Genetic Diversity, Nitrogen Fixation, and Water Use Efficiency in a Panel of Honduran Common Bean (Phaseolus vulgaris L.) Landraces and Modern Genotypes
Abstract
:1. Introduction
2. Results
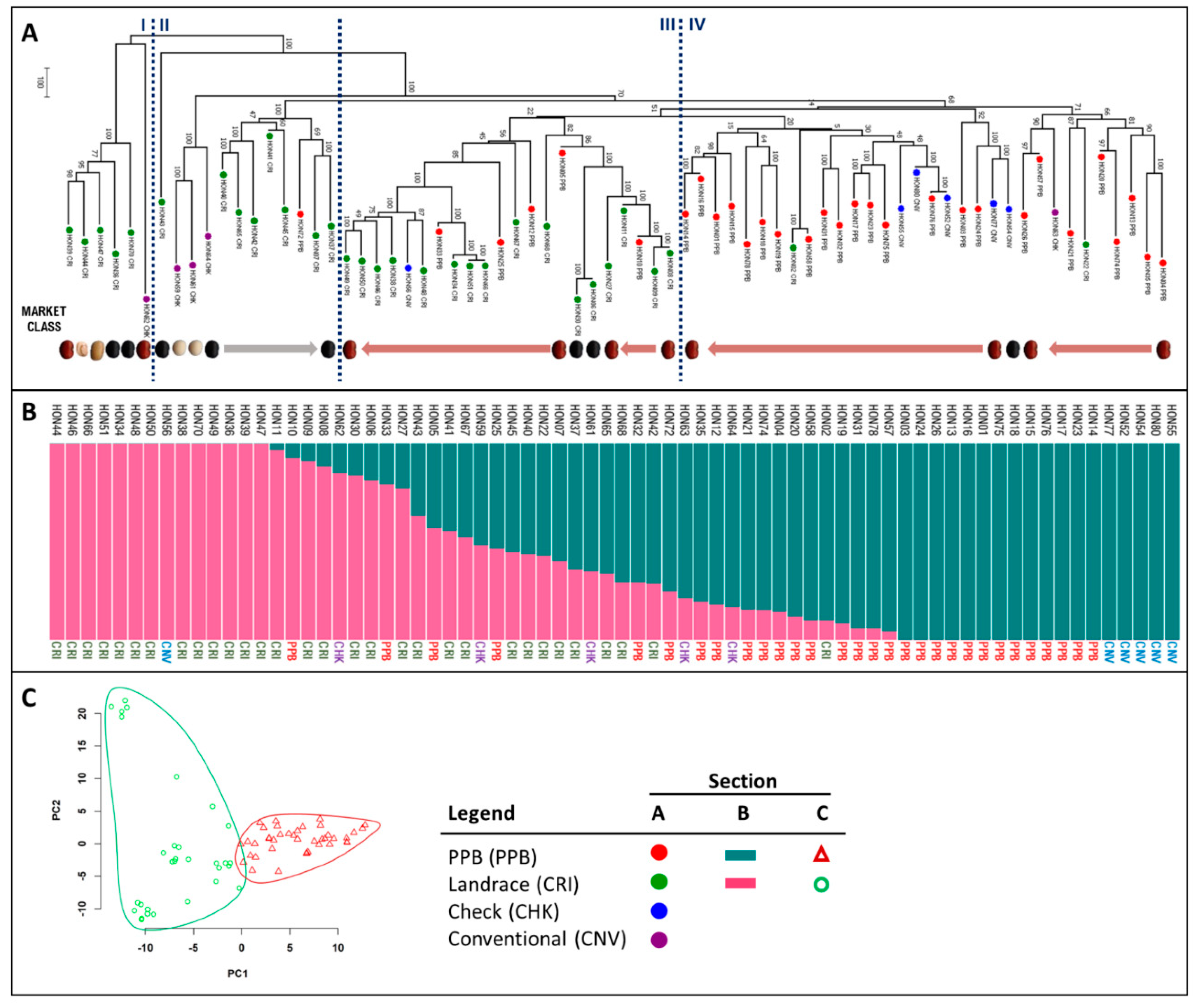
2.1. Analysis of Genetic Relatedness
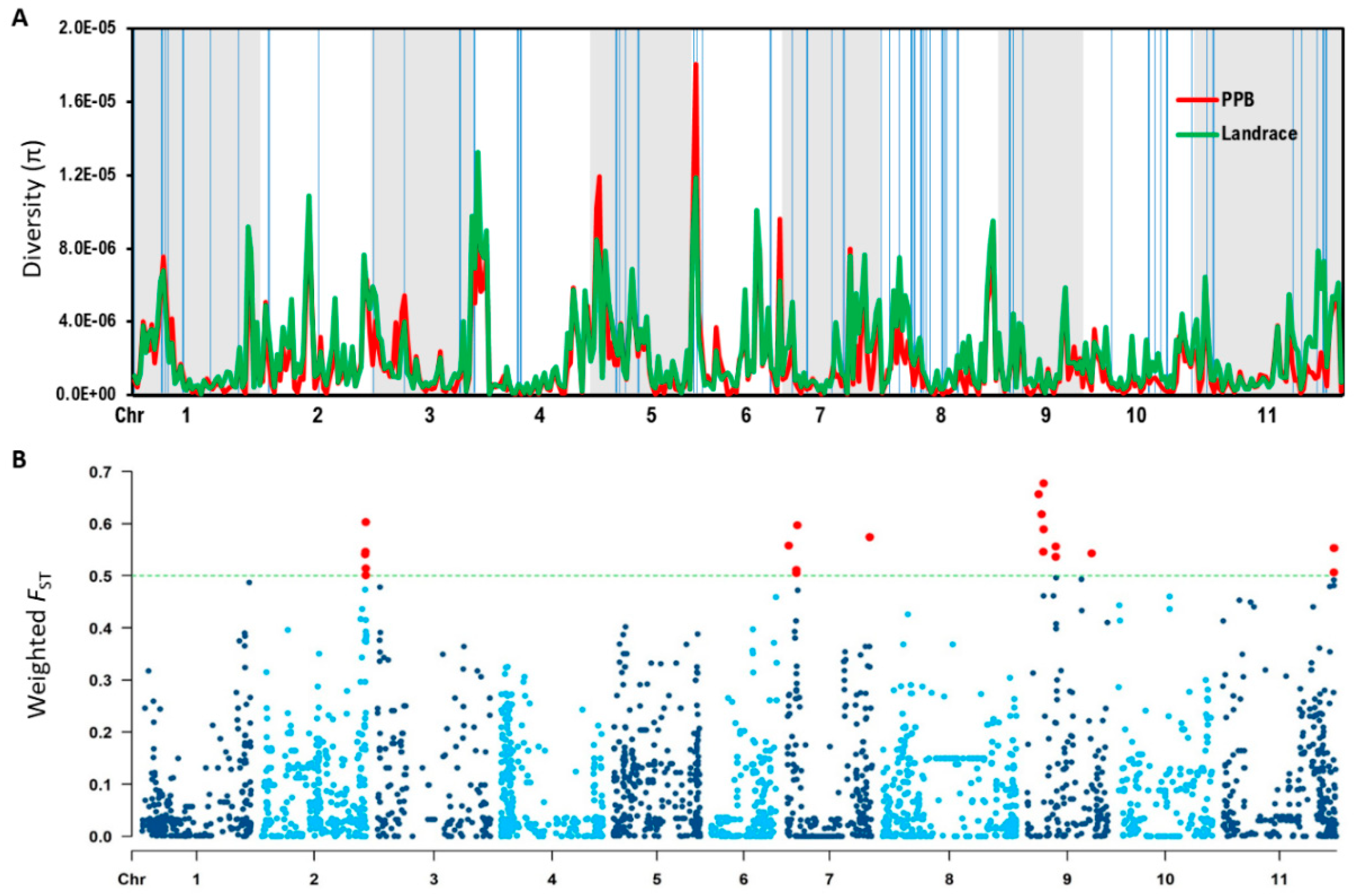
2.2. Nucleotide Diversity and Population Differentiation: Landrace and PPB Categories
2.3. Identification of Candidate Genes
2.4. Diversity for Symbiotic Nitrogen Fixation
2.5. Diversity for Agronomic Traits
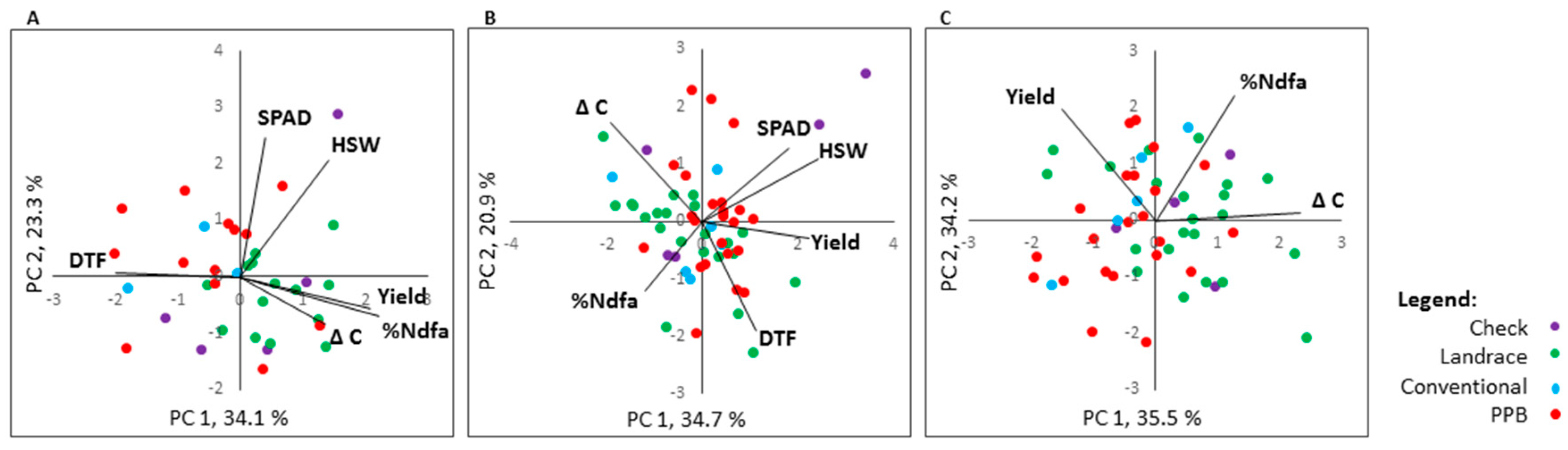
2.6. Trait Correlation and Genotype by Trait Biplot Analyses
2.6.1. %Ndfa
2.6.2. Agronomic Traits
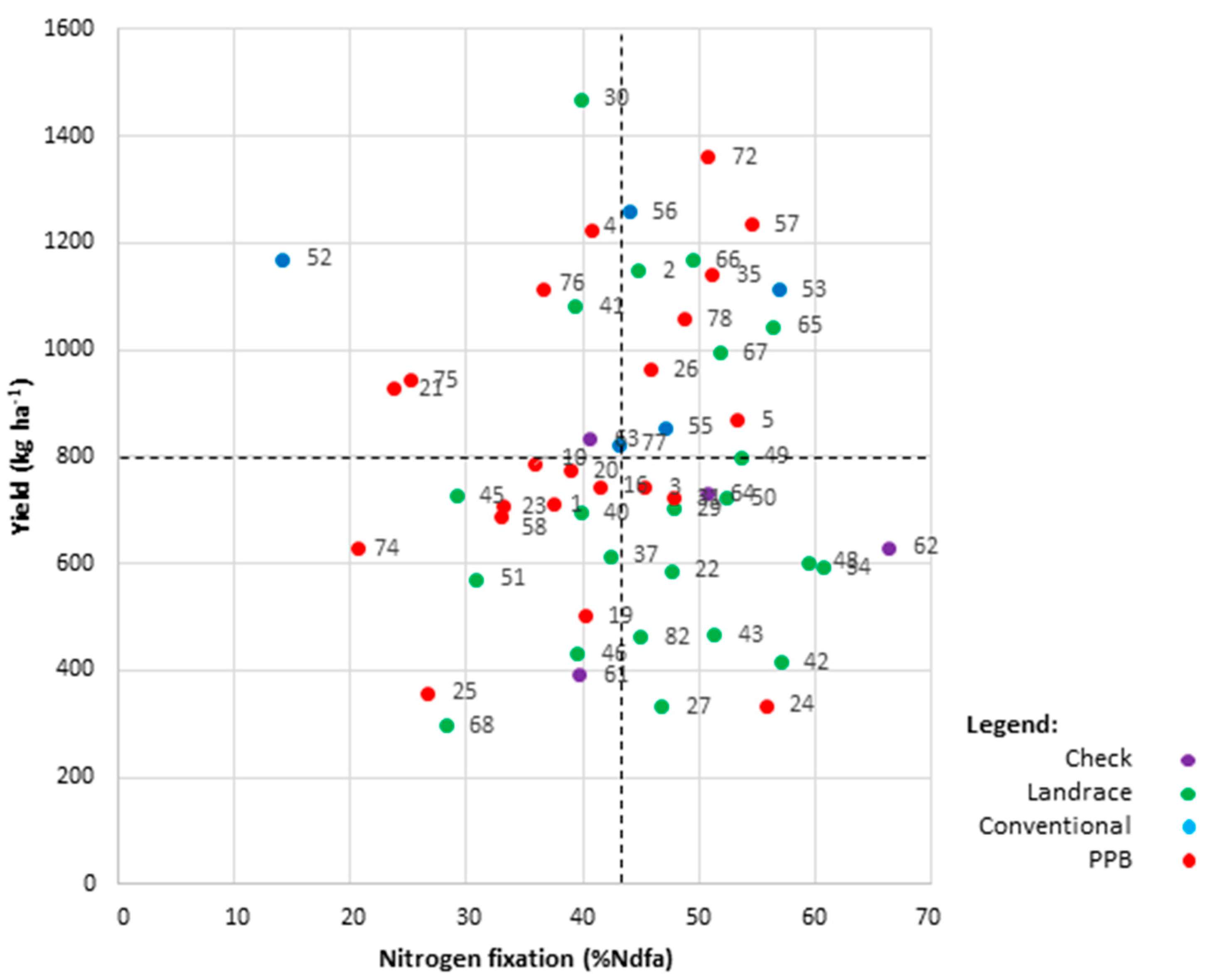
2.6.3. High-Yielding and High-Fixing Genotypes
3. Discussion
3.1. Genotype Origins and Pedigree Explain Honduran Panel Structure
3.2. Optimizing Use of Genetic Diversity of Honduran Landraces and PPB Varieties
3.3. Regions of High Genetic Differentiation Indicate Regions Impacted by Selection
3.4. Landraces are Superior Nitrogen Fixers
3.5. PPB Genotypes Have Superior Water Use Efficiency Values
3.6. Conventional Genotypes Have Superior Yields
3.7. Utility of Panel Genotypes for Breeding
4. Materials and Methods
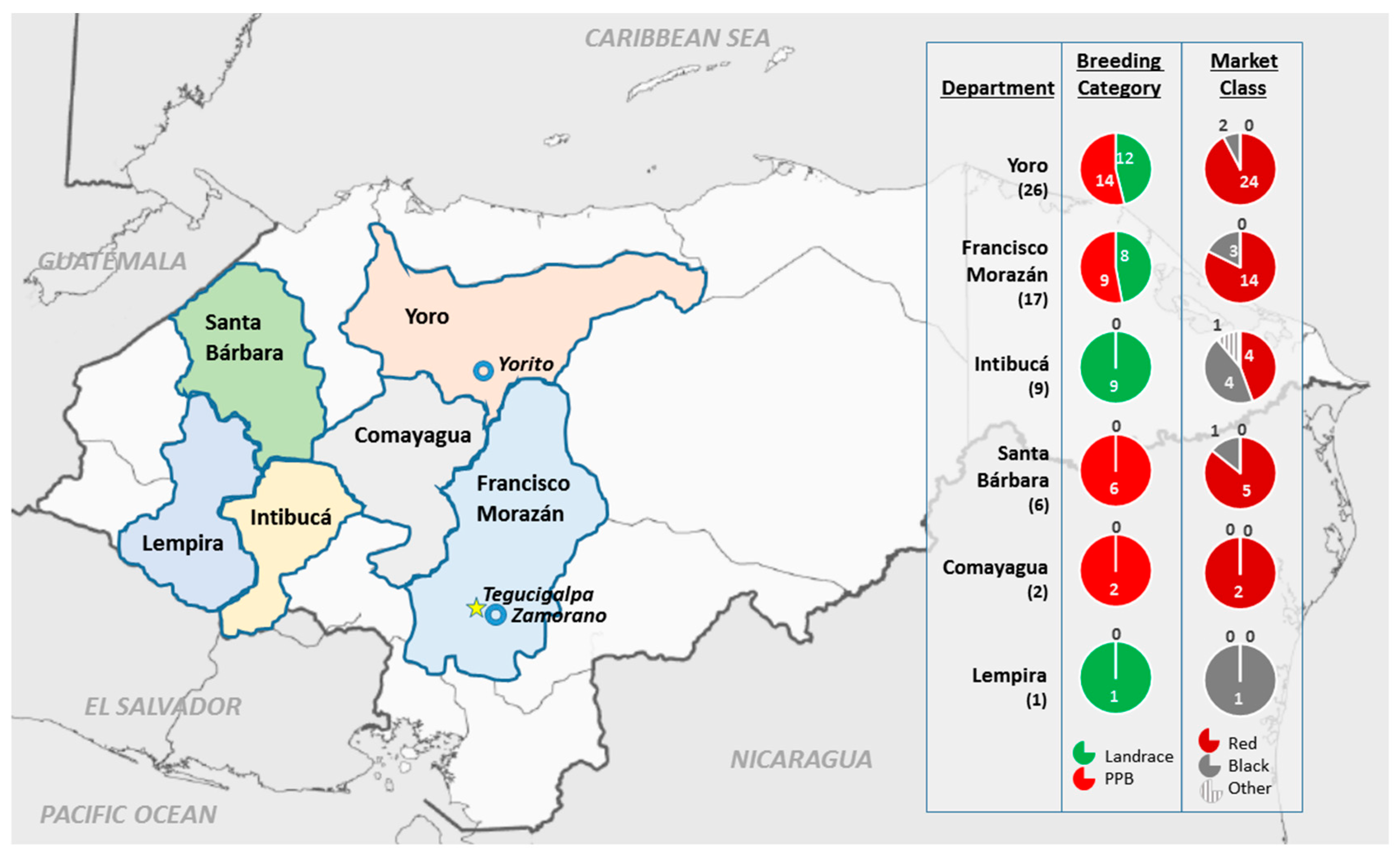
4.1. Plant Material
4.2. Field Experimental Design and Maintenance
4.2.1. Elora
4.2.2. Yorito
4.3. Phenotyping
4.3.1. Elora
4.3.2. Yorito
4.4. Isotope Analysis
4.5. Genotyping
4.6. Population Structure
4.7. Genetic Diversity
4.8. Candidate Gene Investigation
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bitocchi, E.; Rau, D.; Bellucci, E.; Rodriguez, M.; Murgia, M.L.; Gioia, T.; Santo, D.; Nanni, L.; Attene, G.; Papa, R. Beans (Phaseolus ssp.) as a model for understanding crop evolution. Front. Plant Sci. 2017, 8, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bitocchi, E.; Nanni, L.; Bellucci, E.; Rossi, M.; Giardini, A.; Zeuli, P.S.; Logozzo, G.; Stougaard, J.; McClean, P.; Attene, G.; et