In this section, we carry out numerical simulations to verify our theoretical analysis. In order to simulate the optimal time of booster vaccination both in countries with low vaccination rates and high vaccination rates, we choose two groups of actual parameters under different vaccination rates according to official data. We also study the impact of vaccine effectiveness on the epidemic by adding a third set of parameters. Then, we calculate the equilibria and critical values of time delay through MATLAB. After that, we simulate the change of the epidemic with different booster vaccination times. According to the results, we give the conclusion on the most suitable booster vaccination time and give some reasonable suggestions for epidemic control.
5.1. Determination for Parameter Values
In this section, we use statistical methods to analyze the values of parameters according to the actual data obtained from several official websites. Then, we select three groups of parameters with the highest research significance.
- (1)
COVID-19 mortality rate: c
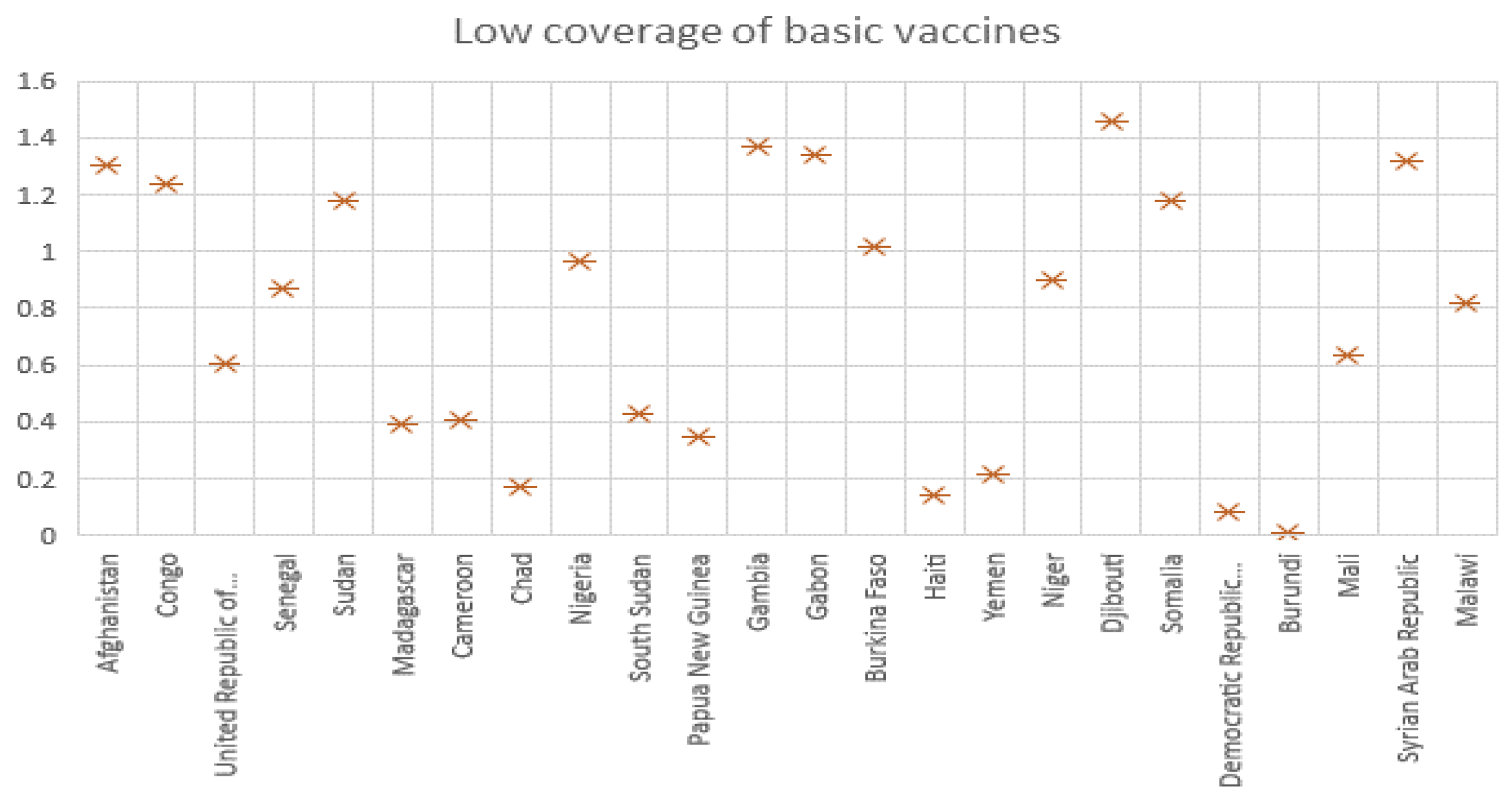
Based on data from the official website of The World Health Organization (
https://www.who.int/, accessed on 14 March 2022), we can obtain the COVID-19 mortality rates of different countries. In order to ensure that the data can reflect the average, we take representative data and eliminate outliers. Finally, we screen the death rates due to disease for 29 countries. According to the data, we make a bar chart, which is presented in
Figure 2.
