transport
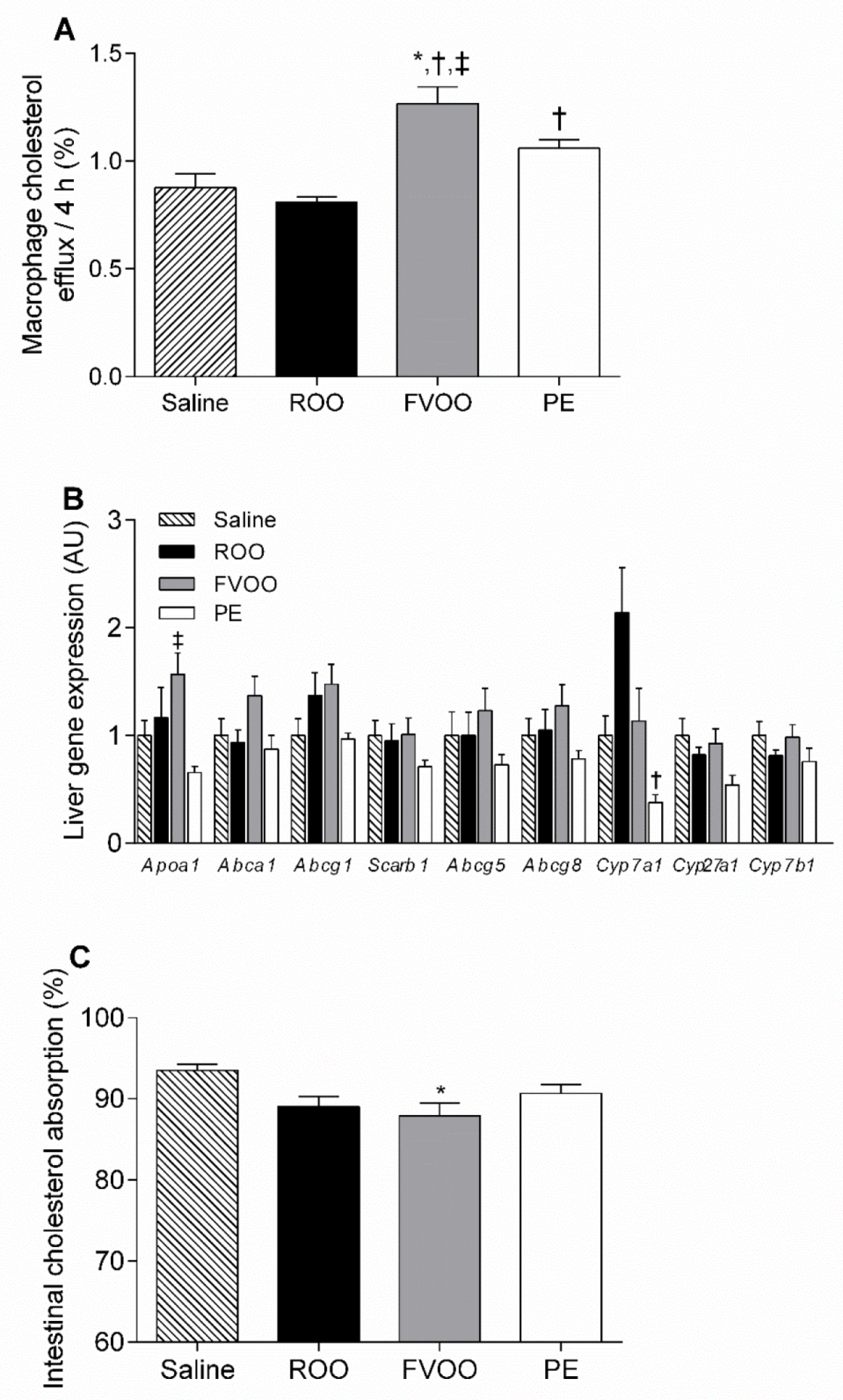
3.3. FVOO and OO Phenolic Compounds Upregulate HDL-Mediated Macrophage Cholesterol Efflux But Do Not Affect Liver Gene Expression Profile or Intestinal Cholesterol Absorption
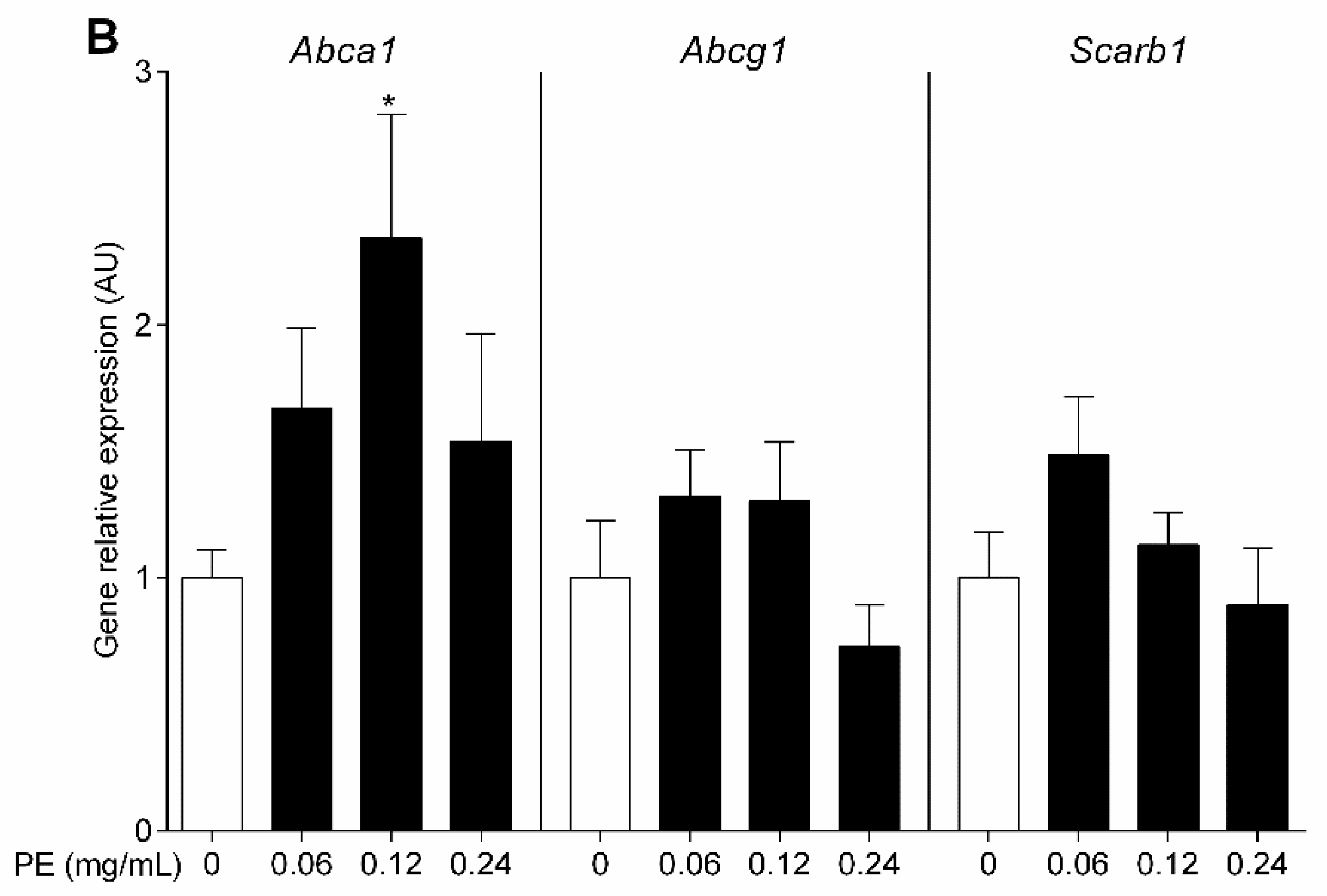
3.4. OO Phenolic Compounds Upregulate Macrophage Abca1 Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marcelino, G.; Hiane, P.A.; Freitas, K.d.C.; Santana, L.F.; Pott, A.; Donadon, J.R.; Guimarães, R.d.C.A. Effects of Olive Oil and Its Minor Components on Cardiovascular Diseases, Inflammation, and Gut Microbiota. Nutrients 2019, 11, 1826. [Google Scholar] [CrossRef] [Green Version]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- Foscolou, A.; Critselis, E.; Panagiotakos, D. Olive oil consumption and human health: A narrative review. Maturitas 2018, 118, 60–66. [Google Scholar] [CrossRef]
- Covas, M.-I.; de la Torre, R.; Fitó, M. Virgin olive oil: A key food for cardiovascular risk protection. Br. J. Nutr. 2015, 113 (Suppl. 2), S19–S28. [Google Scholar] [CrossRef] [Green Version]
- Covas, M.-I.; Ruiz-Gutiérrez, V.; Torre, R.; Kafatos, A.; Lamuela-Raventós, R.M.; Osada, J.; Owen, R.W.; Visioli, F. Minor Components of Olive Oil: Evidence to Date of Health Benefits in Humans. Nutr. Rev. 2006, 64, S20–S30. [Google Scholar] [CrossRef]
- Covas, M.-I.; Nyyssönen, K.; Poulsen, H.E.; Kaikkonen, J.; Zunft, H.-J.F.; Kiesewetter, H.; Gaddi, A.; de la Torre, R.; Mursu, J.; Bäumler, H.; et al. The effect of polyphenols in olive oil on heart disease risk factors: A randomized trial. Ann. Intern. Med. 2006, 145, 333–341. [Google Scholar] [CrossRef]
- Hernáez, Á.; Fernández-Castillejo, S.; Farràs, M.; Catalán, Ú.; Subirana, I.; Montes, R.; Solà, R.; Muñoz-Aguayo, D.; Gelabert-Gorgues, A.; Díaz-Gil, Ó.; et al. Olive oil polyphenols enhance high-density lipoprotein function in humans: A randomized controlled trial. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2115–2119. [Google Scholar] [CrossRef] [Green Version]
- European Food Safety Authority EFSA Panel on Dietetic Products, Nutrition and Allergies. EFSA J. 2011, 9, 2033. [CrossRef]
- Official Journal of the European Union L 136/1 (25.5.2012). COMMISSION REGULATION (EU) No 432/2012 of 16 May 2012 Establishing a List of Permitted Health Claims Made on Foods, Other than Those Referring to the Reduction of Disease Risk and to Children’s Development and Health. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32012R0432 (accessed on 25 May 2012).