al. Mesoamerican origin of the common bean (Phaseolus vulgaris L.) is revealed by sequence data. Proc. Natl. Acad. Sci. USA 2012, 109, E788–E796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mamidi, S.; Rossi, M.; Annam, D.; Moghaddam, S.; Lee, R.; Papa, R.; McClean, P. Investigation of the domestication of common bean (Phaseolus vulgaris) using multilocus sequence data. Funct. Plant Biol. 2011, 38, 953–967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mamidi, S.; Rossi, M.; Moghaddam, S.M.; Annam, D.; Lee, R.; Papa, R.; McClean, P.E. Demographic factors shaped diversity in the two gene pools of wild common bean Phaseolus vulgaris L. Heredity 2013, 110, 267–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmutz, J.; McClean, P.E.; Mamidi, S.; Wu, G.A.; Cannon, S.B.; Grimwood, J.; Jenkins, J.; Shu, S.; Song, Q.; Chavarro, C.; et al. A reference genome for common bean and genome-wide analysis of dual domestications. Nat. Genet. 2014, 46, 707–713. [Google Scholar] [CrossRef] [Green Version]
- Gepts, P.; Osborn, T.C.; Rashka, K.; Bliss, F.A. Phaseolin-Protein Variability in Wild Forms and Landraces of the Common Bean (Phaseolus vulgaris): Evidence for Multiple Centers of Domestication. Econ. Bot. 1986, 40, 451–468. [Google Scholar] [CrossRef]
- Bitocchi, E.; Bellucci, E.; Giardini, A.; Rau, D.; Rodriguez, M.; Biagetti, E.; Santilocchi, R.; Spagnoletti Zeuli, P.; Gioia, T.; Logozzo, G.; et al. Molecular analysis of the parallel domestication of the common bean (Phaseolus vulgaris) in Mesoamerica and the Andes. New Phytol. 2013, 197, 300–313. [Google Scholar] [CrossRef]
- Bernsten, R.H. Bean-Growing Environments in Honduras. Available online: https://msu.edu/~bernsten/beanatlas/CountryPages--withGIS/Honduras/2.BeanGrowingEnvironment/hon_text_bean_growinging_environments.htm (accessed on 28 October 2019).
