Effects of Exercise Intervention on Health-Related Quality of Life in Patients with Systemic Lupus Erythematosus: A Systematic Review and Meta-Analysis of Controlled Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy and Study Selection
2.2. Assessment of Risk of Bias in Included Randomized Controlled Trials
2.3. Statistical Analysis
3. Results
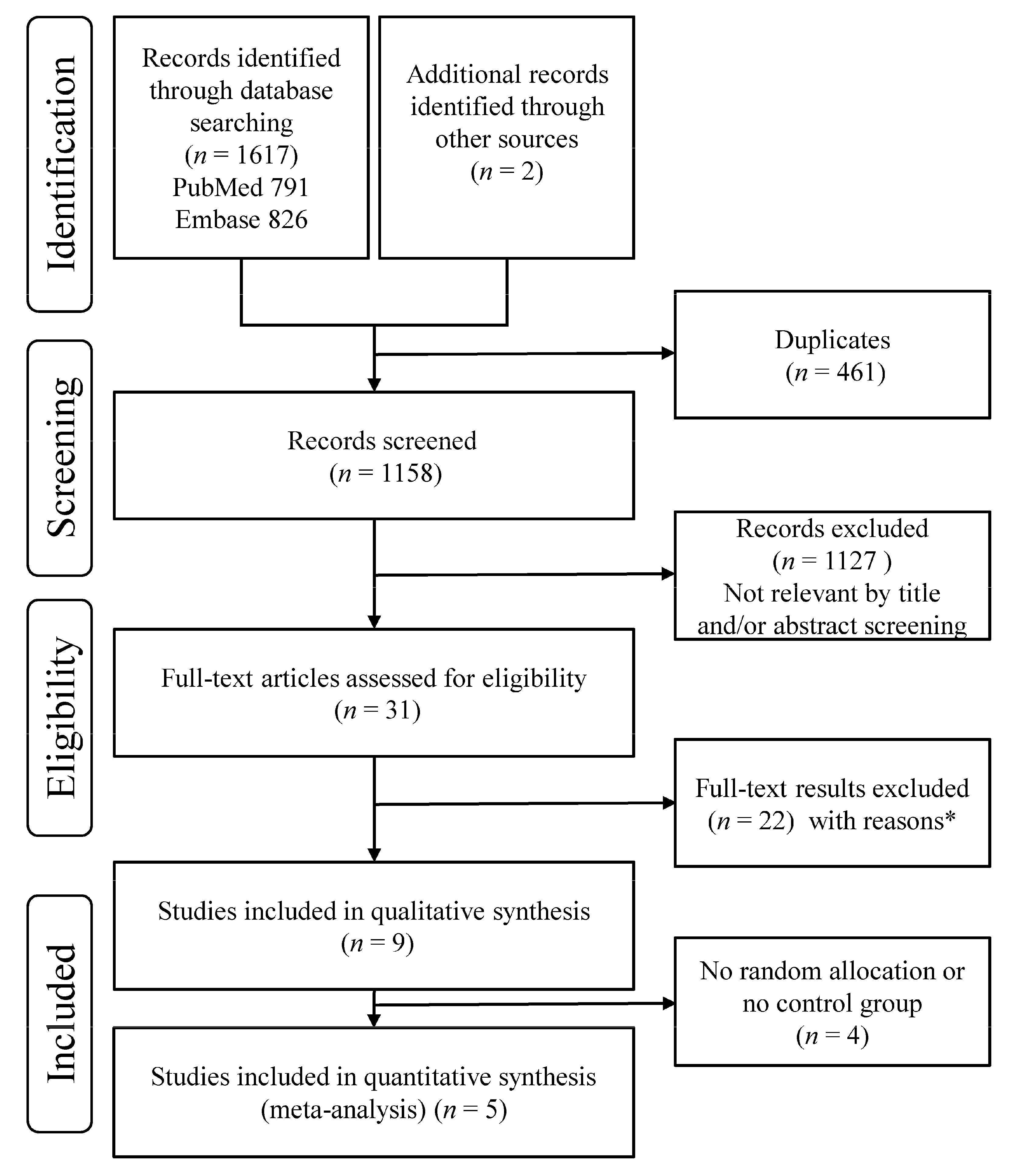
3.1. Search Results
3.2. Characteristics of Included Studies
3.3. Intervention Characteristics of Included Studies
3.4. Characteristics of the Outcome Measures
3.5. Effects of Interventions: Exercise versus Control
3.6. Risk of Bias in Included Randomized Controlled Trials
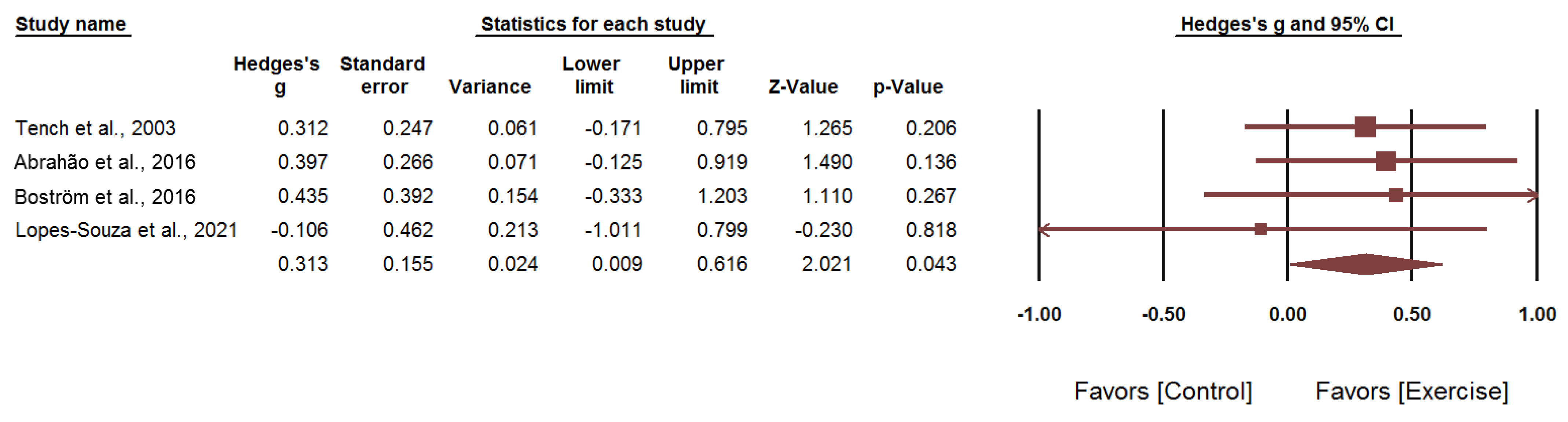
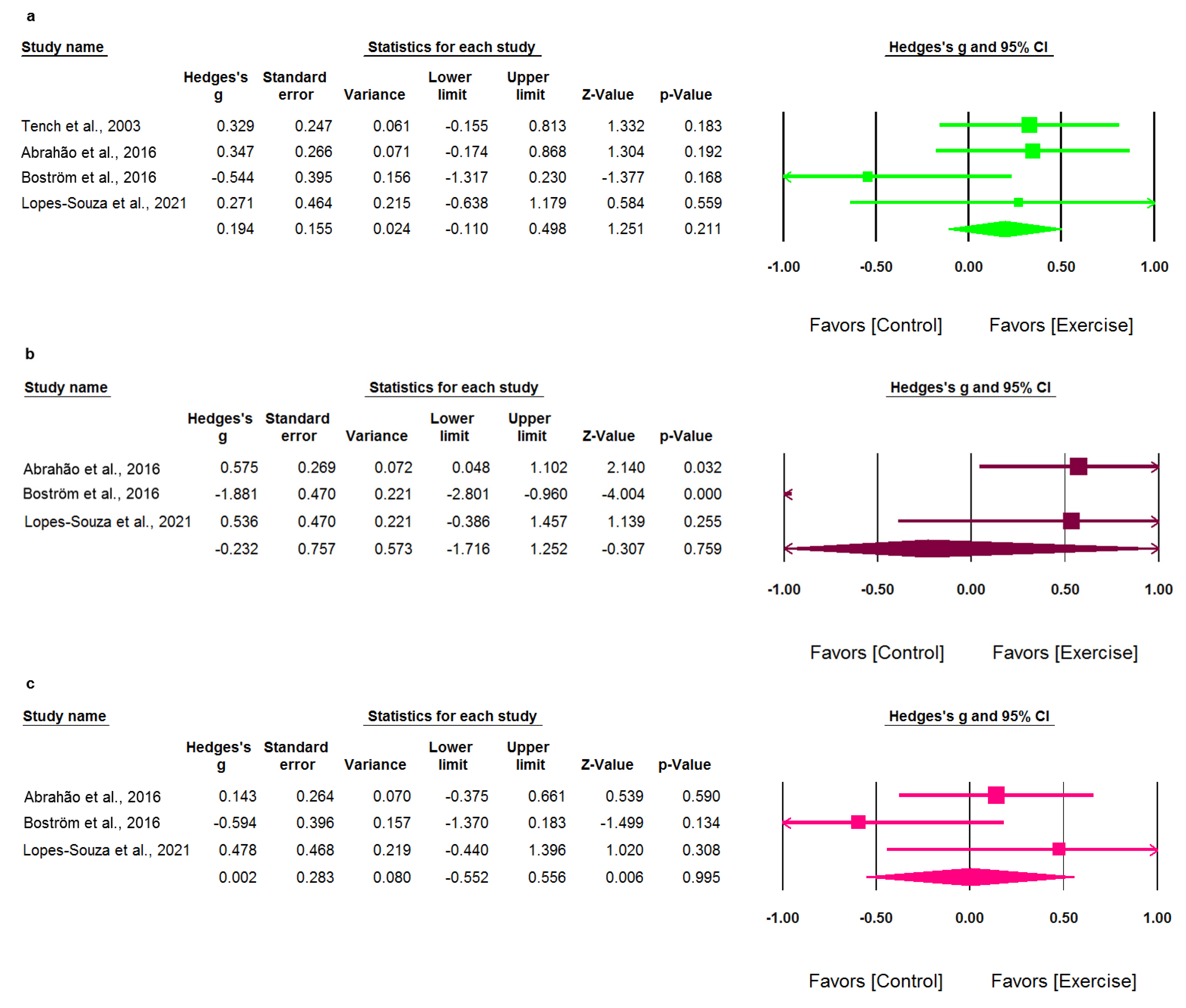
3.7. Meta-Analysis
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pons-Estel, G.J.; Alarcon, G.S.; Scofield, L.; Reinlib, L.; Cooper, G.S. Understanding the epidemiology and progression of systemic lupus erythematosus. Semin. Arthritis Rheum. 2010, 39, 257–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leong, P.-Y.; Huang, J.-Y.; Chiou, J.-Y.; Bai, Y.-C.; Wei, J.C.-C. The prevalence and incidence of systemic lupus erythematosus in Taiwan: A nationwide population-based study. Sci. Rep. 2021, 11, 5631. [Google Scholar] [CrossRef] [PubMed]
- Carter, E.E.; Barr, S.G.; Clarke, A.E. The global burden of SLE: Prevalence, health disparities and socioeconomic impact. Nat. Rev. Rheumatol. 2016, 12, 605–620. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Fu, T.; Yin, R.; Zhang, Q.; Shen, B. Prevalence of depression and anxiety in systemic lupus erythematosus: A systematic review and meta-analysis. BMC Psychiatry 2017, 17, 70. [Google Scholar] [CrossRef] [Green Version]
- Dobkin, P.L.; Fortin, P.R.; Joseph, L.; Esdaile, J.M.; Danoff, D.S.; Clarke, A.E. Psychosocial contributors to mental and physical health in patients with systemic lupus erythematosus. Arthritis Care Res. 1998, 11, 23–31. [Google Scholar] [CrossRef]
- Schipper, H. Quality of Life: Principles of the clinical paradigm. J. Psychosocial. Oncol. 1990, 8, 171–185. [Google Scholar] [CrossRef]
- Jolly, M. How does quality of life of patients with systemic lupus erythematosus compare with that of other common chronic illnesses? J. Rheumatol. 2005, 32, 1706–1708. [Google Scholar]
- Mok, C.C.; Ho, L.Y.; Cheung, M.Y.; Yu, K.L.; To, C.H. Effect of disease activity and damage on quality of life in patients with systemic lupus erythematosus: A 2-year prospective study. Scand. J. Rheumatol. 2009, 38, 121–127. [Google Scholar] [CrossRef]
