New Approach to Chronic Back Pain Treatment: A Case Control Study
Abstract
:1. Introduction
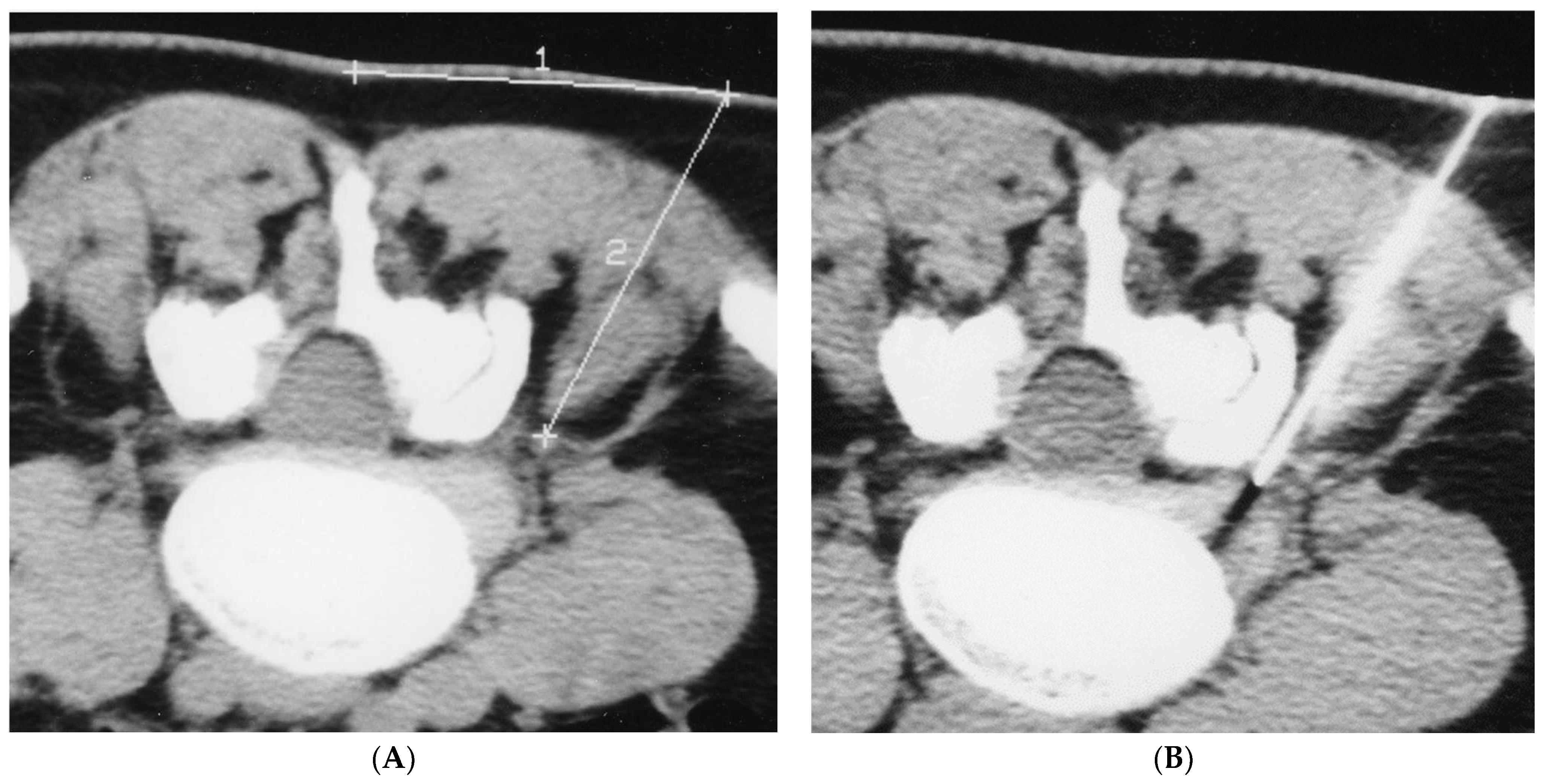
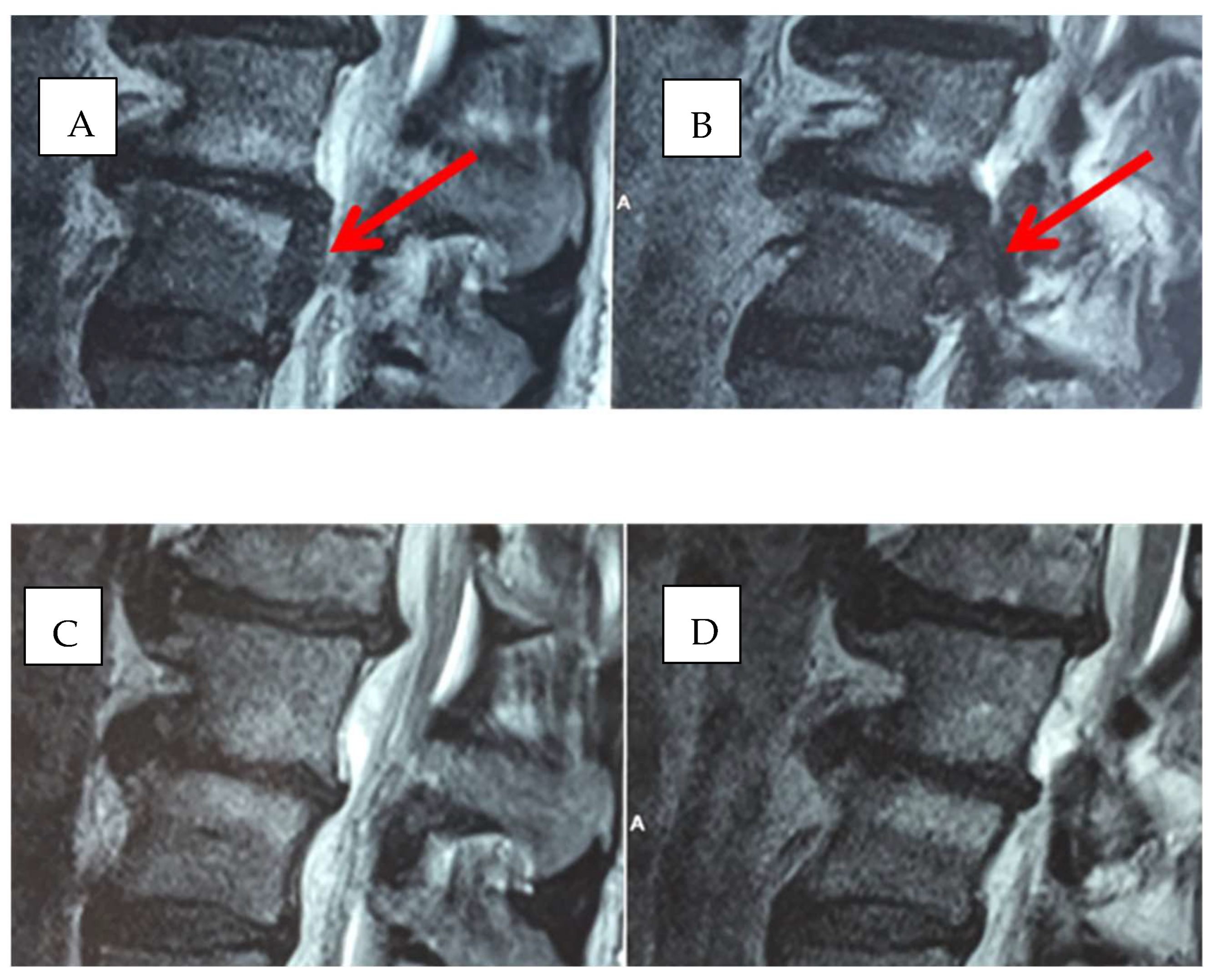