- Rubió, L.; Motilva, M.-J.; Macià, A.; Ramo, T.; Romero, M.-P. Development of a Phenol-Enriched Olive Oil with Both Its Own Phenolic Compounds and Complementary Phenols from Thyme. J. Agric. Food Chem. 2012, 60, 3105–3112. [Google Scholar] [CrossRef]
- Pedret, A.; Fernández-Castillejo, S.; Valls, R.-M.; Catalán, Ú.; Rubió, L.; Romeu, M.; Macià, A.; López de Las Hazas, M.C.; Farràs, M.; Giralt, M.; et al. Cardiovascular Benefits of Phenol-Enriched Virgin Olive Oils: New Insights from the Virgin Olive Oil and HDL Functionality (VOHF) Study. Mol. Nutr. Food Res. 2018, 62, e1800456. [Google Scholar] [CrossRef]
- Lee-Rueckert, M.; Blanco-Vaca, F.; Kovanen, P.T.; Escola-Gil, J.C. The role of the gut in reverse cholesterol transport—Focus on the enterocyte. Prog. Lipid Res. 2013, 52, 317–328. [Google Scholar] [CrossRef]
- Lee-Rueckert, M.; Escola-Gil, J.C.; Kovanen, P.T. HDL functionality in reverse cholesterol transport—Challenges in translating data emerging from mouse models to human disease. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2016. [Google Scholar] [CrossRef]
- Escolà-Gil, J.C.; Rotllan, N.; Julve, J.; Blanco-Vaca, F. In vivo macrophage-specific RCT and antioxidant and antiinflammatory HDL activity measurements: New tools for predicting HDL atheroprotection. Atherosclerosis 2009, 206, 321–327. [Google Scholar] [CrossRef]
- Rubió, L.; Valls, R.-M.; Macià, A.; Pedret, A.; Giralt, M.; Romero, M.-P.; de la Torre, R.; Covas, M.-I.; Solà, R.; Motilva, M.-J. Impact of olive oil phenolic concentration on human plasmatic phenolic metabolites. Food Chem. 2012, 135, 2922–2929. [Google Scholar] [CrossRef]
- Vázquez Roncero, A.; Janer del Valle, C.; Janer del Valle, M.L. Determinación de los polifenoles totales del aceite de oliva. Grasas Aceites 1973, 24, 350–357. [Google Scholar]
- Van Haperen, R.; van Tol, A.; Vermeulen, P.; Jauhiainen, M.; van Gent, T.; van den Berg, P.; Ehnholm, S.; Grosveld, F.; van der Kamp, A.; de Crom, R. Human Plasma Phospholipid Transfer Protein Increases the Antiatherogenic Potential of High Density Lipoproteins in Transgenic Mice. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1082–1088. [Google Scholar] [CrossRef] [Green Version]