- Olesinska, M.; Saletra, A. Quality of life in systemic lupus erythematosus and its measurement. Reumatologia 2018, 56, 45–54. [Google Scholar] [CrossRef]
- Pinto, A.J.; Miyake, C.N.H.; Benatti, F.B.; Silva, C.A.; Sallum, A.M.E.; Borba, E.; de Sa-Pinto, A.L.; Bonfa, E.; Gualano, B. Reduced aerobic capacity and quality of life in physically inactive patients with systemic lupus erythematosus with mild or inactive disease. Arthritis Care Res. 2016, 68, 1780–1786. [Google Scholar] [CrossRef] [Green Version]
- Kernder, A.; Elefante, E.; Chehab, G.; Tani, C.; Mosca, M.; Schneider, M. The patient’s perspective: Are quality of life and disease burden a possible treatment target in systemic lupus erythematosus? Rheumatology 2020, 59 (Suppl. 5), v63–v68. [Google Scholar] [CrossRef]
- Gladman, D.; Urowitz, M.; Fortin, P.; Isenberg, D.; Goldsmith, C.; Gordon, C.; Petri, M. Systemic Lupus International Collaborating Clinics conference on assessment of lupus flare and quality of life measures in SLE. Systemic Lupus International Collaborating Clinics Group. J. Rheumatol. 1996, 23, 1953–1955. [Google Scholar]
- Fangtham, M.; Kasturi, S.; Bannuru, R.R.; Nash, J.L.; Wang, C. Non-pharmacologic therapies for systemic lupus erythematosus. Lupus 2019, 28, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.; Winquist, N.W.; Wescott, A.B.; Lattie, E.G.; Graham, A.K. Systematic review of digital and non-digital non-pharmacological interventions that target quality of life and psychological outcomes in adults with systemic lupus erythematosus. Lupus 2021, 30, 1058–1077. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-L.; Yu, K.-H.; Tsai, J.-C. The effectiveness of exercise in adults with systemic lupus erythematosus: A systematic review and meta-analysis to guide evidence-based practice. Worldviews Evid.-Based Nurs. 2017, 14, 306–315. [Google Scholar] [CrossRef]
- O’Dwyer, T.; Durcan, L.; Wilson, F. Exercise and physical activity in systemic lupus erythematosus: A systematic review with meta-analyses. Semin. Arthritis Rheum. 2017, 47, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Sources to search. In Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2019; pp. 70–79. [Google Scholar]
- Sterne, J.A.C.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [Green Version]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Studies with more than two intervention groups. In Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2019; pp. 585–590. [Google Scholar]