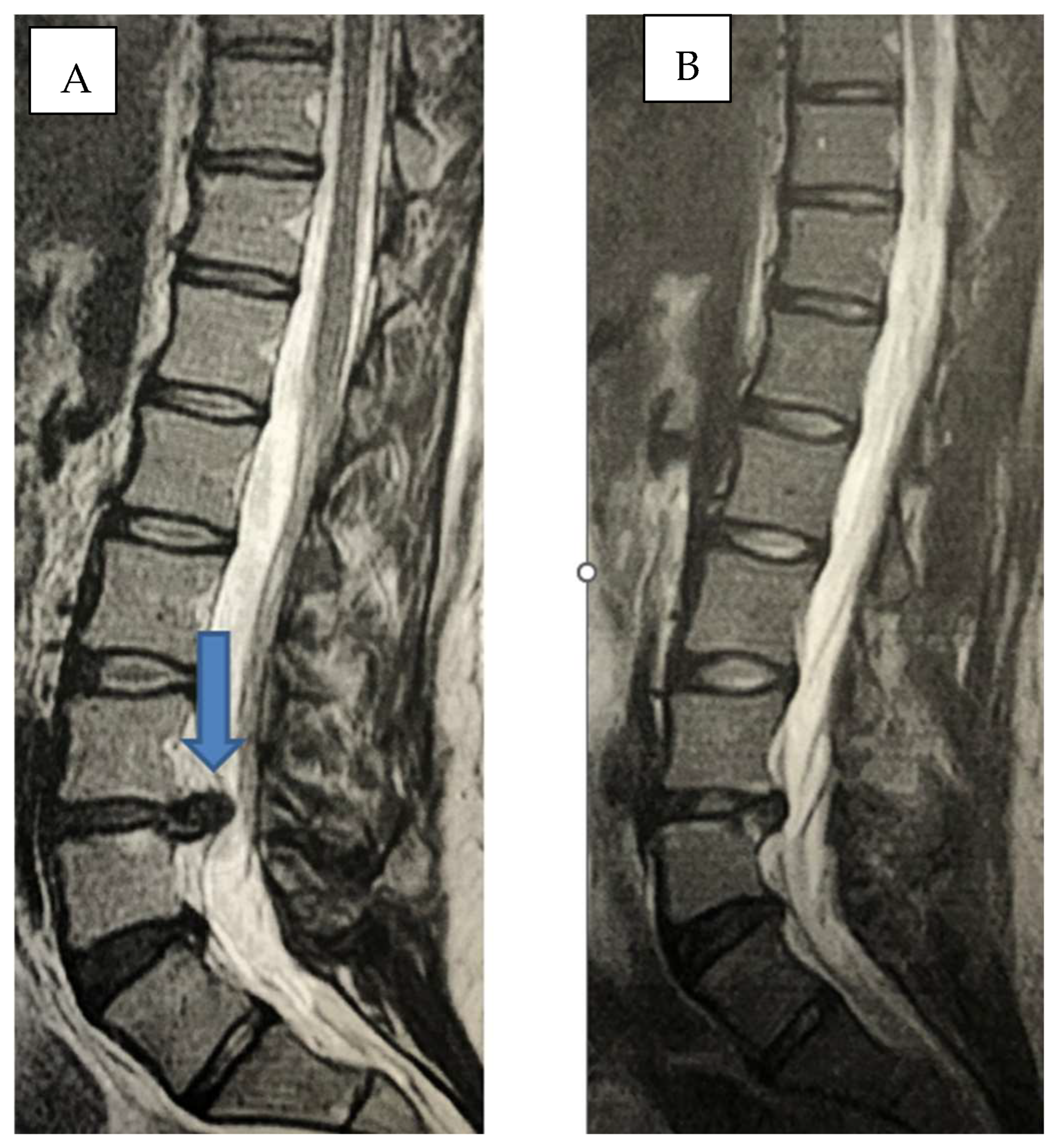
2. Methods
2.1. Infiltration Technique
- (a)
- excellent: resolution of pain, and return to normal activity carried out prior to pain onset;
- (b)
- good or satisfactory: more than 50% reduction of pain;
- (c)
- mediocre or poor: partial reduction of pain below 70%.
