The Feasibility of Using N-Of-1 Trials to Investigate Deprescribing in Older Adults with Dementia: A Pilot Study
Abstract
1. Introduction
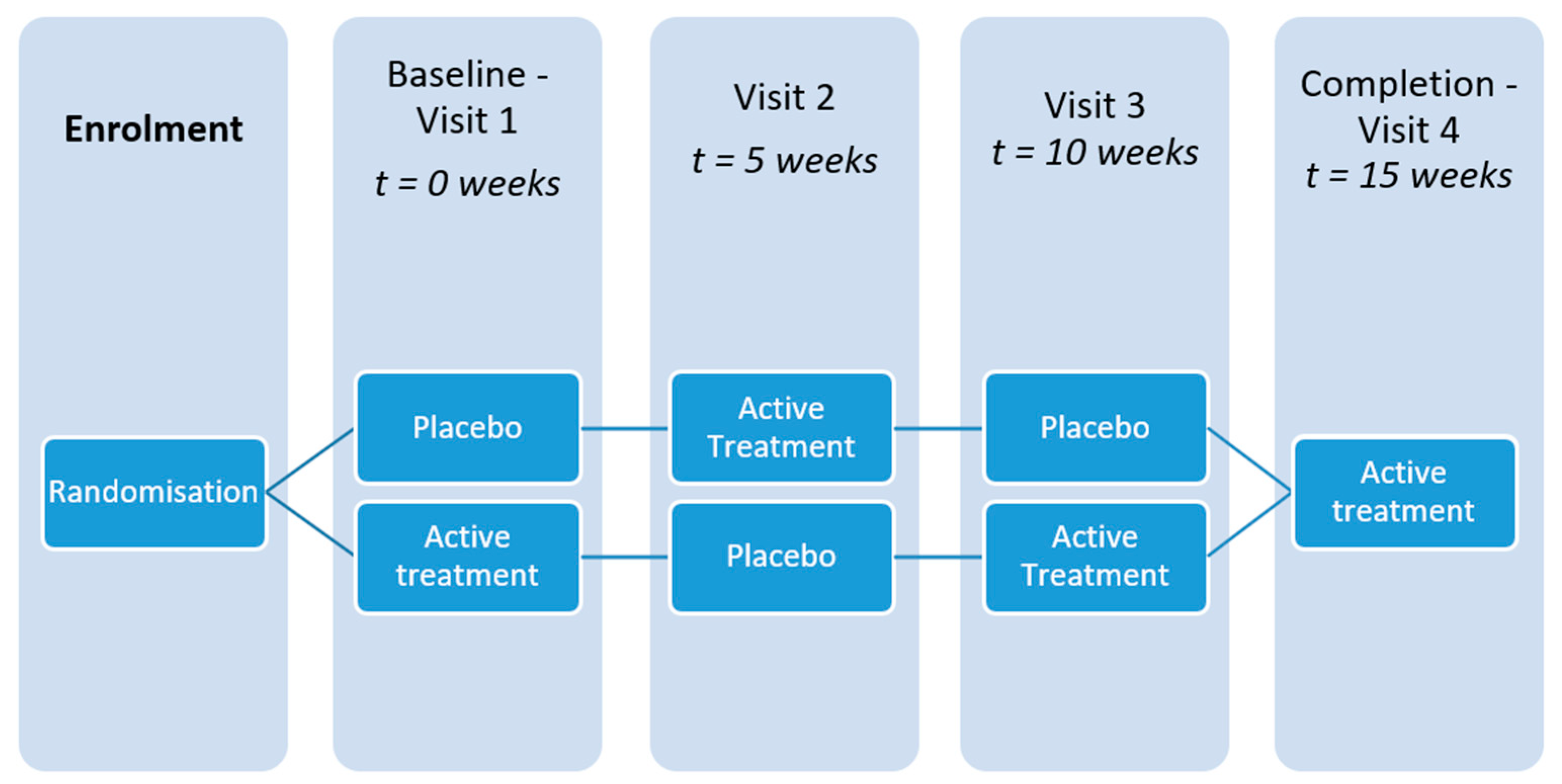
2. Materials and Methods
- Feasibility of using the N-of-1 method for this clinical deprescribing trial, assessed by analysing the actual recruitment numbers compared with screening and eligibility numbers.