From
Figure 2, it is easy to find that the COVID-19 mortality rates of these countries are mostly in the range of 0.0008 to 0.001, so we choose the mean value of 0.0009 as the value of
c.
- (2)
Cure rate:
We obtained the cure rates of COVID-19 in different countries from the website of the WHO. By eliminating the missing values and outliers, we obtain the cure rates of 62 countries (such as the USA, Japan, Germany, Austria, Italy, Canada, South Africa, France and so on) and plot the scatter diagram in
Figure 3.
As for cure rates
, we can clearly see that it is almost at the same level through the dotted line in
Figure 3, so we figure out the average rate of 62 countries: 0.861 as the value of
.
- (3)
Infection rate: ,
Infection rates can vary from country to country because of the spread of the disease and the level of government concern. In addition, while antibodies are produced in vaccinated people, an immune barrier is not yet fully formed. So they also have some rate of transmission, but obviously, the people who get the booster vaccine have a lower rate of infection than the people who just get the basic vaccine. We consult the relevant data from the Centers for Disease Control and Prevention (
https://www.cdc.gov/coronavirus/2019-ncov/index.html, accessed on 14 March 2022) and determine the values or range of
and
in light of the actual situation. Then, we choose
and
.
- (4)
Re-vaccination rate: ,
As we mentioned in the modeling, the level of antibody production after vaccination depends on the individual [
22]. For people such as the elderly or those with underlying diseases who have recovered, the antibodies produced by the vaccine are almost completely disabled, and they need a basic injection to regain active antibodies at a conversion rate of
from
R to
S. In addition, some people still have some antibody activity in their bodies, and they only need to inject enhancers to increase their resistance to SARS-CoV-2 at a conversion rate of
from
R to
V. We think the difference is related to the age structure of the infected person (see
Figure 4).
We find that recovered people between 20 and 50 years old account for 61% of the total, and we assume that this group has better physical fitness than other age groups. So we consider , and choose .
- (5)
Natural mortality rate: d
In order to find the value of natural mortality rate
d, we select population data from the National Bureau of Statistics (
http://www.stats.gov.cn/enGliSH/, accessed on 14 March 2022) from 2006 to 2019, and we forecast a natural mortality rate
in 2022 based on trends (see
Figure 5).
- (6)
Basic vaccination rate:
Due to limited vaccine resources in some countries or insufficient attention to the epidemic, vaccination rates vary significantly among countries. We classify countries in terms of high and low vaccination rates and discuss the impact of booster vaccination on epidemic control in both groups.
As we can see in
Figure 6 and
Figure 7, we classify the data provided by the WHO and select reasonable data to draw scatter plots. For countries with low vaccination rates, we find vaccination rates are around 0.8, so we select
for the first set of parameters. For countries with high vaccination rates, in which people recognize the effectiveness of vaccines for epidemic control, vaccination rates reach 10, so we select
.
- (7)
Booster vaccination rate:
For booster vaccination, although the vaccination process is still going on and the rate of booster vaccination is still a variable, we can still analyze it based on the available data from the WHO because the level of national interest in vaccines does not change very much (see
Figure 8 and
Figure 9).
It is clear that the low booster vaccination rate is between 0.4 and 0.5, so we choose
as the booster vaccination rate for countries with low vaccination rates. In
Figure 9, an average of 0.864 is selected as the booster vaccination rate for countries with high vaccination rates.
- (8)
The failure rate of booster vaccination:
As for , since the booster vaccine has just been developed, there is no exact failure rate and expiry time. Therefore, we refer to other vaccine-related data from the official website and select as the failure rate of vaccines in countries with high vaccination rates and as the failure rate of vaccines in countries with low vaccination rates, according to some experts’ prediction of the effectiveness of COVID-19 booster vaccines. To study the impact of a lower vaccine failure rate on epidemic control, we select in the third group of parameters. This is consistent with the fact that the higher the failure rate, the less willing people are to be vaccinated.