- FAO. FAOSTAT Database, Dry Bean Yield. 2018. Available online: http://www.fao.org/faostat/en/#data (accessed on 22 May 2020).
- Beebe, S.; Ramirez, J.; Jarvis, A.; Rao, I.M.; Mosquera, G.; Bueno, J.M.; Blair, M.W. Genetic Improvement of Common Beans and the Challenges of Climate Change. Crop Adapt. Clim. Chang. 2011, 356–369. [Google Scholar]
- World Bank Group. Climate Change Knowledge Portal, Honduras. Available online: https://climateknowledgeportal.worldbank.org/country/honduras/climate-data-projections (accessed on 14 May 2020).
- Beebe, S. Common Bean Breeding in the Tropics. In Plant Breeding Reviews, Volume 36; Janick, J., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2012; Volume 36, pp. 357–426. ISBN 9781118358566. [Google Scholar]
- Lindstrom, K.; Mousavi, S.A. Effectiveness of nitrogen fixation in rhizobia. Microb. Biotechnol. 2020, 13, 1314–1335. [Google Scholar] [CrossRef] [Green Version]
- Kamfwa, K.; Cichy, K.A.; Kelly, J.D. Genome-wide association analysis of symbiotic nitrogen fixation in common bean. Theor. Appl. Genet. 2015, 128, 1999–2017. [Google Scholar] [CrossRef]
- Diaz, L.M.; Ricaurte, J.; Cajiao, C.; Galeano, C.H.; Rao, I.; Beebe, S.; Raatz, B. Phenotypic evaluation and QTL analysis of yield and symbiotic nitrogen fixation in a common bean population grown with two levels of phosphorus supply. Mol. Breed. 2017, 37, 76. [Google Scholar] [CrossRef]
- Farid, M.; Navabi, A. N2 fixation ability of different dry bean genotypes. Can. J. Plant Sci. 2015, 95, 1243–1257. [Google Scholar] [CrossRef]
- Heilig, J.A.; Wright, E.M.; Kelly, J.D. Symbiotic nitrogen fixation of black and navy bean under organic production systems. Agron. J. 2017, 109, 2223–2230. [Google Scholar] [CrossRef]
- Wilker, J.; Navabi, A.; Rajcan, I.; Marsolais, F.; Hill, B.; Torkamaneh, D.; Pauls, K.P. Agronomic Performance and Nitrogen Fixation of Heirloom and Conventional Dry Bean Varieties Under Low-Nitrogen Field Conditions. Front. Plant Sci. 2019, 10, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanyal, D.; Osorno, J.M.; Chatterjee, A. Influence of Rhizobium inoculation on dry bean yield and symbiotic nitrogen fixation potential and symbiotic nitrogen fixation potential. J. Plant Nutr. 2020, 43, 798–810. [Google Scholar] [CrossRef]
- Aserse, A.A.; Markos, D.; Getachew, G.; Yli-halla, M. Rhizobial inoculation improves drought tolerance, biomass and grain yields of common bean (Phaseolus vulgaris L.) and soybean (Glycine max L.) at Halaba and Boricha in Southern Ethiopia. Arch. Agron. Soil Sci. 2020, 66, 488–501. [Google Scholar] [CrossRef]
- Barbosa, N.; Portilla, E.; Buendia, H.F.; Raatz, B.; Beebe, S.; Rao, I. Genotypic differences in symbiotic nitrogen fixation ability and seed yield of climbing bean. Plant Soil 2018, 428, 223–239. [Google Scholar] [CrossRef] [Green Version]
- Reinprecht, Y.; Schram, L.; Hill, B.; Pauls, K.P. Effects of Nitrogen Application on Nitrogen Fixation in Common Bean Production. Front. Plant Sci. 2020, 11, 1172. [Google Scholar] [CrossRef]
- Humphries, S.; Gallardo, O.; Jiménez, J.; Sierra, F. Linking Small Farmers to the Formal Research Sector: Lessons from a Participatory Bean Breeding Programme in Honduras; AgREN: Odisha, India, 2005. [Google Scholar]
- Villa, T.C.C.; Maxted, N.; Scholten, M.; Ford-Lloyd, B. Defining and identifying crop landraces. Plant Genet. Resour. 2005, 3, 373–384. [Google Scholar] [CrossRef] [Green Version]
- Mather, D.L.; Bernsten, R.; Rosas, J.C.; Viana Ruano, A.; Escoto, D. The economic impact of bean disease resistance research in Honduras. Agric. Econ. 2003, 29, 343–352. [Google Scholar] [CrossRef]
- Reyes, B.A. The Economic Impact of Improved Bean Varieties and Determinants of Market Participation: Evidence from Latin America and Angola; Michigan State University: East Lansing, MI, USA, 2012. [Google Scholar]
- Ceccarelli, S.; Grando, S.; Singh, M.; Michael, M.; Shikho, A.; Al Issa, M.; Al Saleh, A.; Kaleonjy, G.; Al Ghanem, S.M.; Al Hasan, A.L.; et al. A methodological study on participatory barley breeding I. Selection phase. Euphytica 2000, 133, 185–200. [Google Scholar] [CrossRef]
- Almekinders, C.J.M.; Elings, A. Collaboration of farmers and breeders: Participatory crop improvement in perspective. Euphytica 2001, 122, 425–438. [Google Scholar] [CrossRef]
- Humphries, S.; Rosas, J.C.; Gómez, M.; Jiménez, J.; Sierra, F.; Gallardo, O.; Avila, C.; Barahona, M. Synergies at the interface of farmer-scientist partnerships: Agricultural innovation through participatory research and plant breeding in Honduras. Agric. Food Secur. 2015, 4, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Gomez, M.; Rosas, J.C.; Humphries, S.; Jiménez, J.; Barahona, M.; Avila, C.; Orellana, P.; Sierra, F. Pushing back against bureaucracy: Farmers’ role in decentralizing plant breeding and seed production in Honduras. In Farmers and Plant Breeding; Westengen, O.T., Winge, T., Eds.; Routledge: London, UK, 2020. [Google Scholar]
- Rosas, J.C. Contribuciones del Programa de Investigaciones en Frijol en Centro América y El Caribe. Ceiba 2013, 52, 65–73. [Google Scholar] [CrossRef]
- Moghaddam, S.M.; Mamidi, S.; Osorno, J.M.; Lee, R.; Brick, M.; Kelly, J.; Miklas, P.; Urrea, C.; Song, Q.; Cregan, P.; et al. Genome-Wide Association Study Identifies Candidate Loci Underlying Agronomic Traits in a Middle American Diversity Panel of Common Bean. Plant Genome 2016, 9, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Heilig, J.A.; Beaver, J.S.; Wright, E.M.; Song, Q.; Kelly, J.D. QTL analysis of symbiotic nitrogen fixation in a black bean population. Crop Sci. 2017, 57, 118–129. [Google Scholar] [CrossRef]
- Cichy, K.A.; Porch, T.G.; Beaver, J.S.; Cregan, P.; Fourie, D.; Glahn, R.P.; Grusak, M.A.; Kamfwa, K.; Katuuramu, D.N.; McClean, P.; et al. A Phaseolus vulgaris Diversity Panel for Andean Bean Improvement. Crop Sci. 2015, 55, 2149–2160. [Google Scholar] [CrossRef] [Green Version]
- Kamfwa, K.; Cichy, K.A.; Kelly, J.D. Identification of quantitative trait loci for symbiotic nitrogen fixation in common bean. Theor. Appl. Genet. 2019, 132, 1375–1387. [Google Scholar] [CrossRef]
- Farid, M. Symbiotic Nitrogen Fixation in Common Bean; University of Guelph: Guelph, ON, Canada, 2015. [Google Scholar]
- Flores, A.C.; Via, V.D.; Savy, V.; Villagra, U.M.; Zanetti, M.E.; Blanco, F. Comparative phylogenetic and expression analysis of small GTPases families in legume and non-legume plants. Plant Signal. Behav. 2018, 13, e1432956. [Google Scholar] [CrossRef] [Green Version]
- Guillén, G.; Díaz-camino, C.; Loyola-torres, C.A.; Aparicio-fabre, R.; Hernández-lópez, A.; Díaz-sánchez, M.; Sanchez, F. Detailed analysis of putative genes encoding small proteins in legume genomes. Front. Plant Sci. 2013, 4, 208. [Google Scholar]
- Rípodas, C.; Castaingts, M.; Clúa, J.; Blanco, F.; Zanetti, M.E. Annotation, phylogeny and expression analysis of the nuclear factor Y gene families in common bean (Phaseolus vulgaris). Front. Plant Sci. 2015, 5, 761. [Google Scholar]
- Oladzad, A.; Porch, T.; Rosas, J.C.; Moghaddam, S.M.; Beaver, J.; Beebe, S.E.; Burridge, J.; Jochua, C.N.; Miguel, M.A.; Miklas, P.N.; et al. Single and multi-trait GWAS identify genetic factors associated with production traits in common bean under abiotic stress environments. G3 Genes Genomes Genet. 2019, 9, 1881–1892. [Google Scholar]
- Tock, A.J.; Fourie, D.; Walley, P.G.; Holub, E.B.; Soler, A.; Cichy, K.A.; Pastor-Corrales, M.A.; Song, Q.; Porch, T.G.; Hart, J.P.; et al. Genome-wide linkage and association mapping of halo blight resistance in common bean to race 6 of the globally important bacterial pathogen. Front. Plant Sci. 2017, 8, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nova-Franco, B.; Íñiguez, L.P.; Valdés-López, O.; Alvarado-Affantranger, X.; Leija, A.; Fuentes, S.I.; Ramírez, M.; Paul, S.; Reyes, J.L.; Girard, L.; et al. The micro-RNA72c-APETALA2-1 node as a key regulator of the common bean-Rhizobium etli nitrogen fixation symbiosis. Plant Physiol. 2015, 168, 273–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuiderveen, G.H.; Padder, B.A.; Kamfwa, K.; Song, Q.; Kelly, J.D. Genome-Wide association study of anthracnose resistance in andean beans (Phaseolus vulgaris). PLoS ONE 2016, 11, e0156391. [Google Scholar] [CrossRef]
- Duwadi, K.; Austin, R.S.; Mainali, H.R.; Bett, K.; Marsolais, F.; Dhaubhadel, S. Slow darkening of pinto bean seed coat is associated with significant metabolite and transcript differences related to proanthocyanidin biosynthesis. BMC Genom. 2018, 19, 1–13. [Google Scholar] [CrossRef]
- Zhang, C.; Hou, Y.; Hao, Q.; Chen, H.; Chen, L.; Yuan, S.; Shan, Z.; Zhang, X.; Yang, Z.; Qiu, D.; et al. Genome-wide survey of the soybean GATA transcription factor gene family and expression analysis under low nitrogen stress. PLoS ONE 2015, 10, e0125174. [Google Scholar] [CrossRef] [Green Version]
