Zinc-lysine Supplementation Mitigates Oxidative Stress in Rapeseed (Brassica napus L.) by Preventing Phytotoxicity of Chromium, When Irrigated with Tannery Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection and Analysis of Wastewater and Soil
2.2. Pot Experiment
2.3. Plant Harvesting
2.4. Analysis Items and Methods
2.5. Statistical Analysis
3. Results
3.1. Effect of Foliar Application of Zn-lys on Plant Growth and Biomass under Different Levels of Tannery Wastewater
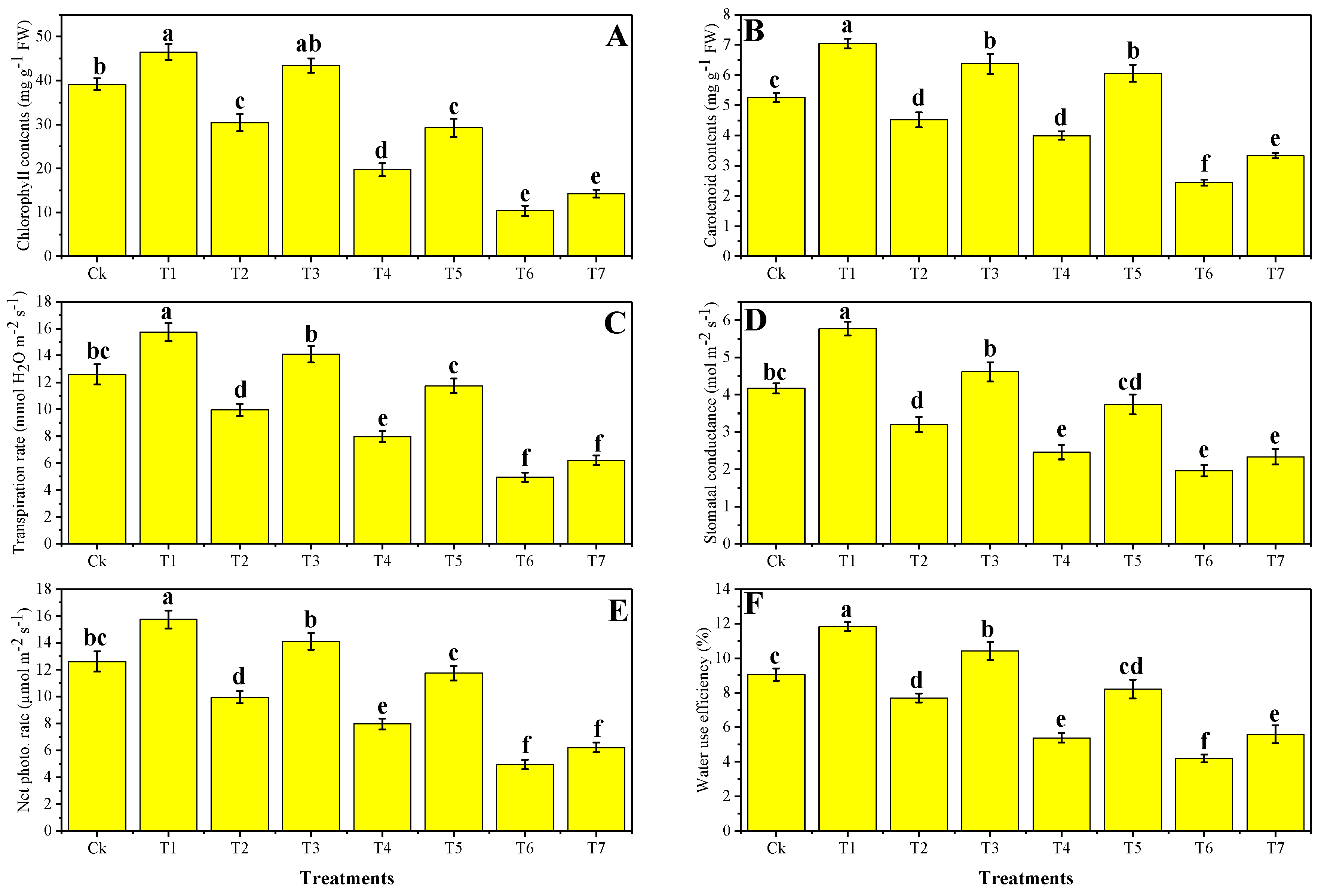
3.2. Effect of Foliar Application of Zn-lys on Photosynthetic Pigments and Gas Exchange Attributes under Different Levels of Tannery Wastewater
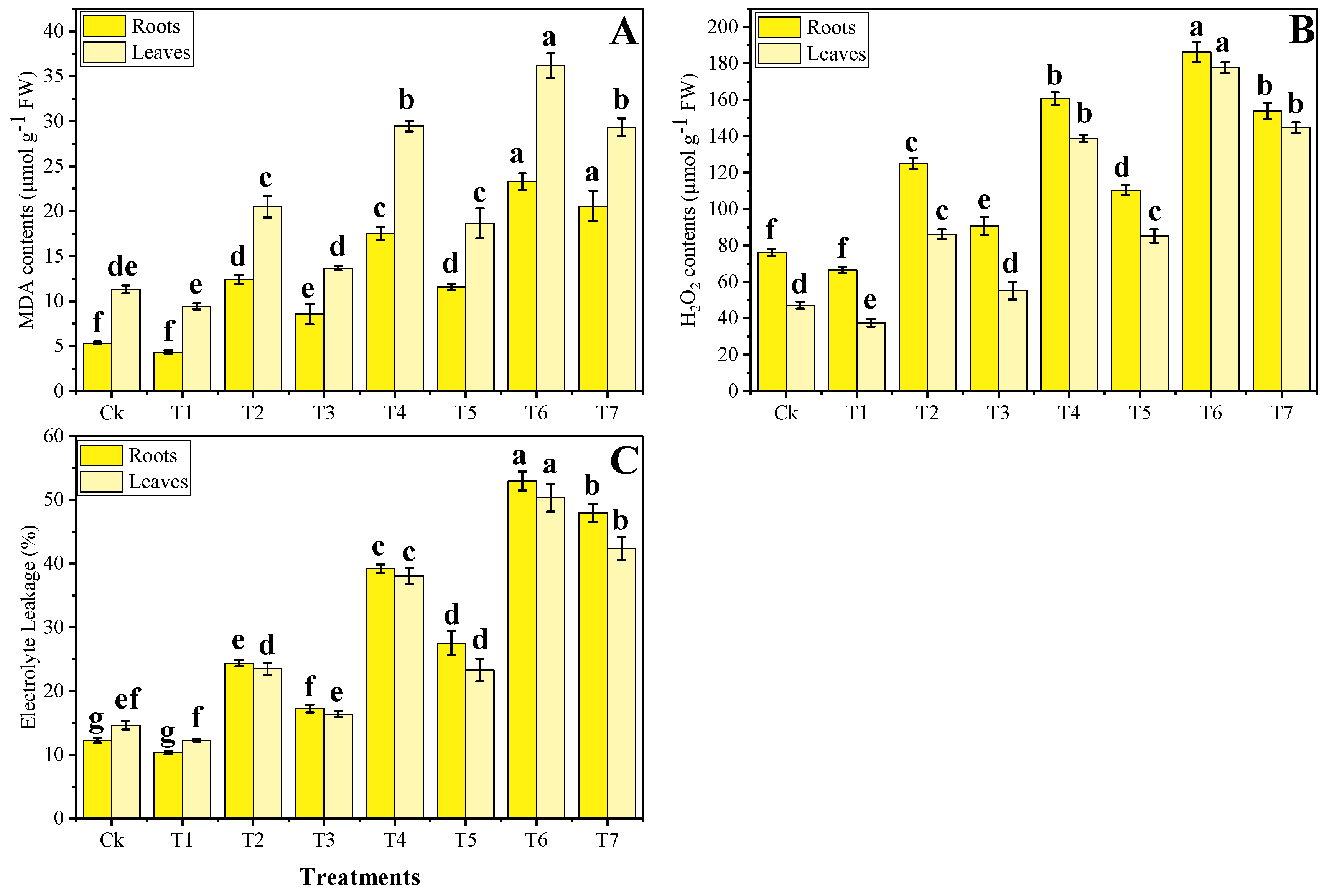
3.3. Effect of Foliar Application of Zn-lys on Oxidative Stress and Antioxidant Response under Different Levels of Tannery Wastewater
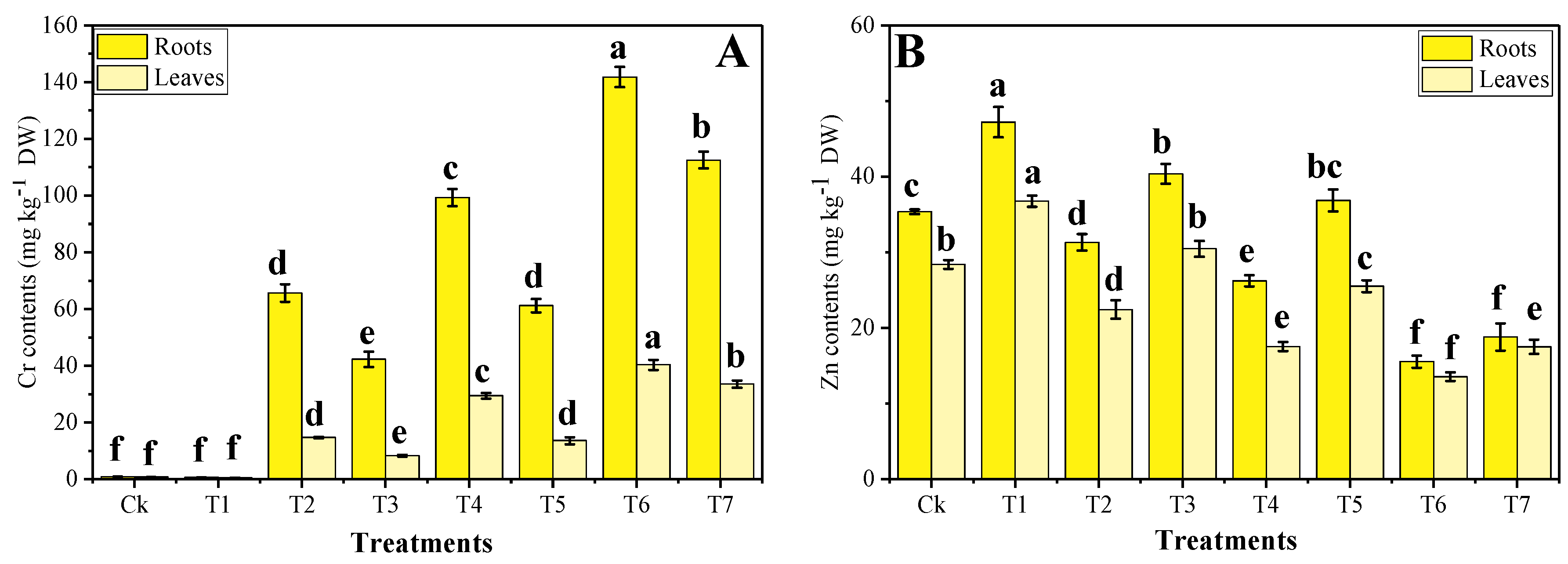
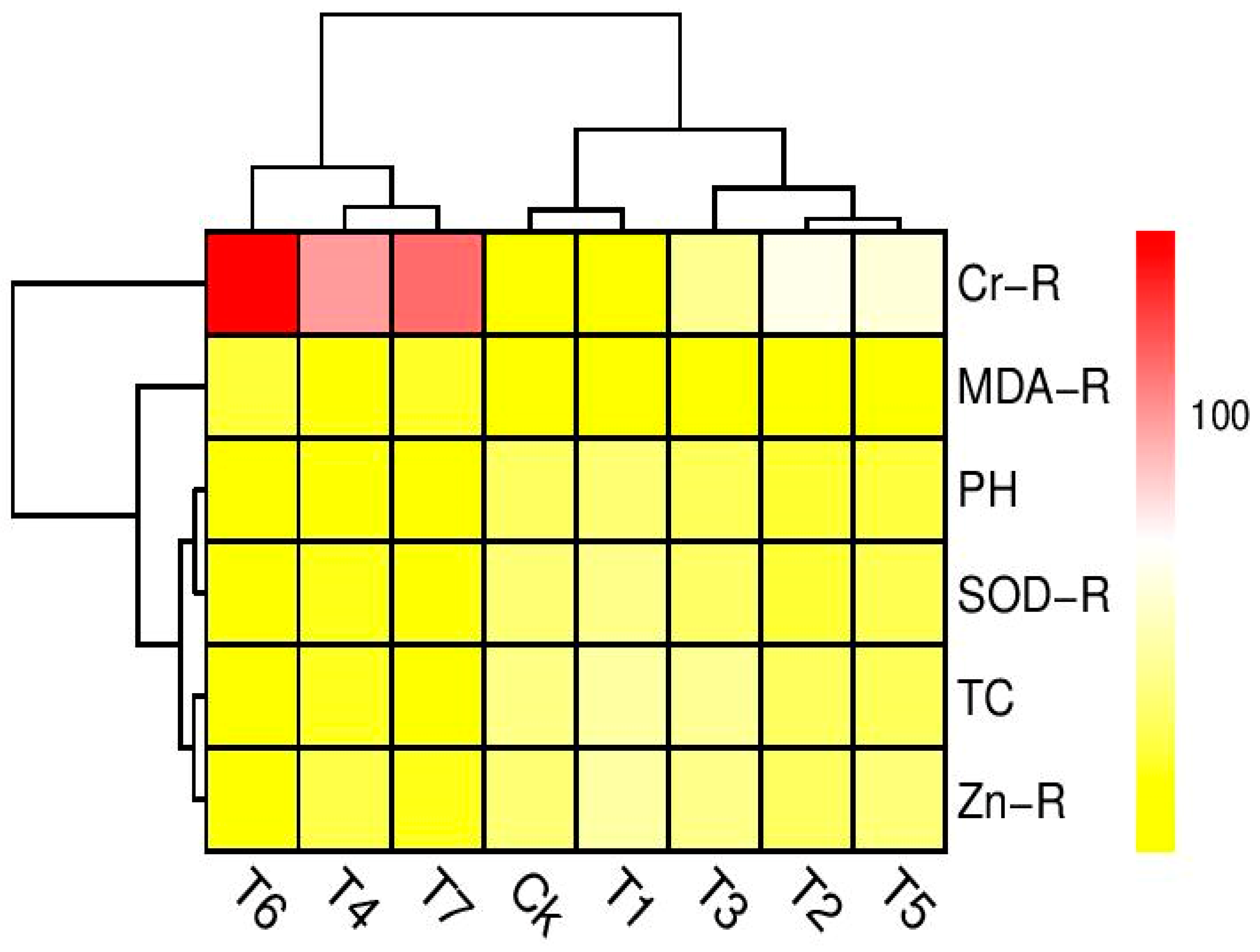
3.4. Effect of Foliar Application of Zn-lys on Uptake and Accumulation of Cr and Zn under Different Levels of Tannery Wastewater
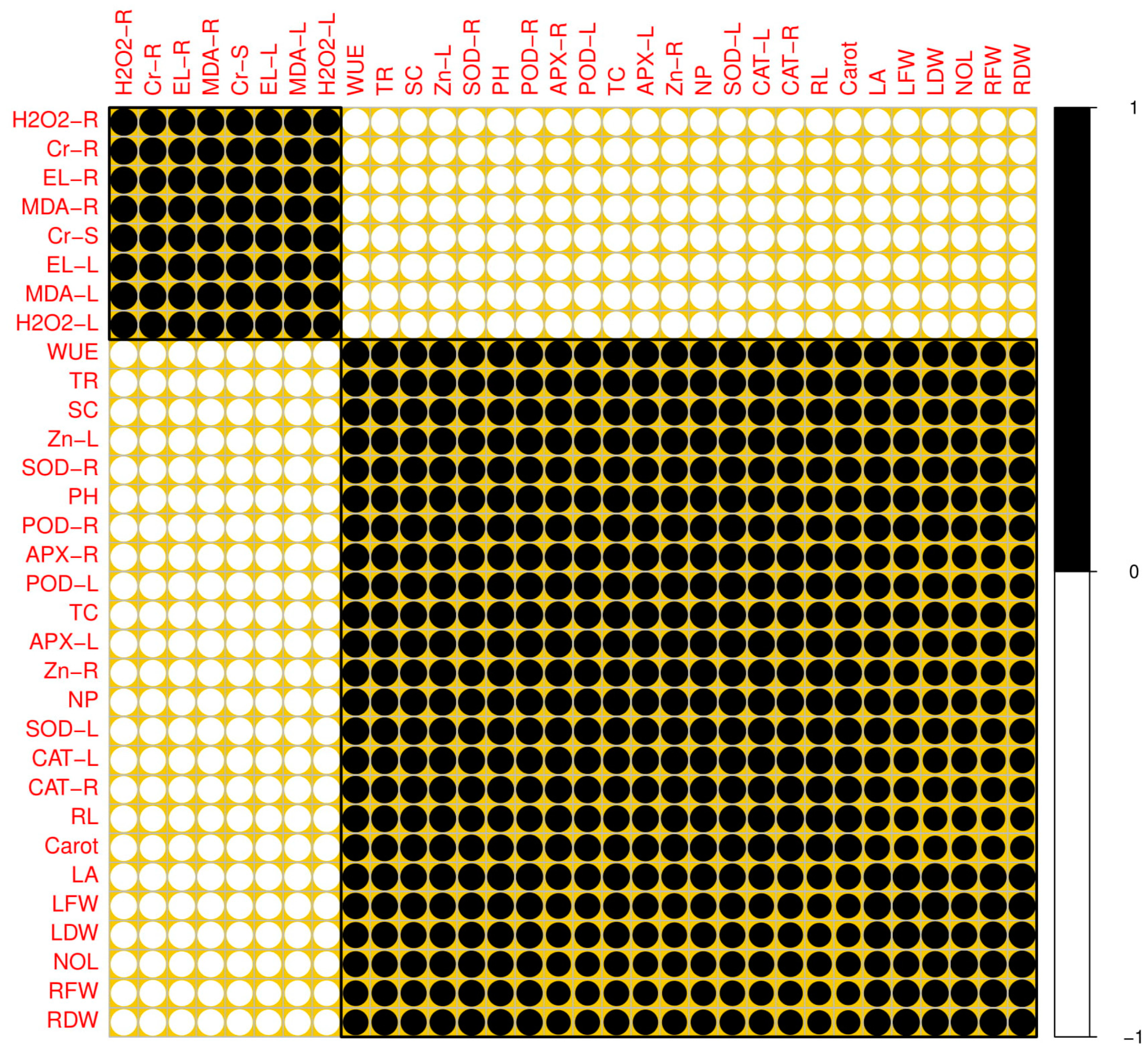
3.5. Correlation between Different Parameters Studied in This Experiment
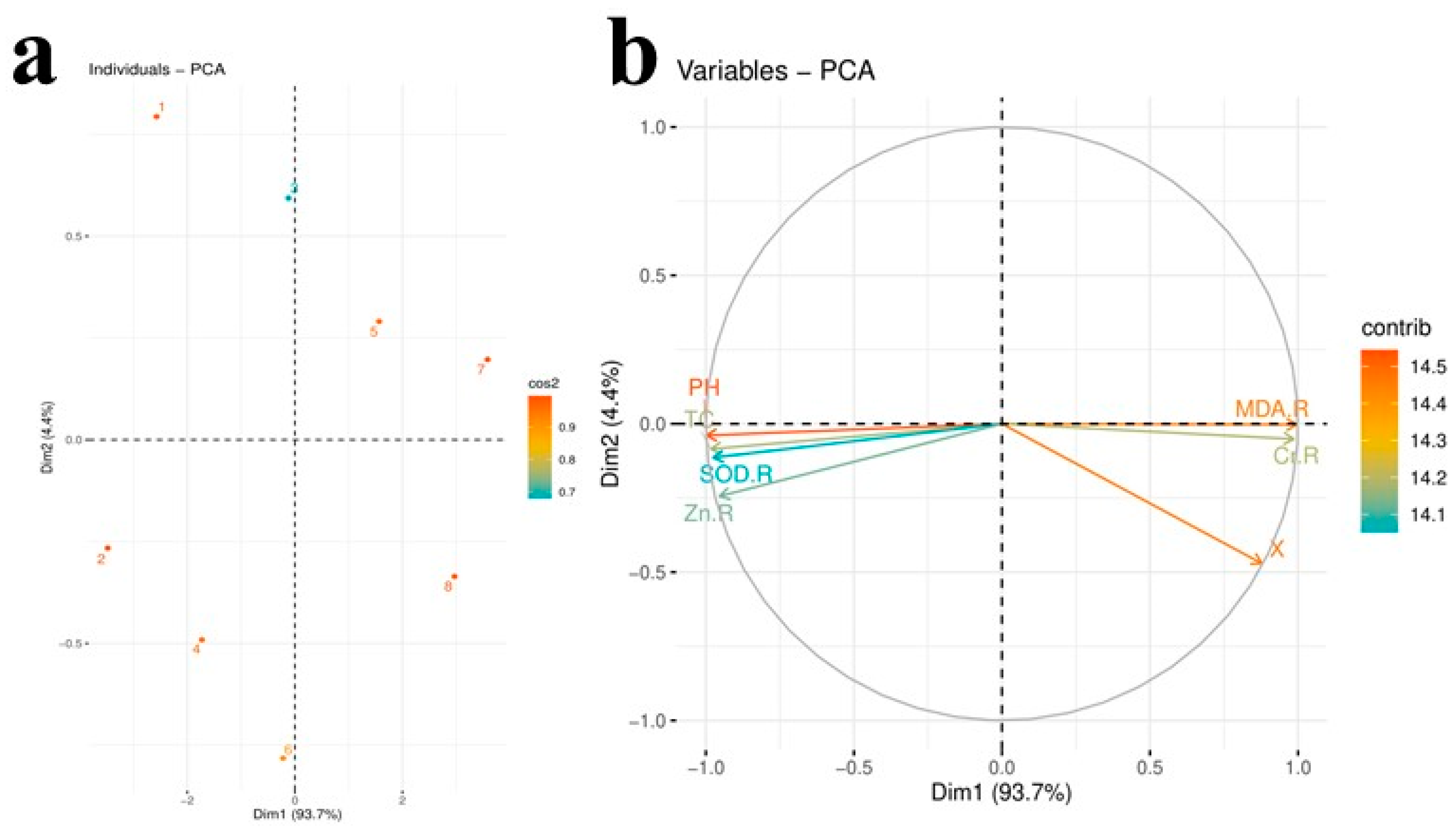
3.6. Principal Component Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Saleem, M.H.; Ali, S.; Rehman, M.; Hasanuzzaman, M.; Rizwan, M.; Irshad, S.; Shafiq, F.; Iqbal, M.; Alharbi, B.M.; Alnusaire, T.S. Jute: A Potential Candidate for Phytoremediation of Metals—A Review. Plants 2020, 9, 258. [Google Scholar] [CrossRef] [Green Version]
- Kamran, M.; Malik, Z.; Parveen, A.; Huang, L.; Riaz, M.; Bashir, S.; Mustafa, A.; Abbasi, G.H.; Xue, B.; Ali, U. Ameliorative Effects of Biochar on Rapeseed (Brassica napus L.) Growth and Heavy Metal Immobilization in Soil Irrigated with Untreated Wastewater. J. Plant Growth Regul. 2019, 39, 266–281. [Google Scholar] [CrossRef]