2.2. Statistical Analysis
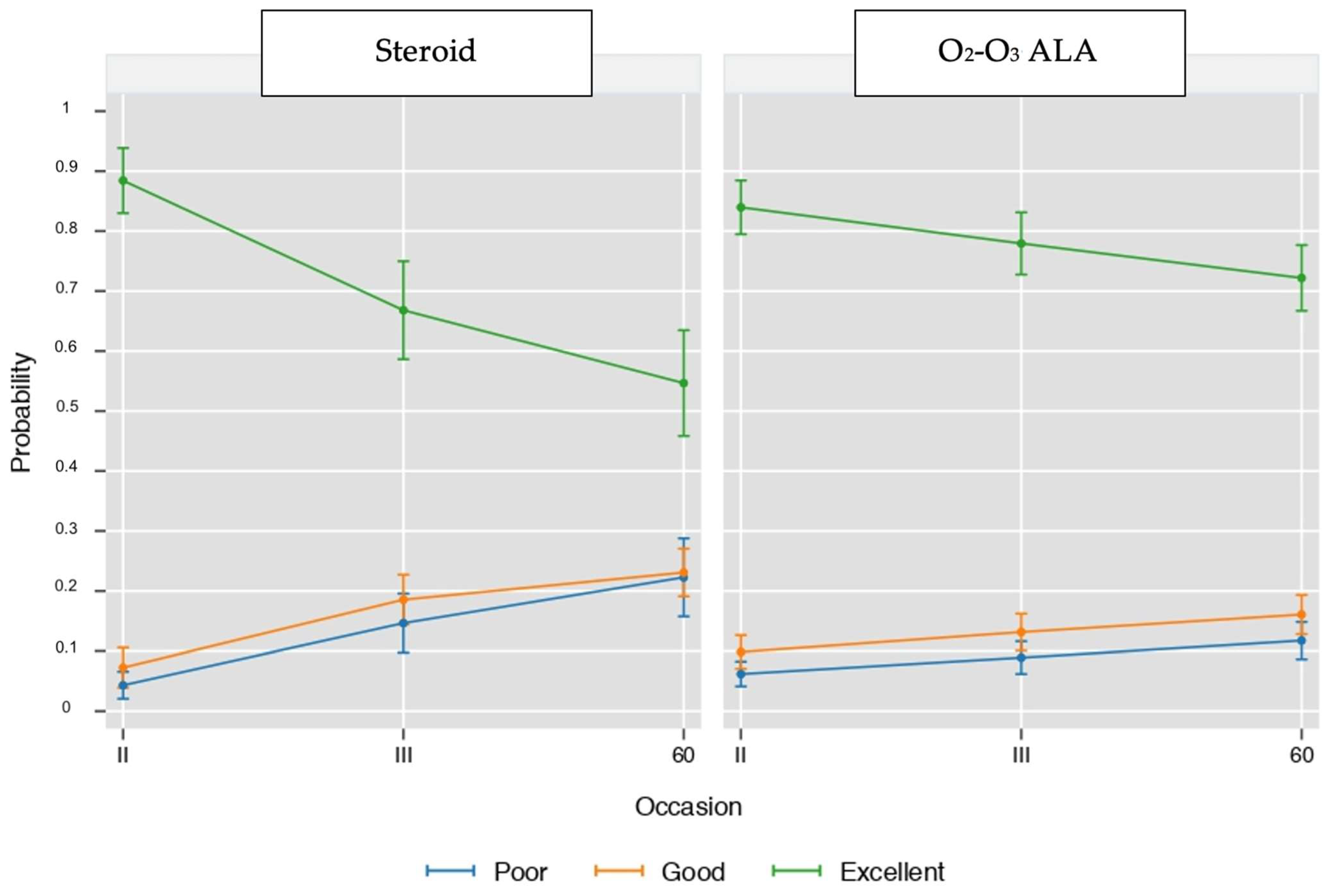
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chou, R. Low back pain (chronic). BMJ Clin. Evid. 2010, 2010, 1116. [Google Scholar] [PubMed]
- Ehrlich, G. Low back pain. Bull. World Health Organ. 2003, 81, 671–676. [Google Scholar] [PubMed]
- Pellicanò, G.; Martinelli, F.; Tavanti, V.; Bonetti, M.; Leonardi, M.; Muto, M.; Villari, N.; Andreula, C. The Italian Oxygen-Ozone Therapy Federation (FIO) study on oxygen- ozone treatment of herniated disc. Int. J. Ozone Ther. 2007, 6, 7–15. [Google Scholar]
- Iliakis, E.; Valadakis, V.; Vynios, D.H.; Tsiganos, C.P.; Agapitos, E. Rationalization of the activity of medical ozone on intervertebral disc and histological and biochemical study. Rivista di Neuroradiologia 2001, 14 (Suppl. S1), 25–30. [Google Scholar] [CrossRef]
- Gallucci, M.; Limbucci, N.; Zugaro, L.; Barile, A.; Stavroulis, E.; Ricci, A.; Galzio, R.; Masciocchi, C. Sciatica: Treatment with Intradiscal and Intraforaminal Injections of Steroid and Oxygen-Ozone versus Steroid Only. Radiology 2007, 242, 907–913. [Google Scholar] [CrossRef]
- Splendiani, A.; Perri, M.; Conchiglia, A.; Fasano, F.; Di Egidio, G.; Masciocchi, C.; Gallucci, M. MR Assessment of Lumbar Disk Herniation Treated with Oxygen-Ozone Diskolysis: The Role of DWI and Related ADC versus Intervertebral Disk Volumetric Analysis for Detecting Treatment Response. Neuroradiol. J. 2013, 26, 347–356. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Ma, Y.; Jiang, J.; Ding, T.; Wang, J. Treatment of the lumbar disc herniation with intradiscal and intraforaminal injection of oxygen-ozone. J. Back Musculoskelet. Rehabil. 2013, 26, 317–322. [Google Scholar] [CrossRef]
- Steppan, J.; Meaders, T.; Muto, M.; Murphy, K.J. A metaanalysis of the effectiveness and safety of ozone treatments for herniated lumbar discs. J. Vasc. Interv. Radiol. JVIR 2010, 21, 534–548. [Google Scholar] [CrossRef]
- Zennaro, H.; Dousset, V.; Viaud, B.; Allard, M.; Dehais, J.; Sénégas, J.; Caillé, J.M. Periganglionic foraminal steroid injections performed under CT control. AJNR Am. J. Neuroradiol. 1998, 19, 349–352. [Google Scholar]
- Cuckler, J.M.; Bernini, P.; Wiesel, S.W.; Booth, R.E., Jr.; Rothman, R.H.; Pickens, G.T. The use of epidural steroids in the treatment of radicular pain. J. Bone Jt. Surg. Am. 1985, 67, 63–66. [Google Scholar] [CrossRef]
- Dougherty, J.H.; Fraser, R.A.R. Complications involving intraspinal injections of steroids. Neurosurg 1978, 48, 1023–1025. [Google Scholar] [CrossRef] [PubMed]