- Recchia, G.H.; Konzen, E.R.; Cassieri, F.; Caldas, D.G.G.; Tsai, S.M. Arbuscular mycorrhizal symbiosis leads to differential regulation of drought-responsive genes in tissue-specific root cells of common bean. Front. Microbiol. 2018, 9, 1–24. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Ehleringer, J.R.; Hubick, K.T. Carbon Isotope Discrimination and Photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 337–503. [Google Scholar] [CrossRef]
- Wallace, D.H.; Yourstone, K.S.; Masaya, P.N.; Zobel, R.W. Photoperiod gene control over partitioning between reproductive and vegetative growth. Theor. Appl. Genet. 1993, 86, 6–16. [Google Scholar] [CrossRef]
- Rosas, J.C.; Beaver, J.S.; Beebe, S.; Viana, A. Nomenclatura de variedades de frijol común liberadas en Centro América y El Caribe. Agron. Mesoam. 2004, 15, 221–224. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.P. Chapter 2: Common Bean (Phaseolus vulgaris L.). In Genetic Resources, Chromosome Engineering, and Crop Improvement: Grain Legumes, Volume 1; Singh, R.J., Jauhar, P.P., Eds.; Taylor & Francis: Boca Raton, FL, USA, 2005; p. 32. [Google Scholar]
- Haudry, A.; Cenci, A.; Ravel, C.; Bataillon, T.; Brunel, D.; Poncet, C.; Hochu, I.; Poirier, S.; Santoni, S.; Glémin, S.; et al. Grinding up wheat: A massive loss of nucleotide diversity since domestication. Mol. Biol. Evol. 2007, 24, 1506–1517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maron, L.G.; Guimarães, C.T.; Kirst, M.; Albert, P.S.; Birchler, J.A.; Bradbury, P.J.; Buckler, E.S.; Coluccio, A.E.; Danilova, T.V.; Kudrna, D.; et al. Aluminum tolerance in maize is associated with higher MATE1 gene copy number. Proc. Natl. Acad. Sci. USA 2013, 110, 5241–5246. [Google Scholar] [CrossRef] [Green Version]
- Bellucci, E.; Bitocchi, E.; Ferrarini, A.; Benazzo, A.; Biagetti, E.; Klie, S.; Minio, A.; Rau, D.; Rodriguez, M.; Panziera, A.; et al. Decreased nucleotide and expression diversity and modified coexpression patterns characterize domestication in the common bean. Plant Cell 2014, 26, 1901–1912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Z.; Jiang, Y.; Wang, Z.; Gou, Z.; Lyu, J.; Li, W.; Yu, Y.; Shu, L.; Zhao, Y.; Ma, Y.; et al. Resequencing 302 wild and cultivated accessions identifies genes related to domestication and improvement in soybean. Nat. Biotechnol. 2015, 33, 408–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nanni, L.; Bitocchi, E.; Bellucci, E.; Rossi, M.; Rau, D.; Attene, G.; Gepts, P.; Papa, R. Nucleotide diversity of a genomic sequence similar to SHATTERPROOF (PvSHP1) in domesticated and wild common bean (Phaseolus vulgaris L.). Theor. Appl. Genet. 2011, 123, 1341–1357. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, S.L.; Ceccarelli, S.; Blair, M.W.; Upadhyaya, H.D.; Are, A.K.; Ortiz, R. Landrace Germplasm for Improving Yield and Abiotic Stress Adaptation. Trends Plant Sci. 2016, 21, 31–42. [Google Scholar] [CrossRef]
- Ceccarelli, S. Adaptation to low/high input cultivation. Euphytica 1996, 92, 203–214. [Google Scholar] [CrossRef]
- Ceccarelli, S. Specific adaptation and breeding for marginal conditions. Euphytica 1994, 77, 205–219. [Google Scholar] [CrossRef]
- Beebe, S.; Skroch, P.W.; Tohme, J.; Duque, M.C.; Pedraza, F.; Nienhuis, J. Structure of genetic diversity among common bean landraces of Middle American origin based on correspondence analysis of RAPD. Crop Sci. 2000, 40, 264–273. [Google Scholar] [CrossRef]
- Muñoz-Perea, C.G.; Terán, H.; Allen, R.G.; Wright, J.L.; Westermann, D.T.; Singh, S.P. Selection for drought resistance in dry bean landraces and cultivars. Crop Sci. 2006, 46, 2111–2120. [Google Scholar] [CrossRef] [Green Version]
- Bliss, F.A. Breding common bean for improved biological nitrogen fixation. Plant Soil 1993, 152, 71–79. [Google Scholar] [CrossRef]
- Broughton, W.J.; Hernández, G.; Blair, M.; Beebe, S.; Gepts, P.; Vanderleyden, J. Beans (Phaseolus spp.)—Model food legumes. Plant Soil 2003, 252, 55–128. [Google Scholar] [CrossRef] [Green Version]
- Hurtado-Gonzales, O.P.; Gilio, T.A.S.; Pastor-Corrales, M.A. Resistant Reaction of Andean Common Bean Lamdrace G19833, Reference Genome, to 13 Races of Uromyces appendiculatus Suggests Broad Spectrum Rust Resistance. In Proceedings of the Annual report of the Bean Improvement Cooperative, East Lansing, MI, USA, 30 October–1 November 2017; pp. 27–28. [Google Scholar]
- Lioi, L.; Zuluaga, D.L.; Pavan, S.; Sonnante, G. Genotyping-by-sequencing reveals molecular genetic diversity in Italian common bean landraces. Diversity 2019, 11, 154. [Google Scholar] [CrossRef] [Green Version]
- Papa, R.; Acosta, J.; Delgado-Salinas, A.; Gepts, P. A genome-wide analysis of differentiation between wild and domesticated Phaseolus vulgaris from Mesoamerica. Theor. Appl. Genet. 2005, 111, 1147–1158. [Google Scholar] [CrossRef] [PubMed]
- Padder, B.A.; Kamfwa, K.; Awale, H.E.; Kelly, J.D. Transcriptome profiling of the Phaseolus vulgaris—Colletotrichum lindemuthianum pathosystem. PLoS ONE 2016, 11, e0165823. [Google Scholar] [CrossRef]
- Ayyappan, V.; Kalavacharla, V.; Thimmapuram, J.; Bhide, K.P.; Sripathi, V.R.; Smolinski, T.G.; Manoharan, M.; Thurston, Y.; Todd, A.; Kingham, B.; et al. Genome-wide profiling of histone modifications (H3K9me2 and H4K12ac) and gene expression in rust (uromyces appendiculatus) inoculated common bean (Phaseolus vulgaris L.). PLoS ONE 2015, 10, e0132176. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, M.; Stiller, J.; Liu, C. A pan-transcriptome analysis shows that disease resistance genes have undergone more selection pressure during barley domestication 06 Biological Sciences 0604 Genetics. BMC Genom. 2019, 20, 1–11. [Google Scholar] [CrossRef]
- Zheng, F.; Wu, H.; Zhang, R.; Li, S.; He, W.; Wong, F.L.; Li, G.; Zhao, S.; Lam, H.M. Molecular phylogeny and dynamic evolution of disease resistance genes in the legume family. BMC Genom. 2016, 17, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Varshney, R.K.; Saxena, R.K.; Upadhyaya, H.D.; Khan, A.W.; Yu, Y.; Kim, C.; Rathore, A.; Kim, D.; Kim, J.; An, S.; et al. Whole-genome resequencing of 292 pigeonpea accessions identifies genomic regions associated with domestication and agronomic traits. Nat. Genet. 2017, 49, 1082–1088. [Google Scholar] [CrossRef]
- Varshney, R.K.; Thudi, M.; Roorkiwal, M.; He, W.; Upadhyaya, H.D.; Yang, W.; Bajaj, P.; Cubry, P.; Rathore, A.; Jian, J.; et al. Resequencing of 429 chickpea accessions from 45 countries provides insights into genome diversity, domestication and agronomic traits. Nat. Genet. 2019, 51, 857–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Zhang, J.; He, X.; Wang, Y.; Ma, X.; Yin, D. Genome-wide association study of major agronomic traits related to domestication in peanut. Front. Plant Sci. 2017, 8, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soltani, A.; Mafimoghaddam, S.; Oladzad-Abbasabadi, A.; Walter, K.; Kearns, P.J.; Vasquez-Guzman, J.; Mamidi, S.; Lee, R.; Shade, A.L.; Jacobs, J.L.; et al. Genetic analysis of flooding tolerance in an andean diversity panel of dry bean (Phaseolus vulgaris L.). Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.; Sun, F.; Gao, R.; Dong, H. RAP2.6L overexpression delays waterlogging induced premature senescence by increasing stomatal closure more than antioxidant enzyme activity. Plant Mol. Biol. 2012, 79, 609–622. [Google Scholar] [CrossRef]
- da Silva, D.A.; Tsai, S.M.; Chiorato, A.F.; da Silva Andrade, S.C.; de Fatima Esteves, J.A.; Recchia, G.H.; Carbonell, S.A.M. Analysis of the common bean (Phaseolus vulgaris L.) transcriptome regarding efficiency of phosphorus use. PLoS ONE 2019, 14, e0210428. [Google Scholar] [CrossRef] [PubMed]
- Henry, R.J.; Nevo, E. Exploring natural selection to guide breeding for agriculture. Plant Biotechnol. J. 2014, 12, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Henry, R.J. Innovations in plant genetics adapting agriculture to climate change. Curr. Opin. Plant Biol. 2019. [Google Scholar] [CrossRef]
- Chaverra, M.H.; Graham, P.H. Cultivar Variation in Traits Affecting Early Nodulation of Common Bean. Crop Sci. 1992, 32, 1432–1436. [Google Scholar] [CrossRef]
- Moghaddam, A.; Raza, A.; Vollmann, J.; Ardakani, M.R.; Wanek, W.; Gollner, G.; Friedel, J.K. Carbon isotope discrimination and water use efficiency relationships of alfalfa genotypes under irrigated and rain-fed organic farming. Eur. J. Agron. 2013, 50, 82–89. [Google Scholar] [CrossRef]
- Muñoz-Perea, C.G.; Allen, R.G.; Westermann, D.T.; Wright, J.L.; Singh, S.P. Water use efficiency among dry bean landraces and cultivars in drought-stressed and non-stressed environments. Euphytica 2007, 155, 393–402. [Google Scholar] [CrossRef]
- Drought-Resistant Bean Variety Offers Hope to Central American Communities. Available online: https://www.canr.msu.edu/legumelab/uploads/files/Success_Drought_resistant_beans_Central_America.pdf (accessed on 9 September 2020).