- Tench, C.M.; McCarthy, J.; McCurdie, I.; White, P.D.; D’Cruz, D.P. Fatigue in systemic lupus erythematosus: A randomized controlled trial of exercise. Rheumatology 2003, 42, 1050–1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abrahão, M.I.; Gomiero, A.B.; Peccin, M.S.; Grande, A.J.; Trevisani, V.F. Cardiovascular training vs. resistance training for improving quality of life and physical function in patients with systemic lupus erythematosus: A randomized controlled trial. Scand. J. Rheumatol. 2016, 45, 197–201. [Google Scholar] [CrossRef]
- Boström, C.; Elfving, B.; Dupré, B.; Opava, C.H.; Lundberg, I.E.; Jansson, E. Effects of a one-year physical activity programme for women with systemic lupus erythematosus—A randomized controlled study. Lupus 2016, 25, 602–616. [Google Scholar] [CrossRef] [PubMed]
- Keramiotou, K.; Anagnostou, C.; Kataxaki, E.; Galanos, A.; Sfikakis, P.P.; Tektonidou, M.G. The impact of upper limb exercise on function, daily activities and quality of life in systemic lupus erythematosus: A pilot randomised controlled trial. RMD Open 2020, 6, e001141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes-Souza, P.; Dionello, C.F.; Bernardes-Oliveira, C.L.; Moreira-Marconi, E.; Marchon, R.M.; Teixeira-Silva, Y.; Paineiras-Domingos, L.L.; da Cunha Sá-Caputo, D.; Xavier, V.L.; Bergmann, A.; et al. Effects of 12-week whole-body vibration exercise on fatigue, functional ability and quality of life in women with systemic lupus erythematosus: A randomized controlled trial. J. Bodyw. Mov. Ther. 2021, 27, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Ramsey-Goldman, R.; Schilling, E.M.; Dunlop, D.; Langman, C.; Greenland, P.; Thomas, R.J.; Chang, R.W. A pilot study on the effects of exercise in patients with systemic lupus erythematosus. Arthritis Care Res. 2000, 13, 262–269. [Google Scholar] [CrossRef]
- de Carvalho, M.R.; Sato, E.I.; Tebexreni, A.S.; Heidecher, R.T.; Schenkman, S.; Neto, T.L. Effects of supervised cardiovascular training program on exercise tolerance, aerobic capacity, and quality of life in patients with systemic lupus erythematosus. Arthritis Rheum. 2005, 53, 838–844. [Google Scholar] [CrossRef]