- Change in Alzheimer’s Disease Assessment Scale-Cognitive Subscale (ADAS-CoG) score of each participant [16]. The ADAS-CoG is a comprehensive psychometric instrument that evaluates: memory, attention, reasoning, language, orientation, and praxis. Scores range from 0 to 70 with a higher score indicating an increased degree of cognitive impairment. A mean difference of at least 4 points between intervention periods was to be considered as clinically significant for this study. This measure was chosen as it has been used in previous Alzheimer’s disease (AD) statin trials [17]. Furthermore, the test is used to inform on the efficacy and cost effectiveness of Pharmaceutical Benefits Scheme (PBS) subsidised medicines for the treatment of AD, and there are correlations between improvement in ADAS-CoG score and clinically meaningful measures in people with moderate dementia [18].
- Self-reported Quality of Life in Alzheimer’s Disease (QoL-AD), as per previous clinical trials in dementia, and as also recommended by the Dementia Collaborative Research Centres, Australia [19]. It is a brief, 13-item measure recording the participant’s quality of life and is completed by the participant as an interview, and by their caregiver as a questionnaire. A higher score indicates greater quality of life [20].
- The Short Performance Physical Battery (SPPB); a series of physical tests mimicking daily activities. It is scored from 0 to 12 with a higher score indicating greater physical performance [21].
- The John Hodges Cambridge Behavioural Inventory—Revised for the carer (CBI-R); a carer-completed questionnaire on observed cognitive and behavioural disturbances that are used to determine carer-related outcomes. It has 45 questions, scored on a scale of 0 to 4 with larger scores indicating more frequent problems [22].
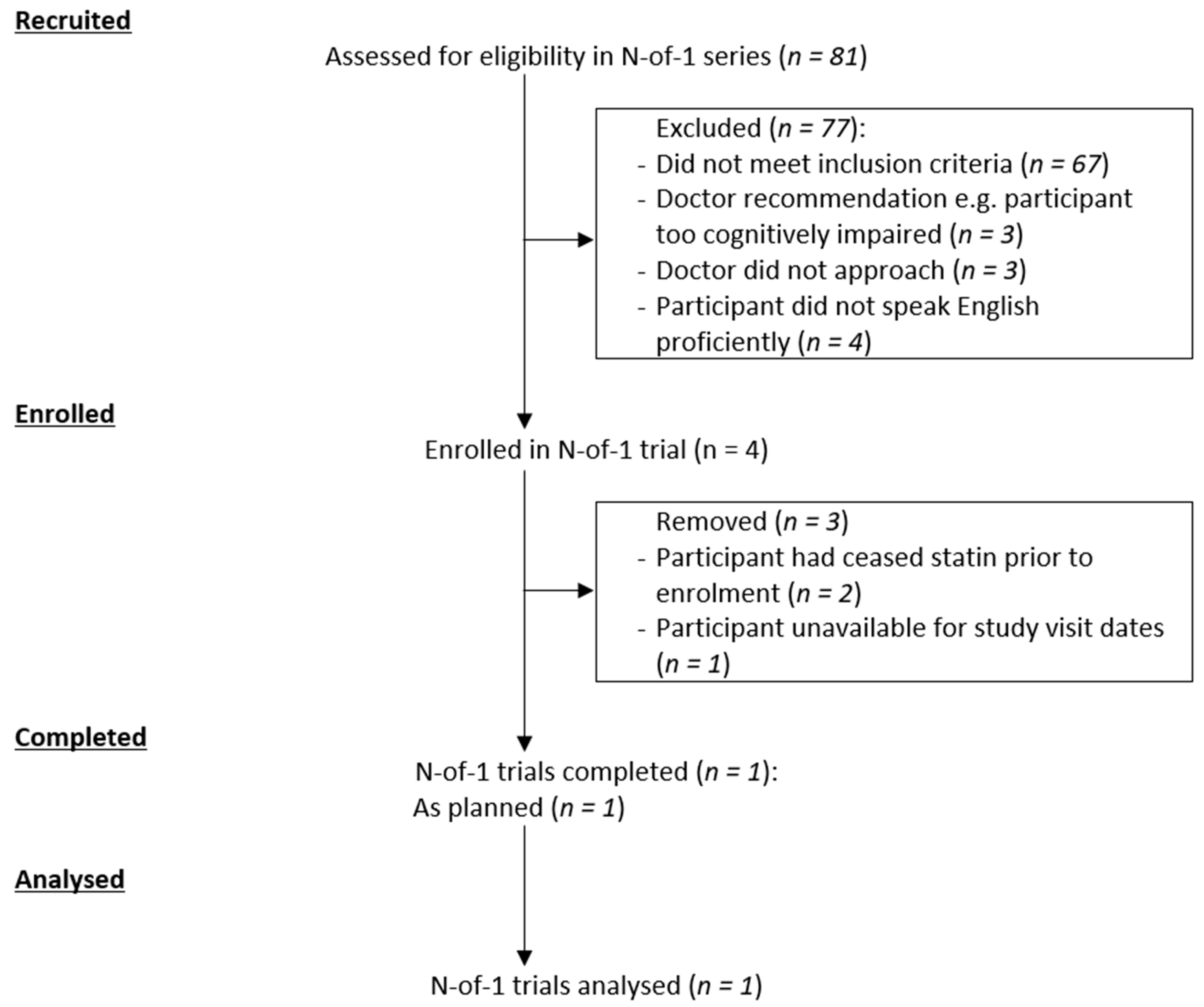
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Reeve, E.; Bell, J.S.; Hilmer, S.N. Barriers to Optimising Prescribing and Deprescribing in Older Adults with Dementia: A Narrative Review. Curr. Clin. Pharm. 2015, 10, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Hajjar, E.R.; Hanlon, J.T.; Artz, M.B.; Lindblad, C.I.; Pieper, C.F.; Sloane, R.J.; Ruby, C.M.; Schmader, K.E. Adverse drug reaction risk factors in older outpatients. Am. J. Geriatr. Pharm. 2003, 1, 82–89. [Google Scholar] [CrossRef]
- Kanagaratnam, L.; Dramé, M.; Trenque, T.; Oubaya, N.; Nazeyrollas, P.; Novella, J.L.; Jolly, D.; Mahmoudi, R. Adverse drug reactions in elderly patients with cognitive disorders: A systematic review. Maturitas 2016, 85, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Strandberg, T.E.; Kolehmainen, L.; Vuorio, A. Evaluation and treatment of older patients with hypercholesterolemia: A clinical review. JAMA 2014, 312, 1136–1144. [Google Scholar] [CrossRef]
