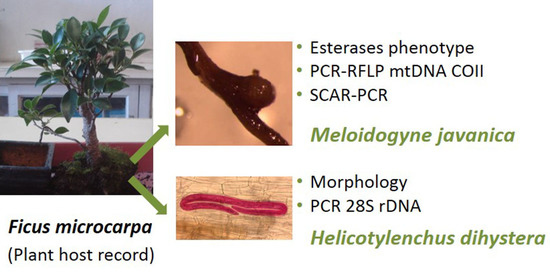
Ficus microcarpa Bonsai “Tiger bark” Parasitized by the Root-Knot Nematode Meloidogyne javanica and the Spiral Nematode Helicotylenchus dihystera, a New Plant Host Record for Both Species
Abstract
:1. Introduction
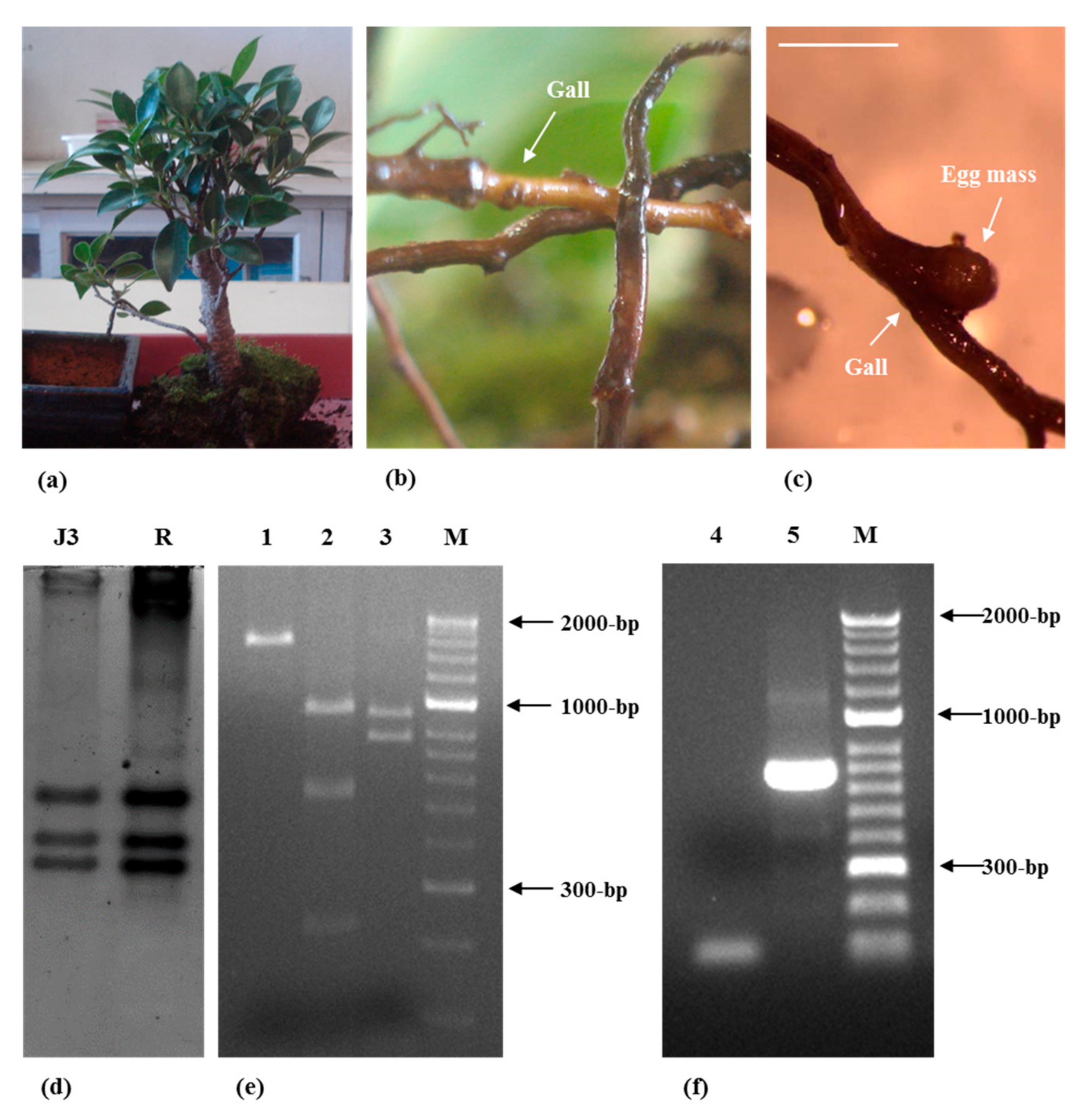