Based on the above consideration, we take the following two groups of parameters (our parameters are all dimensionless):
- (I):
- (II):
- (III):
Parameter (I) simulates countries with low vaccination rates and low vaccine effectiveness, probably due to limited national resources and low level of development; Parameter (II) simulates countries with high vaccination rates and average vaccine effectiveness, which is consistent with the current reality of most countries; In order to study methods that can better control the epidemic, we select a third group of parameters (III), which reduced the failure rate compared with the second group of parameters.
5.2. Simulations and Verification
For the group of parameters (I):
This represents countries with low vaccination rates. We calculate the disease-free equilibrium
. Under this group of parameters,
, so equilibria
do not exist, and
is locally asymptotically stable when
according to Theorem 1 and Theorem 2. When
,
only has one positive root, and
,
,
,
. We choose
and substitute it into Equations (
12) and (
13); we get
,
,
,
. If (H5) holds, the equilibrium
is locally asymptotically stable when
.
When
,
, the equilibrium
is locally asymptotically stable according to Theorem 1;
means the vaccine will fail 24 months after injection and
means that people begin to inject booster vaccinations after 1 month to cope with the decrease of vaccine effectiveness. We choose initial values [50, 50, 10, 10] and picture the number of people in different cabins changing over time in
Figure 10.
The figure above shows that when the vaccine is available for two years, people who get a booster vaccine within a month of getting the basic vaccine can get rid of all infections within 20 months. In other words, herd immunity is achieved before the vaccine wears off. S and V will stabilize after 400 months, and the epidemic will completely disappear.
Remark 1:
Our simulations show that for low-coverage countries, when the vaccine is valid for two years, people need to receive the booster vaccine promptly within one and a half months of receiving the basic vaccine. After 1.5 months, an outbreak will occur. Further, the faster people are vaccinated, the more effectively the epidemic is contained. However, it became clear that getting a booster vaccine after a month would not meet the requirements of the vaccine for the human body. Most of these countries are currently experiencing outbreaks. This is consistent with our simulation results.
For the group of parameters (II):
This represents the situation for countries with high vaccination rates and average vaccine effectiveness. We find
, so equilibrium
make sense and is [104.47, 688.37, 255.14, 367.79]. Equilibrium
is unstable. Substituting this group of parameters into Equation (
14), (H6) is satisfied, so equilibrium
is locally asymptotically stable when
according to Theorem 2. When
,
only has one positive root, and
,
. Selecting
, we obtain
,
,
,
. Substituting the parameters (II) into Equations (
A9) and (
A14), we have
. According to Theorem 3, we can deduce
,
; the periodic solution is stable. This means that the epidemic will fluctuate greatly over time, and people’s means of controlling the epidemic have no obvious effect on controlling the epidemic. However, there will not be a sudden increase in the number of infected people at a certain moment, and the epidemic situation will not be uncontrollable.
Considering the vaccine developed at present is not an instantaneous failure, and vaccines cannot be administered in a short time, is impossible.
According to existing medical research, we believe that the validity period of the vaccine is 23–32 months, so we choose as the validity period of a booster vaccine. Our purpose is to study the impact of different booster vaccine inoculation times on the epidemic situation. Through our simulation under this set of parameters, we find two important time nodes—6 months and 10 months—to get booster vaccination after basic vaccination. Vaccination after 10 months will lead to an outbreak, which is consistent with our theoretical analysis. Vaccination within six months makes a difference in the epidemic compared to the situation in which people get vaccinated after 6 months.
When
that means the vaccine will expire after 25 months and booster vaccination will be carried out after 7 months. We still choose [100, 500, 200, 300] as the initial values; the epidemic situation is shown in
Figure 11.
While the vaccine is still valid for 25 months, people getting booster shots within 7 months will have an overall increase in infections for 500 months, meaning that the number of infections will be high for a long time. This situation can only keep the epidemic under control but does not reduce the number of infected people.
When
that means the vaccine expire after 25 months, and we inject the booster vaccine after 6 months; we choose [100, 500, 200, 300] as the initial values (see
Figure 12).