- Efficacy and safety of statin therapy in older people: A meta-analysis of individual participant data from 28 randomised controlled trials. Lancet 2019, 393, 407–415. [CrossRef]
- Padala, K.P.; Padala, P.R.; McNeilly, D.P.; Geske, J.A.; Sullivan, D.H.; Potter, J.F. The effect of HMG-CoA reductase inhibitors on cognition in patients with Alzheimer’s dementia: A prospective withdrawal and rechallenge pilot study. Am. J. Geriatr. Pharm. 2012, 10, 296–302. [Google Scholar] [CrossRef]
- Page, A.T.; Clifford, R.M.; Potter, K.; Schwartz, D.; Etherton-Beer, C.D. The feasibility and effect of deprescribing in older adults on mortality and health: A systematic review and meta-analysis. Br. J. Clin. Pharm. 2016, 82, 583–623. [Google Scholar] [CrossRef]
- Gurwitz, J.H.; Go, A.S.; Fortmann, S.P. Statins for Primary Prevention in Older Adults: Uncertainty and the Need for More Evidence. JAMA 2016, 316, 1971–1972. [Google Scholar] [CrossRef]
- Scuffham, P.A.; Nikles, J.; Mitchell, G.K.; Yelland, M.J.; Vine, N.; Poulos, C.J.; Pillans, P.I.; Bashford, G.; del Mar, C.; Schluter, P.J.; et al. Using N-of-1 trials to improve patient management and save costs. J. Gen. Intern. Med. 2010, 25, 906–913. [Google Scholar] [CrossRef]
- Gabler, N.B.; Duan, N.; Vohra, S.; Kravitz, R.L. N-of-1 trials in the medical literature: A systematic review. Med. Care 2011, 49, 761–768. [Google Scholar] [CrossRef]
- Marwick, K.F.M.; Stevenson, A.J.; Davies, C.; Lawrie, S.M. Application of n-of-1 treatment trials in schizophrenia: Systematic review. Br. J. Psychiatry 2018, 213, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Joy, T.R.; Monjed, A.; Zou, G.Y.; Hegele, R.A.; McDonald, C.G.; Mahon, J.L. N-of-1 (single-patient) trials for statin-related myalgia. Ann. Intern. Med. 2014, 160, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Clough, A.J.; Hilmer, S.N.; Naismith, S.L.; Kardell, L.D.; Gnjidic, D. N-of-1 trials for assessing the effects of deprescribing medications on short-term clinical outcomes in older adults: A systematic review. J. Clin. Epidemiol. 2018, 93, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Vohra, S.; Shamseer, L.; Sampson, M.J.; Bukutu, C.; Schmid, C.H.; Tate, R.; Nikles, J.; Zucker, D.R.; Kravitz, R.L.; Guyatt, G.H.; et al. CONSORT extension for reporting N-of-1 trials (CENT) 2015 Statement. J. Clin. Epidemiol. 2016, 76, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Rosen, W.G.; Mohs, R.C.; Davis, K.L. A new rating scale for Alzheimer’s disease. Am. J. Psychiatry 1984, 141, 1356–1364. [Google Scholar]
- McGuinness, B.; O’Hare, J.; Craig, D.; Bullock, R.; Malouf, R.; Passmore, P. Statins for the treatment of dementia. Cochrane Database Syst. Rev. 2014, CD007514. [Google Scholar] [CrossRef]
- Verhey, F.R.; Houx, P.; Van Lang, N.; Huppert, F.; Stoppe, G.; Saerens, J.; Böhm, P.; De Vreese, L.; Nordlund, A.; DeDeyn, P.P.; et al. Cross-national comparison and validation of the Alzheimer’s Disease Assessment Scale: Results from the European Harmonization Project for Instruments in Dementia (EURO-HARPID). Int. J. Geriatr. Psychiatry 2004, 19, 41–50. [Google Scholar] [CrossRef]