- Bogdanovic, G.; Stojanovich, L.; Djokovic, A.; Stanisavljevic, N. Physical activity program is helpful for improving quality of life in patients with systemic lupus erythematosus. Tohoku J. Exp. Med. 2015, 237, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Gavilán-Carrera, B.; Vargas-Hitos, J.A.; Morillas-de-Laguno, P.; Rosales-Castillo, A.; Sola-Rodríguez, S.; Callejas-Rubio, J.L.; Sabio, J.M.; Soriano-Maldonado, A. Effects of 12-week aerobic exercise on patient-reported outcomes in women with systemic lupus erythematosus. Disabil. Rehabil. 2020, 1–9. [Google Scholar] [CrossRef]
- McElhone, K.; Abbott, J.; Shelmerdine, J.; Bruce, I.N.; Ahmad, Y.; Gordon, C.; Peers, K.; Isenberg, D.; Ferenkeh-Koroma, A.; Griffiths, B.; et al. Development and validation of a disease-specific health-related quality of life measure, the LupusQol, for adults with systemic lupus erythematosus. Arthritis Rheum. 2007, 57, 972–979. [Google Scholar] [CrossRef]
- Pettersson, S.; Lovgren, M.; Eriksson, L.E.; Moberg, C.; Svenungsson, E.; Gunnarsson, I.; Welin Henriksson, E. An exploration of patient-reported symptoms in systemic lupus erythematosus and the relationship to health-related quality of life. Scand. J. Rheumatol. 2012, 41, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Li, M.; Liu, L.; Wang, Z.; Wang, Y.; Zhao, J.; Wang, Q.; Tian, X.; Li, M.; Zeng, X. Relationship between disease activity, organ damage and health-related quality of life in patients with systemic lupus erythematosus: A systemic review and meta-analysis. Autoimmun. Rev. 2021, 20, 102691. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, K.; Svenungsson, E.; Karreskog, H.; Gunnarsson, I.; Gustafsson, J.; Moller, S.; Pettersson, S.; Bostrom, C. Physical activity in patients with systemic lupus erythematosus and matched controls. Scand. J. Rheumatol. 2012, 41, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Sieczkowska, S.M.; Coimbra, D.R.; Vilarino, G.T.; Andrade, A. Effects of resistance training on the health-related quality of life of patients with rheumatic diseases: Systematic review with meta-analysis and meta-regression. Semin. Arthritis Rheum. 2020, 50, 342–353. [Google Scholar] [CrossRef] [PubMed]
- McElhone, K.; Abbott, J.; Gray, J.; Williams, A.; Teh, L.-S. Patient perspective of systemic lupus erythematosus in relation to health-related quality of life concepts: A qualitative study. Lupus 2010, 19, 1640–1647. [Google Scholar] [CrossRef]
- Leong, K.P.; Kong, K.O.; Thong, B.Y.; Koh, E.T.; Lian, T.Y.; Teh, C.L.; Cheng, Y.K.; Chng, H.H.; Badsha, H.; Law, W.G.; et al. Development and preliminary validation of a systemic lupus erythematosus-specific quality-of-life instrument (SLEQOL). Rheumatology 2005, 44, 1267–1276. [Google Scholar] [CrossRef] [Green Version]