- Jauhiainen, M.; Metso, J.; Pahlman, R.; Blomqvist, S.; van Tol, A.; Ehnholm, C. Human plasma phospholipid transfer protein causes high density lipoprotein conversion. J. Biol. Chem. 1993, 268, 4032–4036. [Google Scholar]
- Jauhiainen, M.; Dolphin, P.J. Human plasma lecithin-cholesterol acyltransferase. An elucidation of the catalytic mechanism. J. Biol. Chem. 1986, 261, 7032–7043. [Google Scholar]
- Escolà-Gil, J.C.; Lee-Rueckert, M.; Santos, D.; Cedó, L.; Blanco-Vaca, F.; Julve, J. Quantification of In Vitro Macrophage Cholesterol Efflux and In Vivo Macrophage-Specific Reverse Cholesterol Transport. In Methods in Mouse Atherosclerosis; Humana Press: New York, NY, USA, 2015; Volume 1339, pp. 211–233. [Google Scholar] [CrossRef]
- Sankaranarayanan, S.; Kellner-Weibel, G.; de la Llera-Moya, M.; Phillips, M.C.; Asztalos, B.F.; Bittman, R.; Rothblat, G.H. A sensitive assay for ABCA1-mediated cholesterol efflux using BODIPY-cholesterol. J. Lipid Res. 2011, 52, 2332–2340. [Google Scholar] [CrossRef] [Green Version]
- Mosele, J.I.; Gosalbes, M.-J.; Macià, A.; Rubió, L.; Vázquez-Castellanos, J.F.; Jiménez Hernández, N.; Moya, A.; Latorre, A.; Motilva, M.-J. Effect of daily intake of pomegranate juice on fecal microbiota and feces metabolites from healthy volunteers. Mol. Nutr. Food Res. 2015, 59, 1942–1953. [Google Scholar] [CrossRef]
- Rubió, L.; Farràs, M.; de La Torre, R.; Macià, A.; Romero, M.-P.; Valls, R.M.; Solà, R.; Farré, M.; Fitó, M.; Motilva, M.-J. Metabolite profiling of olive oil and thyme phenols after a sustained intake of two phenol-enriched olive oils by humans: Identification of compliance markers. Food Res. Int. 2014, 65, 59–68. [Google Scholar] [CrossRef]
- Cedó, L.; Metso, J.; Santos, D.; Sánchez-Quesada, J.L.; Julve, J.; García-León, A.; Mora-Brugués, J.; Jauhiainen, M.; Blanco-Vaca, F.; Escolà-Gil, J.C. Consumption of polyunsaturated fat improves the saturated fatty acid-mediated impairment of HDL antioxidant potential. Mol. Nutr. Food Res. 2015, 59, 1987–1996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escolà-Gil, J.C.; Llaverias, G.; Julve, J.; Jauhiainen, M.; Méndez-González, J.; Blanco-Vaca, F. The Cholesterol Content of Western Diets Plays a Major Role in the Paradoxical Increase in High-Density Lipoprotein Cholesterol and Upregulates the Macrophage Reverse Cholesterol Transport Pathway. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2493–2499. [Google Scholar] [CrossRef] [Green Version]