- Hoe, J.; Katona, C.; Roch, B.; Livingston, G. Use of the QOL-AD for measuring quality of life in people with severe dementia—The LASER-AD study. Age Ageing 2005, 34, 130–135. [Google Scholar] [CrossRef]
- Thorgrimsen, L.; Selwood, A.; Spector, A.; Royan, L.; de Madariaga Lopez, M.; Woods, R.T.; Orrell, M. Whose quality of life is it anyway? The validity and reliability of the Quality of Life-Alzheimer’s Disease (QoL-AD) scale. Alzheimer Dis. Assoc. Disord. 2003, 17, 201–208. [Google Scholar] [CrossRef]
- Puthoff, M.L. Outcome measures in cardiopulmonary physical therapy: Short physical performance battery. Cardiopulm. Phys. J. 2008, 19, 17–22. [Google Scholar] [CrossRef]
- Helen, J.W.; Catherine, J.W.; Eneida, M.; Caroline, H.W.-G.; Sarah, L.M.; Roger, A.B.; John, R.H. The Cambridge Behavioural Inventory revised. Dement. Neuropsychol. 2008, 2, 102–107. [Google Scholar]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Swiger, K.J.; Manalac, R.J.; Blumenthal, R.S.; Blaha, M.J.; Martin, S.S. Statins and cognition: A systematic review and meta-analysis of short- and long-term cognitive effects. Mayo Clin. Proc. 2013, 88, 1213–1221. [Google Scholar] [CrossRef] [PubMed]
- Richardson, K.; Schoen, M.; French, B.; Umscheid, C.A.; Mitchell, M.D.; Arnold, S.E.; Heidenreich, P.A.; Rader, D.J.; deGoma, E.M. Statins and cognitive function: A systematic review. Ann. Intern. Med. 2013, 159, 688–697. [Google Scholar] [CrossRef]
- McGuinness, B.; Craig, D.; Bullock, R.; Passmore, P. Statins for the prevention of dementia. Cochrane Database Syst. Rev. 2016, CD003160. [Google Scholar] [CrossRef]
- Chu, C.S.; Tseng, P.T.; Stubbs, B.; Chen, T.Y.; Tang, C.H.; Li, D.J.; Yang, W.C.; Chen, Y.W.; Wu, C.K.; Veronese, N.; et al. Use of statins and the risk of dementia and mild cognitive impairment: A systematic review and meta-analysis. Sci. Rep. 2018, 8, 5804. [Google Scholar] [CrossRef]
- Rojas-Fernandez, C.H.; Cameron, J.C. Is statin-associated cognitive impairment clinically relevant? A narrative review and clinical recommendations. Ann. Pharm. 2012, 46, 549–557. [Google Scholar] [CrossRef]
- Power, M.C.; Weuve, J.; Sharrett, A.R.; Blacker, D.; Gottesman, R.F. Statins, cognition, and dementia-systematic review and methodological commentary. Nat. Rev. Neurol. 2015, 11, 220–229. [Google Scholar] [CrossRef]
- McGuinness, B.; Cardwell, C.R.; Passmore, P. Statin withdrawal in people with dementia. Cochrane Database Syst. Rev. 2016, 9, CD012050. [Google Scholar]