- Zaheer, I.E.; Ali, S.; Rizwan, M.; Abbas, Z.; Bukhari, S.A.H.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P. Zinc-lysine prevents chromium-induced morphological, photosynthetic, and oxidative alterations in spinach irrigated with tannery wastewater. Environ. Sci. Pollut. Res. 2019, 26, 28951–28961. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, A.; Ali, S.; Rizwan, M.; Ishaque, W.; Rasool, N.; ur Rehman, M.Z.; Bashir, A.; Abid, M.; Wu, L. Management of tannery wastewater for improving growth attributes and reducing chromium uptake in spinach through citric acid application. Environ. Sci. Pollut. Res. 2018, 25, 10848–10856. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.H.; Ali, S.; Hussain, S.; Kamran, M.; Chattha, M.S.; Ahmad, S.; Aqeel, M.; Rizwan, M.; Aljarba, N.H.; Alkahtani, S. Flax (Linum usitatissimum L.): A Potential Candidate for Phytoremediation? Biological and Economical Points of View. Plants 2020, 9, 496. [Google Scholar] [CrossRef] [Green Version]
- Chowdhary, P.; Yadav, A.; Singh, R.; Chandra, R.; Singh, D.; Raj, A.; Bharagava, R.N. Stress response of Triticum aestivum L. and Brassica juncea L. against heavy metals growing at distillery and tannery wastewater contaminated site. Chemosphere 2018, 206, 122–131. [Google Scholar] [CrossRef]
- Ding, H.; Wang, G.; Lou, L.; Lv, J. Physiological responses and tolerance of kenaf (Hibiscus cannabinus L.) exposed to chromium. Ecotoxicol. Environ. Saf. 2016, 133, 509–518. [Google Scholar] [CrossRef]
- Tang, R.; Li, X.; Mo, Y.; Ma, Y.; Ding, C.; Wang, J.; Zhang, T.; Wang, X. Toxic responses of metabolites, organelles and gut microorganisms of Eisenia fetida in a soil with chromium contamination. Environ. Pollut. 2019, 251, 910–920. [Google Scholar] [CrossRef]
- Hussain, A.; Ali, S.; Rizwan, M.; ur Rehman, M.Z.; Hameed, A.; Hafeez, F.; Alamri, S.A.; Alyemeni, M.N.; Wijaya, L. Role of zinc–lysine on growth and chromium uptake in rice plants under Cr stress. J. Plant Growth Regul. 2018, 37, 1413–1422. [Google Scholar] [CrossRef]