- Lopes, M.S.; El-Basyoni, I.; Baenziger, P.S.; Singh, S.; Royo, C.; Ozbek, K.; Aktas, H.; Ozer, E.; Ozdemir, F.; Manickavelu, A.; et al. Exploiting genetic diversity from landraces in wheat breeding for adaptation to climate change. J. Exp. Bot. 2015, 66, 3477–3486. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Gepts, P.; Debouck, D.G. Races of Common Bean (Phaseolus vulgaris, Fabaceae). Econ. Bot. 1991, 45, 379–396. [Google Scholar] [CrossRef]
- Beversdorf, W. OAC Rico Field Bean. Can. J. Plant Sci. 1984, 64, 753–755. [Google Scholar] [CrossRef] [Green Version]
- Park, S.J.; Buttery, B.R. Registration of Ineffective Nodulation Mutant R69 and Nonnodulation Mutant R99 Common Bean Genetic Stocks. Crop Sci. 2006, 46, 1415–1417. [Google Scholar] [CrossRef]
- Park, S.J.; Buttery, B.R. Ethyl-methane sulphonate (EMS) induced nodulation mutants of common bean (Phaseolus vulgaris L.) lacking effective nodules. Plant Soil 1992, 139, 295–298. [Google Scholar] [CrossRef]
- Khanal, R.; Rupert, T.; Navabi, A.; Smith, T.H.; Michaels, T.E.; Burt, A.J.; Pauls, K.P.; Rex, O.A.C. Cultivar Description: Mist Common Bean. Can. J. Plant Sci. 2017, 97, 192–195. [Google Scholar]
- Hosfield, G.L.; Varner, G.V.; Uebersax, M.A.; Kelly, J.D. Registration of ‘Merlot’ Small Red Bean. Crop Sci. 2004, 44, 351–352. [Google Scholar] [CrossRef]
- Aguilera, A.; Smith, T.H.; Pauls, K.P. Cultivar Description: Small Red Bean ‘OAC Rosito’. Available online: https://www.gobeans.ca/downloads/OAC_Rosito-SmallRed.pdf (accessed on 15 November 2019).
- Kelly, J.D.; Varner, G.V.; O’Boyle, P.; Long, B. Registration of ‘Zorro’ Black Bean. J. Plant Regist. 2009, 3, 226–230. [Google Scholar] [CrossRef]
- Jamora, N.; Maredia, M. Database of Improved Beans and Cowpea Varietal Releases in Major Bean and Cowpea Producing Countries in Africa. Lat. Am. US 2010. [Google Scholar]
- Beaver, J.S.; Rosas, J.C.; Myers, J.; Acosta, J.; Kelly, J.D.; Nchimbi-Msolla, S.; Misangu, R.; Bokosi, J.; Temple, S.; Arnaud-Santana, E.; et al. Contributions of the Bean/Cowpea CRSP to cultivar and germplasm development in common bean. Field Crops Res. 2003, 82, 87–102. [Google Scholar] [CrossRef]
- Rosas, J.C.; Vargas, A.G. Rhizobium Biofertilizante para el frijol común.
- Shearer, G.; Kohl, D.H. Natural 15N abundance as a method of estimating the contribution of biologically fixed nitrogen to N2-fixing systems: Potential for non-legumes. Plant Soil 1988, 110, 317–327. [Google Scholar] [CrossRef]
- Peoples, M.B.; Unkovich, M.J.; Herridge, D.F. Measuring Symbiotic Nitrogen Fixation in Legumes. In Nitrogen Fixation in Crop Production; Emerich, D.W., Krishnan, H.B., Eds.; American Society of Agronomy: Madison, WI, USA, 2009. [Google Scholar]
- Song, Q.; Jia, G.; Hyten, D.L.; Jenkins, J.; Hwang, E.Y.; Schroeder, S.G.; Osorno, J.M.; Schmutz, J.; Jackson, S.A.; McClean, P.E.; et al. SNP assay development for linkage map construction, anchoring whole-genome sequence, and other genetic and genomic applications in common bean. G3 Genes Genomes Genet. 2015, 5, 2285–2290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradbury, P.J.; Zhang, Z.; Kroon, D.E.; Casstevens, T.M.; Ramdoss, Y.; Buckler, E.S. TASSEL: Software for association mapping of complex traits in diverse samples. Bioinformatics 2007, 23, 2633–2635. [Google Scholar] [CrossRef] [PubMed]
- Browning, B.L.; Browning, S.R. Genotype Imputation with Millions of Reference Samples. Am. J. Hum. Genet. 2016, 98, 116–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torkamaneh, D.; Belzile, F. Scanning and Filling: Ultra-Dense SNP Genotyping Combining Genotyping-by-Sequencing, SNP Array and Whole-Genome Resequencing Data. PLoS ONE 2015, 10, e0131533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raj, A.; Stephens, M.; Pritchard, J.K. FastSTRUCTURE: Variational inference of population structure in large SNP data sets. Genetics 2014, 197, 573–589. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Felsenstein, J. Confidence Limits on Phylogenies: An Approach Using the Bootstrap. Evolution 1985, 39, 783. [Google Scholar] [CrossRef]
- Tamura, K.; Nei, M.; Kumar, S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc. Natl. Acad. Sci. USA 2004, 101, 11030–11035. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Danecek, P.; Auton, A.; Abecasis, G.; Albers, C.A.; Banks, E.; DePristo, M.A.; Handsaker, R.E.; Lunter, G.; Marth, G.T.; Sherry, S.T.; et al. The variant call format and VCFtools. Bioinformatics 2011, 27, 2156–2158. [Google Scholar] [CrossRef] [PubMed]
- Nei, M.; Li, W.H. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc. Natl. Acad. Sci. USA 1979, 76, 5269–5273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tajima, F. Statistical Method for Testing the Neutral Mutation Hypothesis by DNA Polymorphism. Genetics 1989, 123, 585–595. [Google Scholar] [PubMed]
- Shapiro, A.S.S.; Wilk, M.B. An Analysis of Variance Test for Normality (Complete Samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Yan, W.; Rajcan, I. Biplot analysis of test sites and trait relations of soybean in Ontario. Crop Sci. 2002, 42, 11–20. [Google Scholar] [CrossRef]
- Martin, K.; Singh, J.; Hill, J.H.; Whitham, S.A.; Cannon, S.B. Dynamic transcriptome profiling of Bean Common Mosaic Virus (BCMV) infection in Common Bean (Phaseolus vulgaris L.). BMC Genom. 2016, 17. [Google Scholar] [CrossRef] [Green Version]
- Mohanta, T.K.; Kumar, P.; Bae, H. Genomics and evolutionary aspect of calcium signaling event in calmodulin and calmodulin-like proteins in plants. BMC Plant Biol. 2017, 17, 38. [Google Scholar] [CrossRef] [Green Version]
- De Souza, L.P.; Scossa, F.; Proost, S.; Bitocchi, E.; Papa, R.; Tohge, T.; Fernie, A.R. Multi-tissue integration of transcriptomic and specialized metabolite profiling provides tools for assessing the common bean (Phaseolus vulgaris) metabolome. Plant J. 2019, 97, 1132–1153. [Google Scholar] [CrossRef] [Green Version]
- Konzen, E.R.; Recchia, G.H.; Cassieri, F.; Gomes Caldas, D.G.; Berny Mier, Y.; Teran, J.C.; Gepts, P.; Tsai, S.M. DREB genes from common bean (Phaseolus vulgaris L.) show broad to specific abiotic stress responses and distinct levels of nucleotide diversity. Int. J. Genom. 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, A.M.; Godoy, L.; Santalla, M. Dissection of Resistance Genes to Pseudomonas syringae pv. phaseolicola in UI3 Common Bean Cultivar. Int. J. Mol. Sci. 2017, 18, 2503. [Google Scholar] [CrossRef] [Green Version]
- MacQueen, A.H.; White, J.W.; Lee, R.; Osorno, J.M.; Schmutz, J.; Miklas, P.N.; Myers, J.; McClean, P.E.; Juenger, T.E. Genetic Associations in Four Decades of Multi-Environment Trials Reveal Agronomic Trait Evolution in Common Bean. Genetics 2020, 215, 267–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Przysiecka, Ł.; Książkiewicz, M.; Wolko, B.; Naganowska, B. Structure, expression profile and phylogenetic inference of chalcone isomerase-like genes from the narrow-leafed lupin (Lupinus angustifolius L.) genome. Front. Plant Sci. 2015, 6, 268. [Google Scholar] [CrossRef] [PubMed]