- Tsartsou, E.; Proutsos, N.; Castanas, E.; Kampa, M. Network Meta-Analysis of Metabolic Effects of Olive-Oil in Humans Shows the Importance of Olive Oil Consumption With Moderate Polyphenol Levels as Part of the Mediterranean Diet. Front. Nutr. 2019, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- George, E.S.; Marshall, S.; Mayr, H.L.; Trakman, G.L.; Tatucu-Babet, O.A.; Lassemillante, A.-C.M.; Bramley, A.; Reddy, A.J.; Forsyth, A.; Tierney, A.C.; et al. The effect of high-polyphenol extra virgin olive oil on cardiovascular risk factors: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2019, 59, 2772–2795. [Google Scholar] [CrossRef]
- Li, Y.; Hou, M.J.; Ma, J.; Tang, Z.H.; Zhu, H.L.; Ling, W.H. Dietary fatty acids regulate cholesterol induction of liver CYP7alpha1 expression and bile acid production. Lipids 2005, 40, 455–462. [Google Scholar] [CrossRef]
- Botham, K.M.; Boyd, G.S. The effect of dietary fat on bile salt synthesis in rat liver. Biochim. Biophys. Acta 1983, 752, 307–314. [Google Scholar] [CrossRef]
- Chiang, J.Y.L. Bile acid metabolism and signaling. Compr. Physiol. 2013, 3, 1191–1212. [Google Scholar] [CrossRef] [Green Version]
- Suárez, M.; Valls, R.M.; Romero, M.-P.; Macià, A.; Fernández, S.; Giralt, M.; Solà, R.; Motilva, M.-J. Bioavailability of phenols from a phenol-enriched olive oil. Br. J. Nutr. 2011, 106, 1691–1701. [Google Scholar] [CrossRef] [Green Version]
- Berrougui, H.; Ikhlef, S.; Khalil, A. Extra Virgin Olive Oil Polyphenols Promote Cholesterol Efflux and Improve HDL Functionality. Evid. Based Complement. Altern. Med. ECAM 2015, 2015, 208062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helal, O.; Berrougui, H.; Loued, S.; Khalil, A. Extra-virgin olive oil consumption improves the capacity of HDL to mediate cholesterol efflux and increases ABCA1 and ABCG1 expression in human macrophages. Br. J. Nutr. 2013, 109, 1844–1855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee-Rueckert, M.; Silvennoinen, R.; Rotllan, N.; Judström, I.; Blanco-Vaca, F.; Metso, J.; Jauhiainen, M.; Kovanen, P.T.; Escola-Gil, J.C. Mast cell activation in vivo impairs the macrophage reverse cholesterol transport pathway in the mouse. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 520–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Saline n = 20 | ROO n = 18 | FVOO n = 19 | PE n = 14 | |