- Zaheer, I.E.; Ali, S.; Saleem, M.H.; Noor, I.; El-Esawi, M.A.; Hayat, K.; Rizwan, M.; Abbas, Z.; El-Sheikh, M.A.; Alyemeni, M.N. Iron–Lysine Mediated Alleviation of Chromium Toxicity in Spinach (Spinacia oleracea L.) Plants in Relation to Morpho-Physiological Traits and Iron Uptake When Irrigated with Tannery Wastewater. Sustainability 2020, 12, 6690. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Hussain, A.; Ali, Q.; Shakoor, M.B.; Zia-ur-Rehman, M.; Farid, M.; Asma, M. Effect of zinc-lysine on growth, yield and cadmium uptake in wheat (Triticum aestivum L.) and health risk assessment. Chemosphere 2017, 187, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, S.; Khoshgoftarmanesh, A.H.; Afyuni, M.; Hadadzadeh, H. Iron (II)–amino acid chelates alleviate salt-stress induced oxidative damages on tomato grown in nutrient solution culture. Sci. Hortic. 2014, 165, 91–98. [Google Scholar] [CrossRef]
- Saleem, M.H.; Rehman, M.; Fahad, S.; Tung, S.A.; Iqbal, N.; Hassan, A.; Ayub, A.; Wahid, M.A.; Shaukat, S.; Liu, L.; et al. Leaf gas exchange, oxidative stress, and physiological attributes of rapeseed (Brassica napus L.) grown under different light-emitting diodes. Photosynthetica 2020, 58, 836–845. [Google Scholar] [CrossRef]
- Zaheer, I.E.; Ali, S.; Saleem, M.H.; Imran, M.; Alnusairi, G.S.H.; Alharbi, B.M.; Riaz, M.; Abbas, Z.; Rizwan, M.; Soliman, M.H. Role of iron–lysine on morpho-physiological traits and combating chromium toxicity in rapeseed (Brassica napus L.) plants irrigated with different levels of tannery wastewater. Plant Physiol. Biochem. 2020, 155, 70–84. [Google Scholar] [CrossRef]
- Mohamed, A.; Ibrahim, A.; Shalby, N.; Bai, C.; Qin, M.; Agami, R.A.; Jie, K.; Wang, B.; Zhou, G. Stomatal and Photosynthetic Traits Are Associated with Investigating Sodium Chloride Tolerance of Brassica napus L. Cultivars. Plants 2020, 9, 62. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Zhang, K.; Gill, R.A.; Islam, F.; Farooq, M.A.; Wang, J.; Zhou, W. Ecotoxicological and Interactive Effects of Copper and Chromium on Physiochemical, Ultrastructural, and Molecular Profiling in Brassica napus L. BioMed Res. Int. 2018, 2018, 9248123. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Ran, R.; Nao, W.S.; Feng, Y.; Jia, L.; Sun, K.; Wang, R.; Feng, H. Physiological effects of short-term copper stress on rape (Brassica napus L.) seedlings and the alleviation of copper stress by attapulgite clay in growth medium. Ecotoxicol. Environ. Saf. 2019, 171, 878–886. [Google Scholar] [CrossRef]