- Rovira, E.; Garcia, E.M.; Catala, J.; Garcia, J. Chronic adhesive arachnoiditis following epidural paramethasone. Rev. Neurol. 1997, 25, 2067–2068. [Google Scholar] [PubMed]
- Manfrè, L. Trattamento della lombosciatalgia acuta e cronica con infiltrazione perigangliare di steroide. Riv. Neuroradiol. 2001, 14, 43–46. [Google Scholar] [CrossRef]
- Cyteval, C.; Thomas, E.; Decoux, E.; Sarrabere, M.P.; Cottin, A.; Blotman, F.; Taourel, P. Cervical Radiculopathy: Open Study on Percutaneous Periradicular Foraminal Steroid Infiltration Performed under CT Control in 30 patients. AJNR Am. J. Neuroradiol. 2004, 25, 441–445. [Google Scholar] [PubMed]
- Magalhaes, F.N.; Dotta, L.; Sasse, A.; Teixera, M.J.; Fonoff, E.T. Ozone therapy as a treatment for low back pain secondary to herniated disc: A systematic review and meta-analysis of randomized controlled trials. Pain Physician 2012, 2, 15. [Google Scholar]
- Perri, M.; Grattacaso, G.; Di Tunno, V.; Marsecano, C.; Di Cesare, E.; Splendiani, A.; Gallucci, M. MRI DWI/ADC signal predicts shrinkage of lumbar disc herniation after O2-O3 discolysis. Neuroradiol. J. 2015, 28, 198–204. [Google Scholar] [CrossRef] [Green Version]
- Rahimi-Movaghar, V.; Eslami, V. The major efficient mechanisms of ozone therapy are obtained in intradiscal procedures. Pain Physician 2012, 15, E1007–E1008. [Google Scholar] [CrossRef]
- Leonardi, M.; Simonetti, L.; Raffi, L.; Cenni, P.; Barbara, C. Mini-Invasive Treatment of Herniated Disc by Oxygen-Ozone Injection. Interv. Neuroradiol. 2003, 9 (Suppl S2), 75. [Google Scholar] [CrossRef] [Green Version]
- Lehnert, T.; Naguib, N.N.; Wutzler, S.; Nour-Eldin, N.E.A.; Bauer, R.W.; Kerl, J.M.; Vogel, T.J.; Balzer, J.O. Analysis of Disk Volume before and after CT-guided Intradiscal and Periganglionic Ozone–Oxygen Injection for the Treatment of Lumbar Disk Herniation. J. Vasc. Interv. Radiol. 2012, 23, 1430–1436. [Google Scholar] [CrossRef]
- Re, L.; Sanchez, G.M.; Mawsouf, N. Clinical evidence of ozone interaction with pain mediators. Saudi Med. J. 2010, 31, 1363–1367. [Google Scholar]
- Hidalgo-Tallón, F.J.; Torres-Morera, L.M.; Baeza-Noci, J.; Carrillo-Izquierdo, M.D.; Pinto-Bonilla, R. Updated Review on Ozone Therapy in Pain Medicine. Front. Physiol. 2022, 13, 840623. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, C.L.; Davies, M.J. Direct detection and identification of radicals generated during the hydroxyl radical-induced degradation of hyaluronic acid and related materials. Free Radic. Biol. Med. 1996, 21, 275–290. [Google Scholar] [CrossRef]