|---|---|---|---|---|
| Body weight (g) | 20.00 ± 0.55 | 20.16 ± 0.45 | 20.73 ± 0.70 | 20.36 ± 0.63 |
| Total cholesterol (mmol/L) | 2.28 ± 0.08 | 2.28 ± 0.10 | 2.86 ± 0.15 *,† | 2.74 ± 0.12 * |
| VLDL+LDL cholesterol (mmol/L) | 0.56 ± 0.03 | 0.50 ± 0.04 | 0.57 ± 0.04 | 0.60 ± 0.04 |
| HDL cholesterol (mmol/L) | 1.72 ± 0.08 | 1.79 ± 0.10 | 2.29 ± 0.13 *,† | 2.15 ± 0.10 * |
| APOA1 (g/L) | 1.13 ± 0.04 | 1.22 ± 0.01 | 1.38 ± 0.06 * | 1.20 ± 0.05 |
| Preβ-HDL particles (g/L) | 0.13 ± 0.01 | 0.14 ± 0.01 | 0.18 ± 0.02 *,‡ | 0.13 ± 0.01 |
| PLTP activity (nmol/mL/h) | 15,725 ± 1169 | 17,918 ± 1099 | 16,920 ± 1008 | 18,336 ± 908 |
| LCAT activity (nmol/mL/h) | 18.39 ± 1.28 | 17.55 ± 1.52 | 18.02 ± 0.44 | 19.70 ± 1.03 |
| Saline | ROO | FVOO | PE | |
|---|---|---|---|---|
| Cholic acid (µg/g feces) | 14.67 ± 1.06 | 12.38 ± 1.14 | 10.76 ± 0.67 | 12.42 ± 0.97 |
| Chenodeoxycholic acid (µg/g feces) | 7.68 ± 0.08 | 8.32 ± 0.34 | 8.71 ± 0.06 * | 8.27 ± 0.29 |
| Deoxycholic acid (µg/g feces) | 22.78 ± 0.49 | 20.51 ± 4.06 | 23.43 ± 2.18 | 20.21 ± 1.16 |
| Lithocholic acid (µg/g feces) | 5.76 ± 0.22 | 6.65 ± 0.63 | 7.58 ± 0.40 | 6.42 ± 0.58 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cedó, L.; Fernández-Castillejo, S.; Rubió, L.; Metso, J.; Santos, D.; Muñoz-Aguayo, D.; Rivas-Urbina, A.; Tondo, M.; Méndez-Lara, K.A.; Farràs, M.; et al. Phenol-Enriched Virgin Olive Oil Promotes Macrophage-Specific Reverse Cholesterol Transport In Vivo. Biomedicines 2020, 8, 266. https://doi.org/10.3390/biomedicines8080266
Cedó L, Fernández-Castillejo S, Rubió L, Metso J, Santos D, Muñoz-Aguayo D, Rivas-Urbina A, Tondo M, Méndez-Lara KA, Farràs M, et al. Phenol-Enriched Virgin Olive Oil Promotes Macrophage-Specific Reverse Cholesterol Transport In Vivo. Biomedicines. 2020; 8(8):266. https://doi.org/10.3390/biomedicines8080266
Chicago/Turabian StyleCedó, Lídia, Sara Fernández-Castillejo, Laura Rubió, Jari Metso, David Santos, Daniel Muñoz-Aguayo, Andrea Rivas-Urbina, Mireia Tondo, Karen Alejandra Méndez-Lara, Marta Farràs, and et al. 2020. "Phenol-Enriched Virgin Olive Oil Promotes Macrophage-Specific Reverse Cholesterol Transport In Vivo" Biomedicines 8, no. 8: 266. https://doi.org/10.3390/biomedicines8080266