- Zaheer, I.E.; Ali, S.; Rizwan, M.; Farid, M.; Shakoor, M.B.; Gill, R.A.; Najeeb, U.; Iqbal, N.; Ahmad, R. Citric acid assisted phytoremediation of copper by Brassica napus L. Ecotoxicol. Environ. Saf. 2015, 120, 310–317. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Bouyoucos, G.J. Hydrometer method improved for making particle size analyses of soils 1. Agron. J. 1962, 54, 464–465. [Google Scholar] [CrossRef]
- Apha, A. American Public Health Association/American Water Works Association/Water Environment Federation. In WPCF, Standard Methods for the Examination of Water and Wastewater; The Water Environment Federation: Washington, DC, USA, 1995. [Google Scholar]
- Ayers, R.; Westcot, D. Water Quality for Agriculture; FAO Irrigation and Drainage Paper 29 Rev.1; Food and Agriculture Organization of the United Nations: Roma, Italy, 1985; Volume 15, p. 2016. [Google Scholar]
- Nagata, M.; Yamashita, I. Simple method for simultaneous determination of chlorophyll and carotenoids in tomato fruit. Nippon Shokuhin Kogyo Gakkaishi 1992, 39, 925–928. [Google Scholar] [CrossRef] [Green Version]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Jana, S.; Choudhuri, M.A. Glycolate metabolism of three submersed aquatic angiosperms: Effect of heavy metals. Aquat. Bot. 1981, 11, 67–77. [Google Scholar] [CrossRef]
- Dionisio-Sese, M.L.; Tobita, S. Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 1998, 135, 1–9. [Google Scholar] [CrossRef]
- Chen, C.-N.; Pan, S.-M. Assay of superoxide dismutase activity by combining electrophoresis and densitometry. Bot. Bull. Acad. Sin. 1996, 37, 107–111. [Google Scholar]
- Sakharov, I.Y.; Ardila, G.B. Variations of peroxidase activity in cocoa (Theobroma cacao L.) beans during their ripening, fermentation and drying. Food Chem. 1999, 65, 51–54. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Rehman, M.Z.; Rizwan, M.; Ghafoor, A.; Naeem, A.; Ali, S.; Sabir, M.; Qayyum, M.F. Effect of inorganic amendments for in situ stabilization of cadmium in contaminated soils and its phyto-availability to wheat and rice under rotation. Environ. Sci. Pollut. Res. 2015, 22, 16897–16906. [Google Scholar] [CrossRef]