- de Sire, A.; Agostini, F.; Lippi, L.; Mangone, M.; Marchese, S.; Cisari, C.; Bernetti, A.; Invernizzi, M. Oxygen–Ozone Therapy in the Rehabilitation Field: State of the Art on Mechanisms of Action, Safety and Effectiveness in Patients with Musculoskeletal Disorders. Biomolecules 2021, 11, 356. [Google Scholar] [CrossRef] [PubMed]
- Sconza, C.; Leonardi, G.; Kon, E.; Respizzi, S.; Massazza, G.; Marcacci, M.; Di Matteo, B. Oxygen-ozone therapy for the treatment of low back pain: A systematic review of randomized controlled trials. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 6034–6046. [Google Scholar]
- Bonetti, M.; Lauritano, D.; Ottaviani, G.M.; Fontana, A.; Zambello, A.; Della Gatta, L.; Muto, M.; Carinci, F. Oxygen-Ozone Therapy Associated with Alpha Lipoic Acid Plus Palmitoylethanolamide and Myrrh versus Ozone Therapy in the Combined Treatment of Sciatic Pain Due to Herniated Discs: Observational Study on 318 Patients. Int. J. Environ. Res. Public Health 2022, 19, 5716. [Google Scholar] [CrossRef]
- Costantino, M.; Guaraldi, C.; Costantino, D.; De Grazia, S.; Unfer, V. Peripheral neuropathy in obstetrics: Efficacy and safety of alpha lipoic acid supplementation. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 2766–2771. [Google Scholar] [PubMed]
- Matsugo, S.; Yan, L.J.; Han, D.; Trischler, H.J.; Packer, L. Elucidation of antioxidant activity of alpha-lipoic acid towards hydroxyl radical. Biochem. Biophys. Res. Commun. 1995, 208, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Mijnhout, G.S.; Kollen, B.J.; Alkhalaf, A.; Kleefstra, N.; Bilo, H.J.G. Alpha lipoic acid for symptomatic peripheral neuropathy in patients with diabetes: A meta-analysis of randomized controlled trials. Int. J. Endocrinol. 2012, 201, 456279. [Google Scholar] [CrossRef]
- Park, S.; Karunakaran, U.; Jeoung, N.H.; Jeon, J.H.; Lee, I.K. Physiological effect and therapeutic application of alpha lipoic acid. Curr. Med. Chem. 2014, 21, 3636–3645. [Google Scholar] [CrossRef]
- Salehi, B.; Berkay Yılmaz, Y.; Antika, G.; Boyunegmez Tumer, T.; Fawzi Mahomoodally, M.; Lobine, D.; Akram, M.; Riaz, M.; Capanoglu, E.; Sharopov, F.; et al. Sharifi-Rad Insights on the Use of alpha-Lipoic Acid for Therapeutic Purposes. J. Biomol. 2019, 9, 356. [Google Scholar]