- Bérdi, M.; Köteles, F.; Szabó, A.; Bárdos, G. Placebo effects in sport and exercise: A meta-analysis. Eur. J. Ment. Health 2011, 6, 196–212. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Ye, D.-Q.; Pan, H.-F.; Li, W.-X.; Li, L.-H.; Li, J.; Li, X.-P.; Xu, J.-H. Influence of social support on health-related quality of life in patients with systemic lupus erythematosus. Clin. Rheumatol. 2009, 28, 265–269. [Google Scholar] [CrossRef]
- Pereira, M.G.; Duarte, S.; Ferraz, A.; Santos, M.; Fontes, L. Quality of life in patients with systemic lupus erythematosus: The mediator role of psychological morbidity and disease activity. Psychol. Health Med. 2020, 25, 1247–1257. [Google Scholar] [CrossRef]



| Study | Country | Sample Size at Start | Female (%) | Intervention | Control | Duration (Weeks) | QOL Scale | Main Findings Relating to QOL |
|---|---|---|---|---|---|---|---|---|
| Meta-analysis | ||||||||
| Tench et al., 2003 [23] | United Kingdom | 93 IG: 33 CG1: 32 CG2: 28 | 100 | Home exercise (walking, cycling, swimming), 30–50 min × 3 times/wk × 12 wks | CG1: Usual care CG2: Relaxation audiotape (this group was not included in the meta-analysis) | 12 | SF-36: 3 domains | No significant between-group differences in the physical function, role physical, and vitality domains of SF-36 after 12 wks of treatment |
| Abrahão et al., 2016 [24] | Brazil | 63 IG1: 21 IG2: 21 CG:21 | 97 | IG1: Cardiovascular exercise, 50 min × 3 times/wk × 12 wks IG2: Resistance exercise, 50 min × 3 times/wk × 12 wks | Usual care | 12 | SF-36: 8 domains | Significant improvement in quality of life from baseline to 12 wks in both exercise groups |
| Boström et al., 2016 [25] | Sweden | 35 IG: 18 CG: 17 | 100 | 0–3 months: Supervised aerobic exercise, 60 min × 2 times/wk + education + individual coaching of physical activity + heart rate monitor + physical activity diary 4–12 months: Tapering of coaching, self-managed physical activity | Usual care | 52 | SF-36: 8 domains | Significant improvement in SF-36 mental health domain at 6 months |
| Keramiotou et al., 2020 [26] | Greece | 58 IG: 28 CG: 30 | 94 | Individually tailored 30-min daily upper-limb home exercise program 30 min daily | routine care | 24 | LupusQoL | Significant improvement in LupusQoL physical health and fatigue domains only in the exercise group but not in the control group |