- Saleem, M.; Ali, S.; Rehman, M.; Rana, M.; Rizwan, M.; Kamran, M.; Imran, M.; Riaz, M.; Hussein, M.; Elkelish, A.; et al. Influence of phosphorus on copper phytoextraction via modulating cellular organelles in two jute (Corchorus capsularis L.) varieties grown in a copper mining soil of Hubei Province, China. Chemosphere 2020, 248, 126032. [Google Scholar] [CrossRef]
- Saleem, M.H.; Fahad, S.; Khan, S.U.; Ahmar, S.; Khan, M.H.U.; Rehman, M.; Maqbool, Z.; Liu, L. Morpho-physiological traits, gaseous exchange attributes, and phytoremediation potential of jute (Corchorus capsularis L.) grown in different concentrations of copper-contaminated soil. Ecotoxicol. Environ. Saf. 2020, 189, 109915. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.P.; Mahajan, P.; Kaur, S.; Batish, D.R.; Kohli, R.K. Chromium toxicity and tolerance in plants. Environ. Chem. Lett. 2013, 11, 229–254. [Google Scholar] [CrossRef]
- Saleem, M.H.; Ali, S.; Rehman, M.; Rizwan, M.; Kamran, M.; Mohamed, I.A.; Bamagoos, A.A.; Alharby, H.F.; Hakeem, K.R.; Liu, L. Individual and combined application of EDTA and citric acid assisted phytoextraction of copper using jute (Corchorus capsularis L.) seedlings. Environ. Technol. Innov. 2020, 19, 100895. [Google Scholar] [CrossRef]
- Yu, X.-Z.; Lu, C.-J.; Li, Y.-H. Role of cytochrome c in modulating chromium-induced oxidative stress in Oryza sativa. Environ. Sci. Pollut. Res. 2018, 25, 27639–27649. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Anee, T.I.; Fujita, M. Exogenous silicon attenuates cadmium-induced oxidative stress in Brassica napus L. by modulating AsA-GSH pathway and glyoxalase system. Front. Plant Sci. 2017, 8, 1061. [Google Scholar] [CrossRef]
- Yasmeen, T.; Ali, Q.; Islam, F.; Noman, A.; Akram, M.S.; Javed, M.T. Biologically treated wastewater fertigation induced growth and yield enhancement effects in Vigna radiata L. Agric. Water Manag. 2014, 146, 124–130. [Google Scholar] [CrossRef]