| Region of High Diversity | π Value | Candidate Genes | |||
|---|---|---|---|---|---|
| Chr | Start (Mbp) | End (Mbp) | Landrace | PPB | Number |
| 1 | 23 | 24 | 5.50 × 10−7 | 1.31 × 10−7 | 2 |
| 1 | 40 | 41 | 7.71 × 10−7 | 1.83 × 10−7 | 4 |
| 1 | 41 | 42 | 1.24 × 10−6 | 2.49 × 10−7 | 4 |
| 1 | 42 | 43 | 1.22 × 10−6 | 3.98 × 10−7 | 1 |
| 1 | 47 | 48 | 8.60 × 10−7 | 9.66 × 10−8 | 3 |
| 2 | 4 | 5 | 1.95 × 10−6 | 6.51 × 10−7 | 0 |
| 2 | 22 | 23 | 1.71 × 10−6 | 1.64 × 10−7 | 8 |
| 2 | 32 | 33 | 5.53 × 10−7 | 1.22 × 10−7 | 5 |
| 2 | 48 | 49 | 5.92 × 10−6 | 1.62 × 10−6 | 18 |
| 3 | 34 | 35 | 6.73 × 10−7 | 1.97 × 10−7 | 8 |
| 3 | 48 | 49 | 1.17 × 10−6 | 1.89 × 10−7 | 8 |
| 4 | 12 | 13 | 1.53 × 10−6 | 2.62 × 10−7 | 0 |
| 4 | 17 | 18 | 4.05 × 10−7 | 6.55 × 10−8 | 0 |
| 4 | 38 | 39 | 4.74 × 10−7 | 9.66 × 10−8 | 6 |
| 4 | 40 | 41 | 4.32 × 10−7 | 1.27 × 10−7 | 0 |
| 5 | 24 | 25 | 3.55 × 10−7 | 6.55 × 10−8 | 1 |
| 5 | 25 | 26 | 2.10 × 10−6 | 1.92 × 10−7 | 0 |
| 5 | 27 | 28 | 7.46 × 10−7 | 1.31 × 10−7 | 1 |
| 5 | 32 | 33 | 1.16 × 10−6 | 3.28 × 10−7 | 11 |
| 6 | 9 | 10 | 1.21 × 10−6 | 6.55 × 10−8 | 7 |
| 6 | 13 | 14 | 1.02 × 10−6 | 1.31 × 10−7 | 14 |
| 6 | 15 | 16 | 4.82 × 10−7 | 1.55 × 10−7 | 6 |
| 7 | 8 | 9 | 4.32 × 10−7 | 9.66 × 10−8 | 1 |
| 7 | 20 | 21 | 5.27 × 10−7 | 6.55 × 10−8 | 1 |
| 7 | 26 | 27 | 3.98 × 10−6 | 1.01 × 10−6 | 0 |
| 7 | 35 | 36 | 3.36 × 10−6 | 7.38 × 10−7 | 9 |
| 7 | 39 | 40 | 2.43 × 10−6 | 3.32 × 10−7 | 8 |
| 8 | 11 | 12 | 5.44 × 10−6 | 1.74 × 10−6 | 1 |
| 8 | 15 | 16 | 1.63 × 10−6 | 5.32 × 10−7 | 2 |
| 8 | 18 | 19 | 1.30 × 10−6 | 3.98 × 10−7 | 1 |
| 8 | 23 | 24 | 5.27 × 10−7 | 6.55 × 10−8 | 0 |
| 8 | 29 | 30 | 1.35 × 10−6 | 3.32 × 10−7 | 0 |
| 8 | 38 | 39 | 3.29 × 10−7 | 3.33 × 10−8 | 0 |
| 8 | 41 | 42 | 7.62 × 10−7 | 2.26 × 10−7 | 2 |
| 8 | 44 | 45 | 1.35 × 10−6 | 1.62 × 10−7 | 0 |
| 8 | 45 | 46 | 9.15 × 10−7 | 2.90 × 10−7 | 0 |
| 8 | 51 | 52 | 2.86 × 10−6 | 6.33 × 10−7 | 6 |
| 8 | 52 | 53 | 1.21 × 10−6 | 9.66 × 10−8 | 12 |
| 8 | 57 | 58 | 3.45 × 10−7 | 6.55 × 10−8 | 0 |
| 9 | 16 | 17 | 7.81 × 10−7 | 1.60 × 10−7 | 0 |
| 9 | 18 | 19 | 9.04 × 10−7 | 3.33 × 10−8 | 3 |
| 9 | 21 | 22 | 1.94 × 10−6 | 4.99 × 10−7 | 0 |
| 10 | 17 | 18 | 2.97 × 10−7 | 9.32 × 10−8 | 0 |
| 10 | 33 | 34 | 2.26 × 10−6 | 7.41 × 10−7 | 0 |
| 10 | 35 | 36 | 9.78 × 10−7 | 2.62 × 10−7 | 2 |
| 10 | 37 | 38 | 1.07 × 10−6 | 1.94 × 10−7 | 7 |
| 10 | 39 | 40 | 3.00 × 10−6 | 7.34 × 10−7 | 1 |
| 11 | 2 | 3 | 8.74 × 10−7 | 1.93 × 10−7 | 3 |
| 11 | 7 | 8 | 8.00 × 10−7 | 1.55 × 10−7 | 23 |
| 11 | 10 | 11 | 1.37 × 10−6 | 3.01 × 10−7 | 7 |
| 11 | 37 | 38 | 2.64 × 10−6 | 7.84 × 10−7 | 1 |
| 11 | 40 | 41 | 9.07 × 10−7 | 3.01 × 10−7 | 3 |
| 11 | 45 | 46 | 7.85 × 10−6 | 9.80 × 10−7 | 9 |
| 11 | 47 | 48 | 7.31 × 10−6 | 1.53 × 10−6 | 0 |
| 11 | 48 | 49 | 1.52 × 10−6 | 4.99 × 10−7 | 0 |
| 11 | 53 | 54 | 6.90 × 10−7 | 2.28 × 10−7 | 11 |
| Chr | SNP Position (Mbp) | FST Value | Number of Candidate Genes |
|---|---|---|---|
| 2 | 48.9 | 0.541 | 8 |
| 49.1 | 0.546 | 11 | |
| 49.1 | 0.603 | 13 | |
| 49.2 | 0.514 | 10 | |
| 49.2 | 0.501 | ||
| 7 | 0.6 | 0.558 | 15 |
| 0.7 | 0.558 | ||
| 4.2 | 0.505 | 13 | |
| 4.2 | 0.511 | ||
| 4.7 | 0.597 | 7 | |
| 38.9 | 0.574 | 10 | |
| 9 | 5.5 | 0.656 | 8 |
| 6.9 | 0.618 | 3 | |
| 7.7 | 0.546 | 5 | |
| 7.8 | 0.677 | 9 | |
| 7.9 | 0.589 | ||
| 13.5 | 0.536 | 11 | |
| 13.6 | 0.536 | ||
| 13.7 | 0.556 | 11 | |
| 30.6 | 0.543 | 6 | |
| 11 | 52.4 | 0.553 | 10 |
| 52.4 | 0.506 | ||
| 52.4 | 0.553 | ||
| 52.4 | 0.553 | ||
| 52.5 | 0.553 | ||
| 52.5 | 0.553 |
| N Derived from the Atmosphere (%) | Carbon Discrimination (Δ) (‰) | Flowering (Days) | Maturity (Days) | Yield (kg ha−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F | Pr > F | F | Pr > F | F | Pr > F | F | Pr > F | F | Pr > F | |
| Breeding category | 1.86 | 0.1441 | 1.51 | 0.2195 | 6.13 | 0.0007 | 3.06 | 0.0368 | 7.15 | 0.0004 |
| LSmean * | SE | LSmean * | SE | LSmean * | SE | LSmean * | SE | LSmean * | SE | |
| Check | 51.4 a | 0.04 | 20.2 a | 0.23 | 48.6 ab | 1.44 | 114.9 a | 2.0 | 933.7 a | 53.18 |
| Conventional | 43.3 a | 0.04 | 19.6 a | 0.24 | 51.0 ab | 1.44 | 108.6 a | 2.82 | 700.9 ab | 77.10 |
| Landrace | 52.5 a | 0.02 | 20.1 a | 0.14 | 48.2 b | 0.87 | 109.9 a | 1.19 | 912.8 a | 28.39 |
| PPB | 46.7 a | 0.02 | 19.9 a | 0.14 | 51.6 a | 0.83 | 114.0 a | 1.26 | 721.6 b | 30.51 |
| N Derived from the Atmosphere (%) | Carbon Discrimination (Δ) (‰) | Flowering (Days) | Maturity (Days) | Yield (kg ha−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F | Pr > F | F | Pr > F | F | Pr > F | F | Pr > F | F | Pr > F | |
| Breeding category | 6.69 | 0.0004 | 0.88 | 0.4535 | 0.94 | 0.4225 | 3.27 | 0.0251 | 3.66 | 0.0148 |
| LSmean * | SE | LSmean * | SE | LSmean * | SE | LSmean * | SE | LSmean * | SE | |
| Check | 50.0 a | 0.03 | 19.8a | 0.32 | 50.8 a | 0.83 | 113.1 ab | 1.65 | 1454.7 ab | 145.59 |
| Conventional | 59.0 bc | 0.02 | 19.8a | 0.29 | 49.6 a | 0.75 | 109.9 ab | 1.42 | 1613.2 ab | 121.38 |
| Landrace | 58.3 b | 0.01 | 19.5a | 0.24 | 49.3 a | 0.36 | 109.0 a | 0.63 | 1396.5 a | 58.55 |
| PPB | 53.2 ac | 0.01 | 19.6a | 0.24 | 49.3 a | 0.36 | 112.1 b | 0.65 | 1686.2 b | 62.74 |
| N Derived from the Atmosphere (%) | Carbon Discrimination (Δ) (‰) | Days to Flowering (Days) | Yield (kg ha−1) | |||||
|---|---|---|---|---|---|---|---|---|
| F | Pr > F | F | Pr > F | F | Pr > F | F | Pr > F | |
| Breeding category | 3.72 | 0.0143 | 3.18 | 0.0280 | 3.60 | 0.0163 | 1.34 | 0.2647 |
| LSmean * | SE | LSmean * | SE | LSmean * | SE | LSmean * | SE | |
| Check | 49.5 ab | 0.04 | 18.0 ab | 0.25 | 39.0 ab | 1.28 | 669.9 a | 135.22 |
| Conventional | 40.1 ab | 0.03 | 18.0 ab | 0.22 | 36.8 ab | 1.06 | 956.5 a | 108.87 |
| Landrace | 46.4 a | 0.01 | 18.2 a | 0.10 | 36.2 a | 0.53 | 745.2 a | 52.16 |
| PPB | 40.1 b | 0.01 | 17.7 b | 0.10 | 38.3 b | 0.54 | 823.2 a | 52.62 |
| Origin | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HON Entry | VARIETY | Elora ‘14 | Yorito ‘14-’15 | Elora ‘15 | SNP Genotyped | Breeding Category | Market Class | Genealogy | Institution or Organization | Notes |
| 59 | OAC Rico | x | x | x | Check | Navy | (Ex Rico 23/Narda)/Ex Rico 23 See [84] | University of Guelph | Resistant to BCMV and Anthracnose. Tolerant to white mold. Unremarkable SNF capacity. In other studies it fixed approximately 53% of N [18]. | |
| 60 | R991 | x | x | x | x | Check | Navy | See [85] | Agriculture Agri-Food Canada (AAFC) | Non-nodulating experimental line. Derived from OAC Rico through ethyl methan sulphonate (EMS) mutagesis [86]. Used in natural abundance method to establish a baseline nitrogen level in seed measured for 14N and 15N accumulation. |
| 61 | OAC Mist | x | x | x | x | Check | Navy | See [87] | University of Guelph | High yielding, late season. Resistant to BCMV and CBB. Generally high SNF capacity. Farid and Navabi (2015) reported that OAC Mist fixed as much as 78.5% of N [16]. Wilker et al. [18] reported that OAC Mist fixed an average of 55% N. |