We can see that the epidemic has fluctuated over 500 months. This is consistent with our reality. Currently, we are required to get a booster shot six months after the basic vaccine. Even then, the epidemic does not disappear completely. There are periodic fastigiums in the number of infections. However, by vaccinating we can prevent the number of infections from increasing or staying high and stabilize the epidemic over many years. That is, the booster vaccination has a positive effect on the development of the epidemic, and the trend will be better with the booster vaccination in time.
Remark 2:
This means that under this set of parameters, people will inevitably live with the virus for a long time, and a booster vaccination at the right time will only have a temporary effect on reducing the number of infections.
For the group of parameters (III):
This set of parameters represents the ideal situation in which the outbreak can be well contained. We find
, so equilibrium
makes sense and is [100.92, 812.57, 169.97, 309.34]. Equilibrium
is unstable. Substituting this group of parameters into Equation (
14), (H6) is satisfied, so equilibrium
is locally asymptotically stable when
according to Theorem 2. When
,
only has one positive root, and
,
. Selecting
, we obtain
,
,
,
. Substituting the parameters (II) into Equations (
A9) and (
A14), we have
. According to Theorem 3, we can deduce
,
, which means under this set of parameters, if the equilibrium
is unstable, a stable Hopf bifurcation periodic solution will appear. This means that although there will not be a large number of people infected with the novel coronavirus and the number of cases will surge, people’s methods are still ineffective, and people need to find better ways to control the epidemic.
When
that means the vaccine expires after 32 months and people inject the booster vaccine after 6 months, we choose [100, 1000, 200, 300] as the initial values (see
Figure 13).
Figure 13 shows a declining trend in the number of infections under the third set of parameters, which stabilizes and approaches almost zero after two decades. This suggests that when the validity of the vaccine is increased to 32 months and the vaccine failure rate is reduced to 0.15, people who receive the booster vaccine 6 months after the basic vaccine can control the outbreak more effectively without long-term coexistence with the virus. In other words, if the vaccine is effective enough, we can expect to be free of COVID-19 by 2042 or earlier. However, this is a relatively ideal situation because many factors in reality can cause the values of parameters in the model to change at any time, and our simulation is based on only a set of constant parameters.
To make the simulation results closer to reality, we can change the value of parameters in real-time according to the actual situation of the epidemic development and use our model to predict the development of the epidemic under different factors such as infection rate, cure rate and vaccine effectiveness. We can provide ideas for the country to control the epidemic by analyzing the simulation results.
When
that means the vaccine expires after 32 months, but people inject the booster vaccine after 14.5 months; we choose [100, 1000, 200, 300] as the initial values (see
Figure 14).
Remark 3:
Comparing Figure 13 with Figure 14, it can be found that when the booster vaccination time is 14.5 months, although the system fluctuation trend becomes smaller and the number of infected people also decreases, it takes longer for the system to stabilize than when the booster vaccination time is 6 months. As shown in Figure 14, the system is not stable after 500 months, which has a bad impact on the country’s economy and development. Therefore, it is necessary to implement the booster vaccine as soon as the effectiveness of the vaccine is certain. 5.3. Analysis of Simulations
Based on the above simulations, we have the following conclusions:
(1) When the time of vaccine expiration is determined, the less time people have between a basic vaccine and a booster, the better the outbreak will be contained in both low and high coverage countries, and when the time of booster vaccination exceeds the critical value, System (
1) will be unstable and the epidemic will be out of control. The critical time for the booster vaccination is 1.4 months for countries with low vaccination rates and 10.17 months for countries with high vaccination rates. It is clear that increasing vaccination rates have had a positive impact on epidemic control.
(2) We select the parameters (I) and (II) closest to the current epidemic situation, and the simulation results are consistent with the real situation. Due to limited vaccine resources or other reasons, some countries have low vaccination rates. For them, booster vaccination is not completed effectively and on time. As for the critical time of 1.44 months in our simulation results, it is impossible to complete in reality. We look at the epidemic status of most countries with low vaccination rates and found that most of them are in an uncontrolled state of the epidemic, which is consistent with our simulations. For countries with high vaccination rates, we found that the critical time for booster vaccination is 10.17 months, which can be achieved in reality. Given the physical demands of vaccination, most countries require people to receive the booster vaccine promptly 6 months after receiving the basic vaccine. In our simulations, 6 months is also considered to be the most suitable optimal time for booster vaccination. In this case, there will be some fluctuations in the current epidemic, but the number of infections will not be at a high level all the time, and people will be able to control the epidemic within a certain range and eventually stabilize it. When the inoculation time is 7 months, although the epidemic does not fluctuate greatly in the near stage and eventually tends to stabilize, the number of infected people will remain at a high level. This means that under the second set of parameters, the effectiveness of the vaccine will not be enough to eliminate the epidemic, and even if people are actively vaccinated and have high vaccination rates, they will inevitably live with COVID-19 for a long time.