| Lopes-Souza et al., 2021 [27] | Brazil | 21 IG: 11 CG: 10 | 100 | Whole-body vibration exercise, 2 times/wk × 12 wks | Isometric stance with 130° knee flexion, 2 times/wk × 12 wks | 12 | SF-36: 8 domains | No significant differences in any of the 8 domains of SF-36 either between wk 0 and wk 12 or between groups |
| Systematic review | ||||||||
| Ramsey-Goldman et al., 2000 [28] 1 | USA | 10 IG1: 5 IG2: 5 | 100 | IG1: Aerobic exercise Phase1: Group exercise, 50 min × 3 times/wk × 2 months Phase2: Home exercise × 6 months IG2: Range of motion/muscle strengthening exercise Phase1: Group exercise, 50 min × 3 times/wk × 2 months Phase2: Home exercise × 6 months | – | 32 | SF-36: physical function domain | No significant differences in physical function of quality of life |
| de Carvalho et al., 2005 [29] 2 | Brazil | 60 IG: 41 CG: 19 | 100 | Supervised cardiovascular exercise, 60 min × 3 times/wk × 12 wks | Usual care | 12 | SF-36: 8 domains | Significant between-group differences in the physical fitness and vitality domains of SF-36 |
| Bogdanovic et al., 2015 [30] 1,2 | Serbia | 60 IG1: 30 IG2: 30 | 100 | IG1: Aerobic exercise, 15 min × 3 times/wk × 6 wks IG2: Isotonic exercises, 30 min × 3 times/wk × 6 wks | – | 6 | SF-36: 8 domains | Significant improvement in all areas of SF-36 after aerobic or isotonic exercise, but no differences between the two types of exercise. |
| Gavilán-Carrera et al., 2020 [31] 2 | Spain | 58 IG: 26 CG: 32 | 100 | Aerobic exercise on a treadmill, 75 min × 2 times/wk × 12 wks | Usual care | 12 | SF-36: physical health and mental health domains | No significant between-group differences in the changes in quality of life. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, M.-C.; Koo, M. Effects of Exercise Intervention on Health-Related Quality of Life in Patients with Systemic Lupus Erythematosus: A Systematic Review and Meta-Analysis of Controlled Trials. Healthcare 2021, 9, 1215. https://doi.org/10.3390/healthcare9091215
Lu M-C, Koo M. Effects of Exercise Intervention on Health-Related Quality of Life in Patients with Systemic Lupus Erythematosus: A Systematic Review and Meta-Analysis of Controlled Trials. Healthcare. 2021; 9(9):1215. https://doi.org/10.3390/healthcare9091215
Chicago/Turabian StyleLu, Ming-Chi, and Malcolm Koo. 2021. "Effects of Exercise Intervention on Health-Related Quality of Life in Patients with Systemic Lupus Erythematosus: A Systematic Review and Meta-Analysis of Controlled Trials" Healthcare 9, no. 9: 1215. https://doi.org/10.3390/healthcare9091215