| 62 | Merlot | x | x | x | x | Check | Small red | See [88] | United States Department of Agriculture—Agriculture Research Service (USDA-ARS) | Intense red seed color. Larger seed size than Honduran beans (mean HSW 39.2 g). Resistant to rust, BCMV, and BCMNV. Susceptible to anthracnose. Moderate SNF capacity. Wilker et al. (unpublished) found Merlot fixed as much as 64.9 % of its N. |
| 63 | OAC Rosito | x | x | x | x | Check | Small red | See [89] | University of Guelph | Developed from a diverse landrace originating in El Salvador [89]. Dark red seed color. Similar seed size to Honduran beans (mean HSW 21.7 g). Resistant to BCMV. Susceptible to Anthracnose and CBB. The SNF capacity of OAC Rosito has not been examined previous to the current study. |
| 64 | Zorro | x | x | x | x | Check | Black | See [90] | Michigan State University | Resistant to rust and anthracnose and is less affected by white mold. Moderate SNF capacity. Wilker et al. [18] reported that Zorro fixed an average of 59%, and Wilker et al. (unpublished) found that Zorro fixed as much as 46.9% of its N. |
| 80 | CENTA San Andrés 3 | x | x | Conventional | Small red | EAP 9510-77, [MD 30-75/DICTA 105] | PIF/Zamorano, UPR, CENTA, El Salvador; 2003 | Same breeding line as Amadeus-77 [49]. Resistant to BGYMV and BCMV. Heat tolerant and adapted for production in lower-altitude coastal areas [49]. | ||
| 52 | Amadeus-77 3 | x | x | x | x | Conventional | Small red | EAP 9510-77, [MD 30-75/DICTA 105] | PIF/Zamorano, UPR, DICTA, Honduras; 2003 | Same breeding line as CENTA San Andres [49]. Resistant to BGYMV and BCMV. Heat tolerant and performs well in low altitude, coastal areas. Widely adopted across Central America, and in 2010, accounted for around 50% of commercial production in the region [26]. |
| 56 | Dorado 3 | x | x | x | x | Conventional | Small red | DOR 364, [BAT 1215 x (RAB 166 x DOR 125)] | Profrijol, DICTA, Honduras; 1990 | Also known as ‘DOR 364’. Resistant to BGYMV and BCMV [49]. Yields well across environments and has mid-range maturity; however, it has a dark red seed coat [49]. |
| 53 | DEORHO 2,3 | x | x | x | Conventional | Small red | SRC 2-18-1, [Milenio/MD 30-75] | PIF/Zamorano, UPR, DICTA, Honduras; 2007 | Also known as ‘DEHORO’ and ‘INTA Matagalpa’. Resistant to BGYMV and BCMV. Higher yielding and desirable light red seed coat color [91]. Popular with Honduran growers, accounting for 23% of the red bean acreage in 2010 [26]. DEORHO was not grown for DNA extraction and consequently was not included in the genetic analyses carried out for this study. | |
| 55 | Tio Canela 75 2,3 | x | x | x | x | Conventional | Small red | MD 30-75, [DOR 483//DOR 391/Pompadour J] | PIF/Zamorano, UPR, Honduras; 1996 | Resistant to BGYMV and BCMV [49]. Yields well across environments, has mid-range maturity, and has a shiny red seed [49]. Tio Canela 75 is a parent line of Amadeus-77 and Carrizalito. |
| 77 | Carrizalito 3 | x | x | x | Conventional | Small red | EAP 9510-1, [MD 30-75/DICTA 105] | PIF/Zamorano, UPR, DICTA, Honduras; 2003 | Resistant to BGYMV and BCMV. Early maturity (68–70 DAP) and upright plant architecture. High yielding variety, adapted to mid-altitude production [91]. | |
| 54 | Aifi Wuriti 2 | x | x | x | x | Conventional | Black | EAP 9712-13, Tio Canela 75/DICTA 105/BG12WB12//Tio Canela 75/DICTA 105/AL12 | PIF/Zamorano, UPR, SNS, Haiti; 2008 | Also known as ‘Negro Olfirwit’. Resistant to BGYMV, BCMV, is tolerant of low soil fertility, and is early-maturing [92]. Popular in Haiti and the Dominican Republic and was successfully adopted by growers in southeast Guatemala [92]. |
| Origin | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HON Entry | VARIETY | Elora ‘14 | Yorito ‘14–’15 | Elora ‘15 | SNP Genotyped | Breeding Category | Market Class | Institution, Farmer, or Organization | Locality | Municipality | Department |
| 02 | Concha Rosada 2 | x | x | x | x | Landrace | Small red | FIPAH | Yorito | Yorito | Yoro |
| 06 | Negro Pedreño | x | x | x | x | Landrace | Black | Odir Palma | La Esperanza | Yorito | Yoro |
| 07 | Negro Concha Blanca 2 | x | x | x | x | Landrace | Black | Odir Palma | La Esperanza | Yorito | Yoro |
| 08 | Balin Rojo 2 | x | x | x | x | Landrace | Small red | Edy Hernandez | La Patastera | Yorito | Yoro |
| 09 | Carmelita 1 | x | x | Landrace | Small red | Francisco Murillo | La Esperanza | Yorito | Yoro | ||
| 11 | Estica | x | x | x | Landrace | Small red | Irene Hernandez | La Esperanza | Yorito | Yoro | |
| 22 | Rosado 2 | x | x | x | x | Landrace | Small red | Odir Palma | La Esperanza | Yorito | Yoro |
| 27 | Chapin Rojo | x | x | x | x | Landrace | Small red | Daniel Vargas | El Injerto | Comayagua | Francisco Morazán |
| 29 | Uva | x | x | x | Landrace | Black | Alonso Gutierrez | San Jose | Vallecillo | Francisco Morazán | |
| 30 | Chapin Negro 2 | x | x | x | x | Landrace | Black | Ovidio Valeriano | Nocoro | Vallecillo | Francisco Morazán |
| 34 | Vaina Rosada | x | x | x | x | Landrace | Small red | CIAL San Jose | San Jose | Vallecillo | Francisco Morazán |
| 36 | Milpero Negro 1 | x | x | Landrace | Black | Bertilio Antonio Rodriguez | San Pedrito | Opalaca | Intibucá | ||
| 37 | Negro Vaina Blanca | x | x | x | x | Landrace | Small red | Carmen Azucenaa Giron | Guayabal | Jesus de Otoro | Intibucá |
| 38 | Mano de Piedra | x | x | x | x | Landrace | Small red | Maria Laines | Maye | Jesus de Otoro | Intibucá |
| 39 | Milpero Rojo 1 | x | x | Landrace | Small red | Bertilio Antonio Rodriguez | San Pedrito | Opalaca | Intibucá | ||
| 40 | Vaina Blanca | x | x | x | x | Landrace | Black | Tiburcio Dias | Pueblo Viejo | NA | NA |
| 41 | Negro Arbolito | x | x | x | x | Landrace | Black | Armando Pineda | Crucita Oriente | Jesus de Otoro | Intibucá |
| 42 | Negro Cuarenteño | x | x | x | x | Landrace | Black | Maria Laines | Maye | Jesus de Otoro | Intibucá |
| 43 | Negro 2 | x | x | x | x | Landrace | Black | Antonio Espinosa | Iguala | Lempira | Lempira |
| 44 | Milpero Gatiador 1 | x | x | Landrace | Carioca | Evelino Sanchez | La Vegas | NA | NA | ||
| 45 | Ponga la Olla | x | x | x | x | Landrace | Black | Estalin Diaz | Pueblo Viejo | NA | NA |
| 46 | Madura Parejo | x | x | x | x | Landrace | Small red | Maria Lainez | Maye | Jesus de Otoro | Intibucá |
| 47 | Milpero Blanco 1 | x | x | Landrace | White | Maria Juana Gutierrez | Monte Verde | Opalaca | Intibucá | ||
| 48 | Cincuenteño | x | x | x | x | Landrace | Small red | FIPAH | Yorito | Yorito | Yoro |
| 49 | Paraísito | x | x | x | x | Landrace | Small red | FIPAH | Yorito | Yorito | Yoro |
| 50 | Rojo de Seda | x | x | x | x | Landrace | Small red | FIPAH | Yorito | Yorito | Yoro |
| 51 | Marciano | x | x | x | x | Landrace | Small red | FIPAH | Yorito | Yorito | Yoro |
| 65 | Olanchano Negro | x | x | x | Landrace | Black | CIAL San Jose de la Mora | San Jose de la Mora | Vallecillo | Francisco Morazán | |
| 66 | Seda-Vallecillo | x | x | x | Landrace | Small red | CIAL San José de la Mora | San Jose de La Mora | Vallecillo | Francisco Morazán | |
| 67 | Chirineño | x | x | x | Landrace | Small red | CIAL Chirinos | Chirinos | Cedros | Francisco Morazán | |