- Ziegler, D.; Ametov, A.S.; Barinov, A.; Dyck, P.J.; Gurieva, I.; Low, P.A.; Munzel, U.; Yakhno, N.; Raz, I.; Novosadova, M.; et al. Oral treatment with alpha-lipoic acid improves symptomatic diabetic polyneuropathy. Diabetes Care 2006, 29, 2365–2370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziegler, D.; Hanefeld, M.; Ruhnau, K.-J.; Lobisch, M.; Schütte, K.; Gries, F.A. Treatment of symptomatic diabetic peripheral neuropathy with the antioxidant alpha-lipoic acid: A 3-week multicentre randomized controlled trial (ALADIN study). Diabetologia 1995, 38, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Bilska, A.; Wlodek, L. Lipoic acid: The drug of the future? Pharm. Rep. 2005, 57, 570–577. [Google Scholar]
- Han, T.; Bai, J.; Liu, W.; Hu, Y. A systematic review and metanalysis of α-lipoic acid in the treatment of diabetic peripheral neuropathy. Eur. J. Endocrinol. 2012, 167, 465–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagamatsu, M.; Nickander, K.K.; Schmelzer, J.D.; Raya, A.; Wittrock, D.A.; Tritschler, H.; Low, P.A. Lipoic acid improves nerve blood flow, reduces oxidative stress, and improves distal nerve conduction in experimental diabetic neuropathy. Diabetes Care 1995, 18, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- El Ashry, E.S.; Rashed, N.; Salama, O.M.; Saleh, A. Components, therapeutic value, and uses of myrrh. Pharmazie 2003, 58, 163–168. [Google Scholar]
- Nomicos, E.Y. Myrrh: Medical marvel or myth of the Magi? Holist Nurs. Pract. 2007, 21, 308–323. [Google Scholar] [CrossRef]
- Fernandez-Cuadros, M.E.; Albaladejo-Florin, M.J.; Martin-Martin, L.M.; Alava-Rabasa, S.; Pérez-Moro, O.S. Effectiveness of Physical Therapy Plus TIOBEC®(α-Lipoic Acid Vitamin B, C, and E) on Complex Regional Pain Syndrome (CRPS): A Small Case Series/Pilot Study. Middle East J. Rehabil. Health Stud. 2020, 7, e100636. [Google Scholar] [CrossRef]
- Paladini, A.; Fusco, M.; Cenacchi, T.; Schievano, P.C.; Piroli, A.; Varrassi, G. Palmitoylethanolamide, a special food for medical purposes, in the treatment of chronic pain: A pooled data meta-analysis. Pain Physician 2016, 19, 11–24. [Google Scholar]
- Dolara, P.; Luceri, C.; Ghelardini, C.; Monserrat, C.; Aiolli, S.; Luceri, F.; Lodovici, M.; Menichetti, S.; Romanelli, M.N. Analgesic effects of myrrh. Nature 1996, 379, 29. [Google Scholar] [CrossRef]
- Petrosino, S.; Di Marzo, V. The pharmacology of palmitoylethanolamide and first data on the therapeutic efficacy of some of its new formulations. J. Pharmacol. 2017, 174, 1349–1365. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Cuadros, M.E.; Albaladejo-Florín, M.J.; Álava-Rabasa, S.; Casique-Bocanegra, L.O.; López-Muñoz, M.J.; Rodríguez-de-Cía, J.; Pérez-Moro, O.S. A nutraceutical combination of Alpha-lipoic acid, Palmitoylethanolamide and Myrrha might be effective in COVID-19 prevention. Lett. Ed./Med. Health Sci. 2020. [Google Scholar] [CrossRef]