- Mohamed, I.A.; Shalby, N.; MA El-Badri, A.; Saleem, M.H.; Khan, M.N.; Nawaz, M.A.; Qin, M.; Agami, R.A.; Kuai, J.; Wang, B. Stomata and Xylem Vessels Traits Improved by Melatonin Application Contribute to Enhancing Salt Tolerance and Fatty Acid Composition of Brassica napus L. Plants. Agronomy 2020, 10, 1186. [Google Scholar] [CrossRef]
- Saleem, M.H.; Ali, S.; Kamran, M.; Iqbal, N.; Azeem, M.; Tariq Javed, M.; Ali, Q.; Zulqurnain Haider, M.; Irshad, S.; Rizwan, M. Ethylenediaminetetraacetic Acid (EDTA) Mitigates the Toxic Effect of Excessive Copper Concentrations on Growth, Gaseous Exchange and Chloroplast Ultrastructure of Corchorus capsularis L. and Improves Copper Accumulation Capabilities. Plants 2020, 9, 756. [Google Scholar] [CrossRef]
- Mallhi, Z.I.; Rizwan, M.; Mansha, A.; Ali, Q.; Asim, S.; Ali, S.; Hussain, A.; Alrokayan, S.H.; Khan, H.A.; Alam, P. Citric Acid Enhances Plant Growth, Photosynthesis, and Phytoextraction of Lead by Alleviating the Oxidative Stress in Castor Beans. Plants 2019, 8, 525. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, M.A.; Hussain, I.; Rasheed, R.; Iqbal, M.; Riaz, M.; Arif, M.S. Advances in microbe-assisted reclamation of heavy metal contaminated soils over the last decade: A review. J. Environ. Manag. 2017, 198, 132–143. [Google Scholar] [CrossRef]
- Lajayer, B.A.; Moghadam, N.K.; Maghsoodi, M.R.; Ghorbanpour, M.; Kariman, K. Phytoextraction of heavy metals from contaminated soil, water and atmosphere using ornamental plants: Mechanisms and efficiency improvement strategies. Environ. Sci. Pollut. Res. 2019, 26, 8468–8484. [Google Scholar] [CrossRef] [PubMed]
- Medda, S.; Mondal, N.K. Chromium toxicity and ultrastructural deformation of Cicer arietinum with special reference of root elongation and coleoptile growth. Ann. Agrar. Sci. 2017, 15, 396–401. [Google Scholar] [CrossRef]
- Ghasemi, S.; Khoshgoftarmanesh, A.H.; Hadadzadeh, H.; Jafari, M. Synthesis of iron-amino acid chelates and evaluation of their efficacy as iron source and growth stimulator for tomato in nutrient solution culture. J. Plant Growth Regul. 2012, 31, 498–508. [Google Scholar] [CrossRef]