| 68 | Roseño | x | x | x | Landrace | Small red | Adan Bustillo | La Fortuna | Victoria | Yoro | |
| 70 | Negro Opalaca | x | x | x | Landrace | Black | NA | Monte Verde | San Francisco de Opalaca | Intibucá | |
| 82 | Olanchano Rojo | x | Landrace | Small red | San Jose de la Mora | San Jose de la Mora | Vallecillo | Francisco Morazán | |||
| Seed Origin | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HON Entry | VARIETY | Elora ‘14 | Yorito ‘14–’15 | Elora ‘15 | SNP Genotyped | Breeding Category | Market Class | Genealogy | Institution, Farmer, or Organization | Locality | Municipality | Department |
| 01 | Macuzalito | x | x | x | x | PPB (PPB)3 | Small red | PPB-9911-44-5-13M, [Concha Rosada//SRC 1-1-18/SRC 1-12-1] | PIF/Zamorano, FIPAH, Honduras; 2004 | Yorito | Yorito | Yoro |
| 13 | FPY-722-53 1 | x | x | PPB (PPB) | Small red | FPY-722-53, Tio Canela 75/ Estica | PIF/Zamorano, FIPAH, CIAL Santa Cruz | Santa Cruz | Yorito | Yoro | ||
| 14 | FPY-722-38 | x | x | x | x | PPB (PPB) | Small red | FPY-722-38, Tio Canela 75/Estica | PIF/Zamorano, FIPAH, CIAL Santa Cruz | Santa Cruz | Yorito | Yoro |
| 15 | FPY-722-13 1 | x | x | x | PPB (PPB) | Small red | FPY-722-13, Tio Canela 75/Estica | PIF/Zamorano, FIPAH, CIAL Santa Cruz | Santa Cruz | Yorito | Yoro | |
| 16 | FPY-724-43 1 | x | x | x | x | PPB (PPB) | Small red | FPY-724-43, Macuzalito/Estica | PIF/Zamorano, FIPAH, CIAL Santa Cruz | Santa Cruz | Yorito | Yoro |
| 18 | FPY-721-16 1 | x | x | PPB (PPB) | Small red | FPY-721-16, Amadeus 77/Estica | PIF/Zamorano, FIPAH, CIAL Santa Cruz | Yorito | Yorito | Yoro | ||
| 19 | FPY-722-41 2 | x | x | x | x | PPB (PPB) | Small red | FPY-722-41, Tio Canela 75/Estica | PIF/Zamorano, FIPAH, CIAL Santa Cruz, Amilcar Orellana | La Esperanza | Yorito | Yoro |
| 23 | PM2-Don Rey 2 | x | x | x | x | PPB (PPB) | Small red | IBC-302-29, Carrizalito//Carrizalito/Paraísito | PIF/Zamorano, UPR, DICTA, ASOCIAL Vallecillo, Reinaldo Funez; 2014 | San Isidro | Vallecillo | Francisco Morazán |
| 25 | FPV-921-4 | x | x | x | x | PPB (PPB) | Small red | FPV-921-4, Vaina Rosada/Amadeus 77 | CIAL San Isidro | San Isidro | Vallecillo | Francisco Morazán |
| 26 | Quebradeño | x | x | x | PPB (PPB) | Small red | IBC-307-7, [TC75//TC75/Cincuenteño] | CIAL Quebrada | Trinidad de Quebrada | Vallecillo | Francisco Morazán | |
| 28 | FPV-921-61 1 | x | PPB (PPB) | Small red | FPV-921-61, Vaina Rosada/Amadeus 77 | CIAL San Isidro | San Isidro | Vallecillo | Francisco Morazán | |||
| 31 | FPV-923-25 2 | x | x | x | x | PPB (PPB) | Small red | FPV-923-25, Vaina Rosada/Conan 33 | CIAL San Isidro | San Isidro | Vallecillo | Francisco Morazán |
| 32 | FPV-923-21 1 | x | x | PPB (PPB) | Small red | FPV-923-21, Vaina Rosada/Conan 33 | CIAL San Isidro | San Isidro | Vallecillo | Francisco Morazán | ||
| 33 | FPV 921-65 1 | x | x | PPB (PPB) | Small red | FPV 921-65, Vaina Rosada/Amadeus 77 | CIAL San Isidro | San Isidro | Vallecillo | Francisco Morazán | ||
| 03 | Cedrón | x | x | x | x | PPB (PVS)4 | Small red | EAP 9508-93, [Bribri//MD 30-37//PR 9177-214-1/Tio Canela 75] | PIF/Zamorano, FIPAH, Honduras; 2005 | Yorito | Yorito | Yoro |
| 04 | Chepe | x | x | x | x | PPB (PVS) | Small red | 703-SM15216-11-5 | PIF/Zamorano, CIAT, FIPAH, Honduras; 2012 | Yorito | Yorito | Yoro |
| 05 | Amilcar 2 | x | x | x | x | PPB (PVS) | Small red | IBC 308-24, Amadeus 77//Amadeus 77/Cincuenteño | PIF/Zamorano, FIPAH, Honduras; 2012 | Yorito | Yorito | Yoro |
| 10 | Marcelino 2 | x | x | x | x | PPB (PVS) | Small red | EAP 9508-41, Bribri/MD 30-37//PR 9177-214-1/Tio Canela 75 | PIF/Zamorano, FIPAH, Edy Hernandez; 2012 | La Patastera | Yorito | Yoro |
| 12 | Esperanceño 2 | x | x | x | x | PPB (PVS) | Small red | PR 0310-26-3-3, VAX 6 | PIF/Zamorano, UPR, CIAT, FIPAH, Pablo Orellana; 2011 | La Esperanza | Yorito | Yoro |
| 17 | Paisano PF | x | x | x | x | PPB (PVS) | Small red | MER-2212-28, Milenio/Amadeus 77 | PIF/Zamorano, PRR, CIAL Palmicha Fatima; 2011 | Palmichal Fatima | Siguatepeque | Comayagua |
| 20 | 523-DFBS 15092-04-4 | x | x | x | x | PPB (PVS) | Small red | 523-DFBS 15092-04-4 | PIF/Zamorano, CIAT, FIPAH | Yorito | Yorito | Yoro |
| 21 | 519-DFBZ 15094-39-4 2 | x | x | x | x | PPB (PVS) | Small red | 519-DFBZ 15094-39-4 | PIF/Zamorano, CIAT, FIPAH | Yorito | Yorito | Yoro |
| 24 | Conan 33 | x | x | x | x | PPB (PVS) | Small red | PRF-9659-25B-1, [EAP 9503/RS3//Bribri/MD 30-37////EAP 9503/RS3//A429/K2///V8025/XR 16492//APN83/CNC] | PIF/Zamorano, FIPAH, Asocial Vallecillo; 2005 | Trinidad de Quebrada | Vallecillo | Francisco Morazán |
| 35 | San Jose | x | x | x | x | PPB (PVS) | Small red | X0-233-171-4, VAX 3 | PIF/Zamorano, UPR, CIAT, FIPAH, CIAL San José; date NA | San Jose | Vallecillo | Francisco Morazán |
| 57 | Campechano | x | x | x | x | PPB (PVS) | Small red | SX14825-7-1 | PIF/Zamorano, CIAT, PRR, ASOCIALAYO; 2012 | La Buena Fe | Zacapa | Santa Bárbara |
| 58 | Don Kike | x | x | x | x | PPB (PVS) | Small red | MDSX14797-6-1 | PIF/Zamorano, CIAT, PRR, ASOCIALAYO; 2012 | La Buena Fe | Zacapa | Santa Bárbara |
| 72 | Arbolito Negro | x | x | x | PPB (PVS) | Black | SJC 729-89, Negro Vaina Blanca/PRF 9924-50N | PIF/Zamorano, PRR, ASOCIALAYO; 2013 | La Buena Fe | Zacapa | Santa Bárbara | |
| 74 | Rojo Delicia | x | x | x | PPB (PVS) | Small red | 703-SM15216-11-4-VR | PIF/Zamorano, CIAT, PRR, ASOCIALAYO; 2015 | La Buena Fe | Zacapa | Santa Bárbara | |
| 75 | Don Cristóbal | x | x | x | PPB (PVS) | Small red | SRC1-12-1-8, [DOR476//XAN155/DOR364] | PIF/Zamorano, CIAT, PRR, CIAL Laguna Seca; 2015 | Laguna Seca | Taulabe | Comayagua | |
| 76 | Victoria | x | x | x | PPB (PVS) | Small red | SRS 56-3, [Amadeus77/SEA5] | PIF/Zamorano, PRR, CIAL Nueva Esperanza; 2015 | Nueva Esperanza | Concepción Sur | Santa Bárbara | |
| 78 | Nueva Esperanza | x | x | x | PPB (PVS) | Small red | DICZA 9801, UPR9606-2-2/MD 30-37 | PIF/Zamorano, PRR, CIAL Nueva Esperanza; 2005 | Nueva Esperanza | Concepción Sur | Santa Bárbara | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilker, J.; Humphries, S.; Rosas-Sotomayor, J.C.; Gómez Cerna, M.; Torkamaneh, D.; Edwards, M.; Navabi, A.; Pauls, K.P. Genetic Diversity, Nitrogen Fixation, and Water Use Efficiency in a Panel of Honduran Common Bean (Phaseolus vulgaris L.) Landraces and Modern Genotypes. Plants 2020, 9, 1238. https://doi.org/10.3390/plants9091238
Wilker J, Humphries S, Rosas-Sotomayor JC, Gómez Cerna M, Torkamaneh D, Edwards M, Navabi A, Pauls KP. Genetic Diversity, Nitrogen Fixation, and Water Use Efficiency in a Panel of Honduran Common Bean (Phaseolus vulgaris L.) Landraces and Modern Genotypes. Plants. 2020; 9(9):1238. https://doi.org/10.3390/plants9091238
Chicago/Turabian StyleWilker, Jennifer, Sally Humphries, Juan Carlos Rosas-Sotomayor, Marvin Gómez Cerna, Davoud Torkamaneh, Michelle Edwards, Alireza Navabi, and K. Peter Pauls. 2020. "Genetic Diversity, Nitrogen Fixation, and Water Use Efficiency in a Panel of Honduran Common Bean (Phaseolus vulgaris L.) Landraces and Modern Genotypes" Plants 9, no. 9: 1238. https://doi.org/10.3390/plants9091238