- Cooper, C.; Ketley, D.; Livingston, G. Systematic review and meta-analysis to estimate potential recruitment to dementia intervention studies. Int. J. Geriatr. Psychiatry 2014, 29, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Fargo, K.N.; Carrillo, M.C.; Weiner, M.W.; Potter, W.Z.; Khachaturian, Z. The crisis in recruitment for clinical trials in Alzheimer’s and dementia: An action plan for solutions. Alzheimers Dement. 2016, 12, 1113–1115. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.; Stowasser, D.; Freeman, C.; Scott, I. Prescriber barriers and enablers to minimising potentially inappropriate medications in adults: A systematic review and thematic synthesis. BMJ Open 2014, 4, e006544. [Google Scholar] [CrossRef] [PubMed]
- Qi, K.; Reeve, E.; Hilmer, S.N.; Pearson, S.A.; Matthews, S.; Gnjidic, D. Older peoples’ attitudes regarding polypharmacy, statin use and willingness to have statins deprescribed in Australia. Int. J. Clin. Pharm. 2015, 37, 949–957. [Google Scholar] [CrossRef]
- Ni Chroinin, D.; Chroinin, C.N.; Beveridge, A. Factors influencing deprescribing habits among geriatricians. Age Ageing 2015, 44, 704–708. [Google Scholar] [CrossRef]
- Van der Ploeg, M.A.; Floriani, C.; Achterberg, W.P.; Bogaerts, J.M.K.; Gussekloo, J.; Mooijaart, S.P.; Streit, S.; Poortvliet, R.K.E.; Drewes, Y.M. Recommendations for (Discontinuation of) Statin Treatment in Older Adults: Review of Guidelines. J. Am. Geriatr. Soc. 2019. [Google Scholar] [CrossRef]
| Characteristic | All Participants |
|---|---|
| Mean Age (Years) | 85.1 ± 3.4 |
| Females, n (%) | 3 (75) |
| Statin, type (daily dose) (n) | Atorvastatin (20 mg (1), 10 mg (1)) Rosuvastatin (5 mg (1)) 1 |
| Mean Mini Mental State Examination Score | 17.5 ± 2.9 |
| Intervention | Visit Number (time-point) | ADAS-CoG 1 (/70) | SPPB 2 (/12) | QoL-AD 3 (/52) | CBI-R 4 (/180) |
|---|---|---|---|---|---|
| Active 5 | One (baseline) | 15 | 9 | 40 | 20 |
| Placebo | Two (5-weeks) | 12 | 7 | 39 | 19 |
| Active | Three (10-weeks) | 15 | 5 | 34 | 22 |
| Placebo | Four (15-weeks) | 19 | 6 | 29 | 32 |
| Overall change (mean active − mean placebo) | −0.5 | 0.5 | 3 | −4.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clough, A.J.; Hilmer, S.N.; Naismith, S.L.; Gnjidic, D. The Feasibility of Using N-Of-1 Trials to Investigate Deprescribing in Older Adults with Dementia: A Pilot Study. Healthcare 2019, 7, 161. https://doi.org/10.3390/healthcare7040161
Clough AJ, Hilmer SN, Naismith SL, Gnjidic D. The Feasibility of Using N-Of-1 Trials to Investigate Deprescribing in Older Adults with Dementia: A Pilot Study. Healthcare. 2019; 7(4):161. https://doi.org/10.3390/healthcare7040161
Chicago/Turabian StyleClough, Alexander J., Sarah N. Hilmer, Sharon L. Naismith, and Danijela Gnjidic. 2019. "The Feasibility of Using N-Of-1 Trials to Investigate Deprescribing in Older Adults with Dementia: A Pilot Study" Healthcare 7, no. 4: 161. https://doi.org/10.3390/healthcare7040161
APA StyleClough, A. J., Hilmer, S. N., Naismith, S. L., & Gnjidic, D. (2019). The Feasibility of Using N-Of-1 Trials to Investigate Deprescribing in Older Adults with Dementia: A Pilot Study. Healthcare, 7(4), 161. https://doi.org/10.3390/healthcare7040161