- Kosar, F.; Akram, N.; Ashraf, M. Exogenously-applied 5-aminolevulinic acid modulates some key physiological characteristics and antioxidative defense system in spring wheat (Triticum aestivum L.) seedlings under water stress. South Afr. J. Bot. 2015, 96, 71–77. [Google Scholar] [CrossRef] [Green Version]
- Saleem, M.H.; Kamran, M.; Zhou, Y.; Parveen, A.; Rehman, M.; Ahmar, S.; Malik, Z.; Mustafa, A.; Anjum, R.M.A.; Wang, B.; et al. Appraising growth, oxidative stress and copper phytoextraction potential of flax (Linum usitatissimum L.) grown in soil differentially spiked with copper. J. Environ. Manag. 2020, 257, 109994. [Google Scholar] [CrossRef]
- Teixeira, W.F.; Fagan, E.B.; Soares, L.H.; Umburanas, R.C.; Reichardt, K.; Neto, D.D. Foliar and seed application of amino acids affects the antioxidant metabolism of the soybean crop. Front. Plant Sci. 2017, 8, 327. [Google Scholar] [CrossRef] [Green Version]

| Texture | Clay Loam |
|---|---|
| Silt | 12.9 |
| Sand | 63.4 |
| Clay | 22.3 |
| pH (H2O mixture) | 7.1 |
| Electrical conductivity (dS m−1) | 3.83 |
| Cation exchange capacity (cmol kg−1) 4.9 | 4.89 |
| Soluble CO3−2 (mmol L−1) | 0.87 |
| Soluble HCO3 (mmol L−1) | 3.78 |
| Soluble Cl− (mmol L−1) | 6.31 |
| Soluble Ca2+ + Mg2+ (mmol L−1) | 15.89 |
| Organic matter (%) | 0.49 |
| Ni (mg kg−1) | 0.21 |
| Cu (mg kg−1) | 0.35 |
| Zn (mg kg−1) | 0.84 |
| Cr (mg kg−1) | 0.24 |
| Parameters | Values | Permissible Limits * |
|---|---|---|
| EC (dS m−1) | 1.41 | <1.5 |
| Sodium absorption rate (mmol L−1)1/2 | 4.02 | <7.5 |
| Residual sodium carbonate (mmol c L−1) | 2.24 | <2.0 |
| Ni (mg L−1) | 0.09 | 0.20 |
| Cd (mg L−1) | 0.04 | 0.01 |
| Pb (mg L−1) | 1.24 | 5.0 |
| Co (mg L−1) | 0.02 | 0.05 |
| Cr (mg L−1) | 4.03 | 0.10 |
| Zn (mg L−1) | 1.95 | 2.00 |
| Treatments | Plant Height (cm) | Number of Leaves | Leaf Area (cm2) | Root Length (cm) | Root Fresh Weight (mg) | Root Dry Weight (mg) | Leaf Fresh Weight (mg) | Leaf Dry Weight (mg) |