- Lo Verme, J.; La Rana, G.; Russo, R.; Calignano, A.; Piomelli, D. The search for the palmitoylethanolamide receptor. Life Sci. 2005, 77, 1685–1698. [Google Scholar] [CrossRef] [Green Version]
- Lo Verme, J.; Fu, J.; Astarita, G.; La Rana, G.; Russo, R.; Calignano, A.; Piomelli, D. The nuclear receptor peroxisome proliferator-activated receptor-α mediates the anti-inflammatory actions of palmitoylethanolamide. Mol. Pharmacol. 2005, 67, 15–19. [Google Scholar] [CrossRef]
- Rankin, L.; Fowler, C.J. The basal pharmacology of palmitoylethanolamide. Int. J. Mol. Sci. 2020, 21, 7942. [Google Scholar] [CrossRef]
- Gabrielsson, L.; Mattsson, S.; Fowler, C.J. Palmitoylethanolamide for the treatment of pain: Pharmacokinetics, safety and efficacy. Br. J. Clin. Pharmacol. 2016, 82, 932–942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alhouayek, M.; Muccioli, G.G. Harnessing the anti-inflammatory potential of palmitoylethanolamide. Drug Discov. Today 2014, 19, 1632–1639. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Cuzzocrea, S. Palmitoylethanolamide is a New Possible Pharmacological Treatment for the Inflammation Associated with Trauma. Mini-Rev. Med. Chem. 2013, 13, 237–255. [Google Scholar]
- Hesselink, J.M.K.; de Boer, T.; Witkamp, R.F. Palmitoylethanolamide: A Natural Body-Own Anti-Inflammatory Agent, Effective and Safe against Influenza and Common Cold. Int. J. Inflamm. 2013, 2013, 151028. [Google Scholar] [CrossRef] [Green Version]
- Hesselink, J.M.K.; Hekker, T.A. Therapeutic utility of palmitoylethanolamide in the treatment of neuropathic pain associated with various pathological conditions: A case series. J. Pain Res. 2012, 5, 437. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonetti, M.; Lauritano, D.; Ottaviani, G.M.; Fontana, A.; Frigerio, M.; Zambello, A.; Della Gatta, L.; Muto, M.; Carinci, F. New Approach to Chronic Back Pain Treatment: A Case Control Study. Biomedicines 2023, 11, 73. https://doi.org/10.3390/biomedicines11010073
Bonetti M, Lauritano D, Ottaviani GM, Fontana A, Frigerio M, Zambello A, Della Gatta L, Muto M, Carinci F. New Approach to Chronic Back Pain Treatment: A Case Control Study. Biomedicines. 2023; 11(1):73. https://doi.org/10.3390/biomedicines11010073
Chicago/Turabian StyleBonetti, Matteo, Dorina Lauritano, Gian Maria Ottaviani, Alessandro Fontana, Michele Frigerio, Alessio Zambello, Luigi Della Gatta, Mario Muto, and Francesco Carinci. 2023. "New Approach to Chronic Back Pain Treatment: A Case Control Study" Biomedicines 11, no. 1: 73. https://doi.org/10.3390/biomedicines11010073