(3) Due to the short development time of the vaccine, its effectiveness is still unclear. In our numerical simulations, different parameters are used to study the impact of vaccine effectiveness on the epidemic. We choose parameters (III) to simulate a better epidemic scenario. Compared with the groups of parameters (I) and (II), the third group has a higher vaccination rate and lower vaccine failure rate, the basal shot is less likely to fail, and the proportion of recovered patients who retain antibodies from the basal shot increased. Through our simulation, we found that under the third set of parameters, when the validity of the vaccine is 32 months and the booster vaccination time is controlled within 20 months, the number of infections decreased and eventually approached zero, the system stabilized, and the epidemic almost disappeared. Changing the timing of the booster vaccine, we found that when the booster vaccine is given at 6 months, the epidemic could be virtually eliminated by 2042. Even though the parameters can change over time in the real world, and this is an ideal situation for us to simulate with a constant set of parameters, we can still conclude that the longer the interval between actual vaccinations, the longer it takes for the epidemic to stabilize. Therefore, considering the economic level of the country and the requirements of the vaccine for the human body, we believe that under the third group of parameters we selected, timely vaccination after 6 months is the ideal epidemic control means.
Compared with the second group of parameters (II), the failure rate of the third group (III) of enhanced vaccines is reduced, the validity period is longer, and the epidemic can be effectively controlled, or even almost disappear. Under the second set of parameters (II), the vaccine is not effective enough, and the epidemic continues. This shows the importance of vaccine effectiveness in controlling outbreaks. In order to better control the epidemic, we need to work to develop a more effective vaccine.
(4) In our simulations above, we select parameters consistent with the current epidemic situation in 2022 and obtain simulation results consistent with the real situation. In fact, our simulations can change with reality, which means that our models are very broad. For example, if a country wants to study epidemic prevention and control strategies for itself, we can bring in the country’s data, take into account the comprehensive strength of the country and the requirements of economic development, analyze the data and select reasonable parameters for simulation to obtain the best time for strengthening vaccination and provide targeted strategies for epidemic control. Our model can also simulate the situation as the virus mutates by changing the infection rate and . Global vaccination is still ongoing, so vaccination rates are constantly changing, and we can change the vaccination rates in the parameters to change our conclusions in real-time. Once the parameters are determined, we can calculate the corresponding critical booster timing and make recommendations that are appropriate to the current epidemic situation.
5.4. Recommendations for Countries
- (i)
For countries with low vaccination rates:
Based on our simulations, it is clear that good control of the epidemic requires people to get the booster vaccine within 1.5 months of getting the basic vaccine. However, a 1.5-month interval between basic and booster vaccination is not feasible in real life given the requirements of the vaccine for people’s health conditions. That means it is very difficult to control COVID-19 in these countries. Therefore, we call on countries with low vaccination rates to increase their vaccination rates as soon as possible so that people pay enough attention to COVID-19;otherwise, it will be difficult to control the epidemic.
- (ii)
For countries with high vaccination rates:
(1) It is clear that timely booster vaccination has a positive impact on controlling the outbreak. Controlling booster vaccination time within a critical period (10.2 months) can make sure the epidemic is under control. Considering the requirements of booster vaccination on the body, we believe that 6 months is the most appropriate time for booster vaccination.
(2) In countries that are already able to get the majority of people who get the basic vaccine on time to get the booster vaccine 6 months later, we can see that there is an upper bound in the number of infections in those countries, which means that the epidemic is contained, and the number of infections does not peak all the time. However, the epidemic is not completely under control. In these countries, the epidemic is cyclical at this stage, with the number of cases going up and down. However, when we improve the effectiveness of the vaccine, which means the duration of the vaccine is longer and the failure rate of the vaccine is lower, the epidemic will be better controlled. The number of cases tends to decrease and almost stabilize after 20 years. So we suggest that research into an effective vaccine should continue, both to increase its longevity and to reduce the vaccine failure rate.
(3) Considering that the virus is still mutating, we suggest that countries make timely policy changes based on the real-time situation of the epidemic.