|---|---|---|---|---|---|---|---|---|
| Ck | 31 ± 0.6ab | 11 ± 1ab | 110 ± 7ab | 13 ± 0.4cd | 4025 ± 55b | 837 ± 36b | 19,336 ± 740b | 2457 ± 50b |
| T1 | 34 ± 1.0a | 13 ± 1a | 126 ± 5a | 17 ± 1a | 5038 ± 70a | 999 ± 46a | 23,675 ± 1200a | 2658 ± 64a |
| T2 | 22 ± 1.6de | 6 ± 0.6bc | 72 ± 5d | 12 ± 0.6de | 1661 ± 57d | 334 ± 13d | 11,991 ± 467d | 1573 ± 56d |
| T3 | 29 ± 1.2bc | 8 ± 0.6b | 91 ± 4c | 15 ± 0.7ab | 2191 ± 64c | 470 ± 18c | 15,599 ± 561c | 1804 ± 50c |
| T4 | 17 ± 0.6ef | 6 ± 1c | 38 ± 3f | 10 ± 0.3e | 798 ± 37f | 198 ± 12e | 5005 ± 100f | 729 ± 30f |
| T5 | 24 ± 1.7cd | 8 ± 0.6b | 53 ± 3e | 14 ± 0.8bc | 1179 ± 53e | 292 ± 19d | 6872 ± 170e | 909 ± 30e |
| T6 | 10 ± 0.6g | 5 ± 0.6c | 17 ± 1g | 8 ± 0.5f | 414 ± 13g | 88 ± 6f | 3142 ± 70g | 433 ± 24g |
| T7 | 11 ± 2.8fg | 6 ± 0.6bc | 19 ± 3g | 10 ± 0.6e | 505 ± 19g | 103 ± 10f | 3665 ± 75fg | 507 ± 28g |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaheer, I.E.; Ali, S.; Saleem, M.H.; Arslan Ashraf, M.; Ali, Q.; Abbas, Z.; Rizwan, M.; El-Sheikh, M.A.; Alyemeni, M.N.; Wijaya, L. Zinc-lysine Supplementation Mitigates Oxidative Stress in Rapeseed (Brassica napus L.) by Preventing Phytotoxicity of Chromium, When Irrigated with Tannery Wastewater. Plants 2020, 9, 1145. https://doi.org/10.3390/plants9091145
Zaheer IE, Ali S, Saleem MH, Arslan Ashraf M, Ali Q, Abbas Z, Rizwan M, El-Sheikh MA, Alyemeni MN, Wijaya L. Zinc-lysine Supplementation Mitigates Oxidative Stress in Rapeseed (Brassica napus L.) by Preventing Phytotoxicity of Chromium, When Irrigated with Tannery Wastewater. Plants. 2020; 9(9):1145. https://doi.org/10.3390/plants9091145
Chicago/Turabian StyleZaheer, Ihsan Elahi, Shafaqat Ali, Muhammad Hamzah Saleem, Muhammad Arslan Ashraf, Qurban Ali, Zohaib Abbas, Muhammad Rizwan, Mohamed A. El-Sheikh, Mohammed Nasser Alyemeni, and Leonard Wijaya. 2020. "Zinc-lysine Supplementation Mitigates Oxidative Stress in Rapeseed (Brassica napus L.) by Preventing Phytotoxicity of Chromium, When Irrigated with Tannery Wastewater" Plants 9, no. 9: 1145. https://doi.org/10.3390/plants9091145