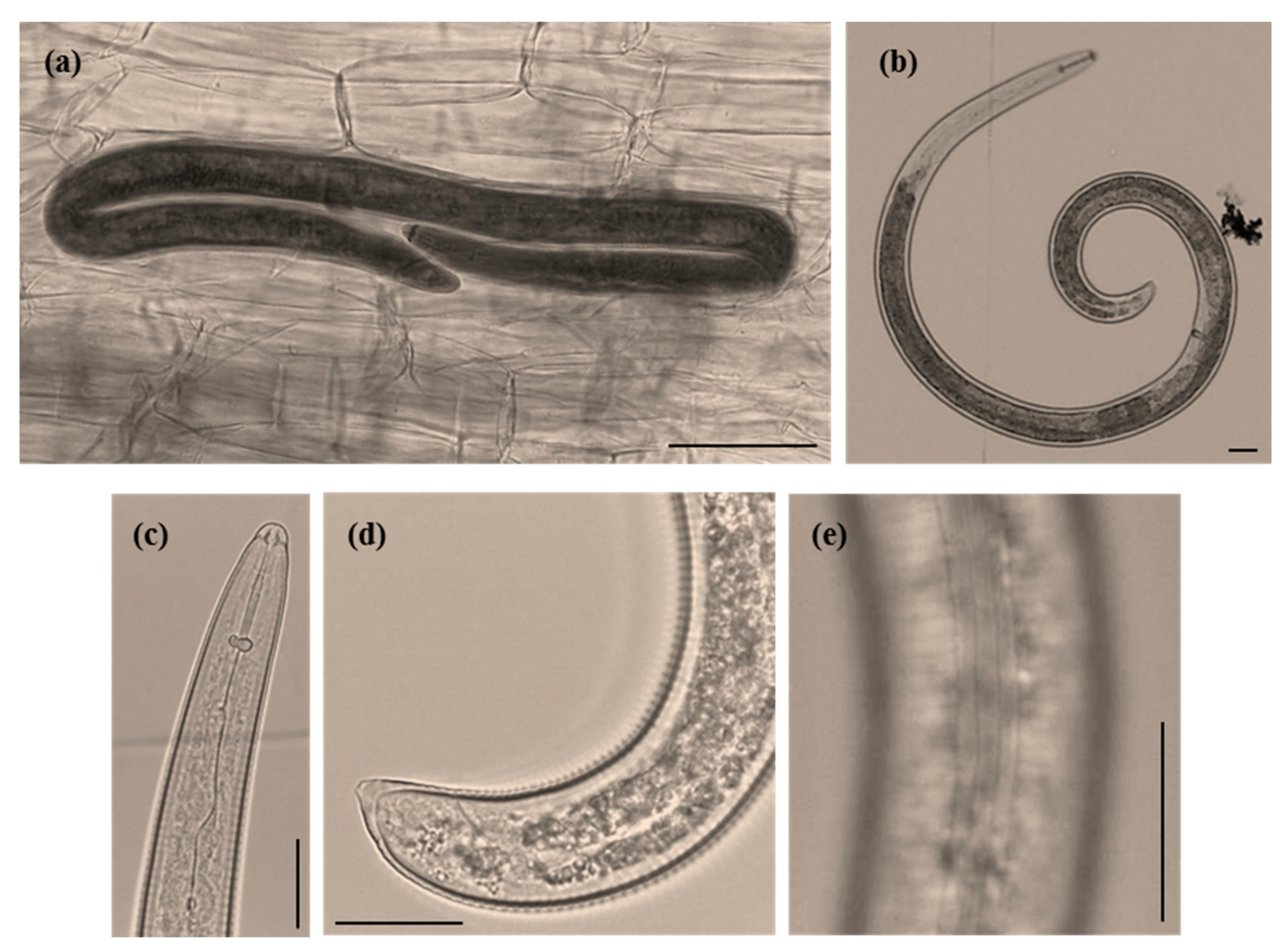
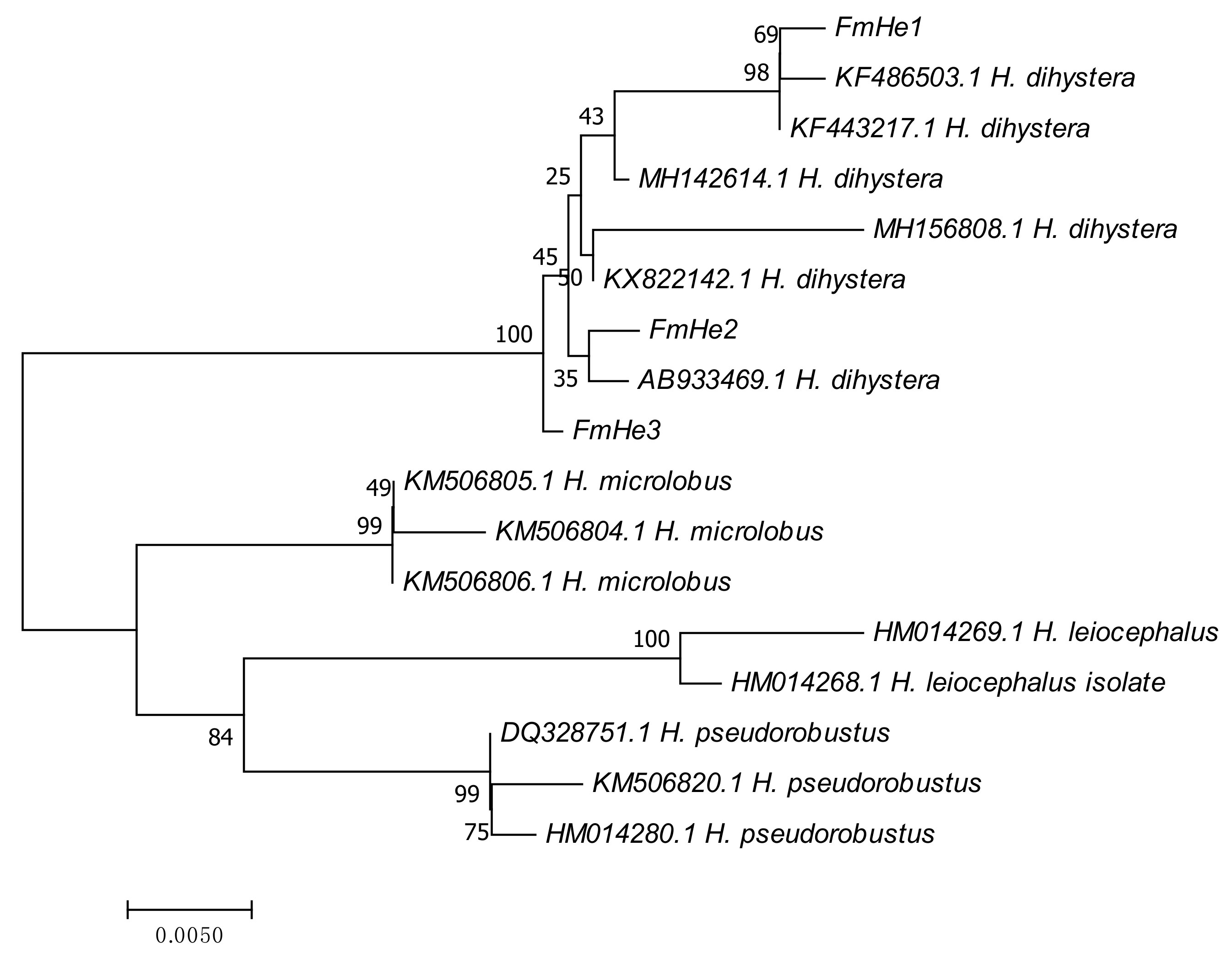
2. Results and Discussion
3. Materials and Methods
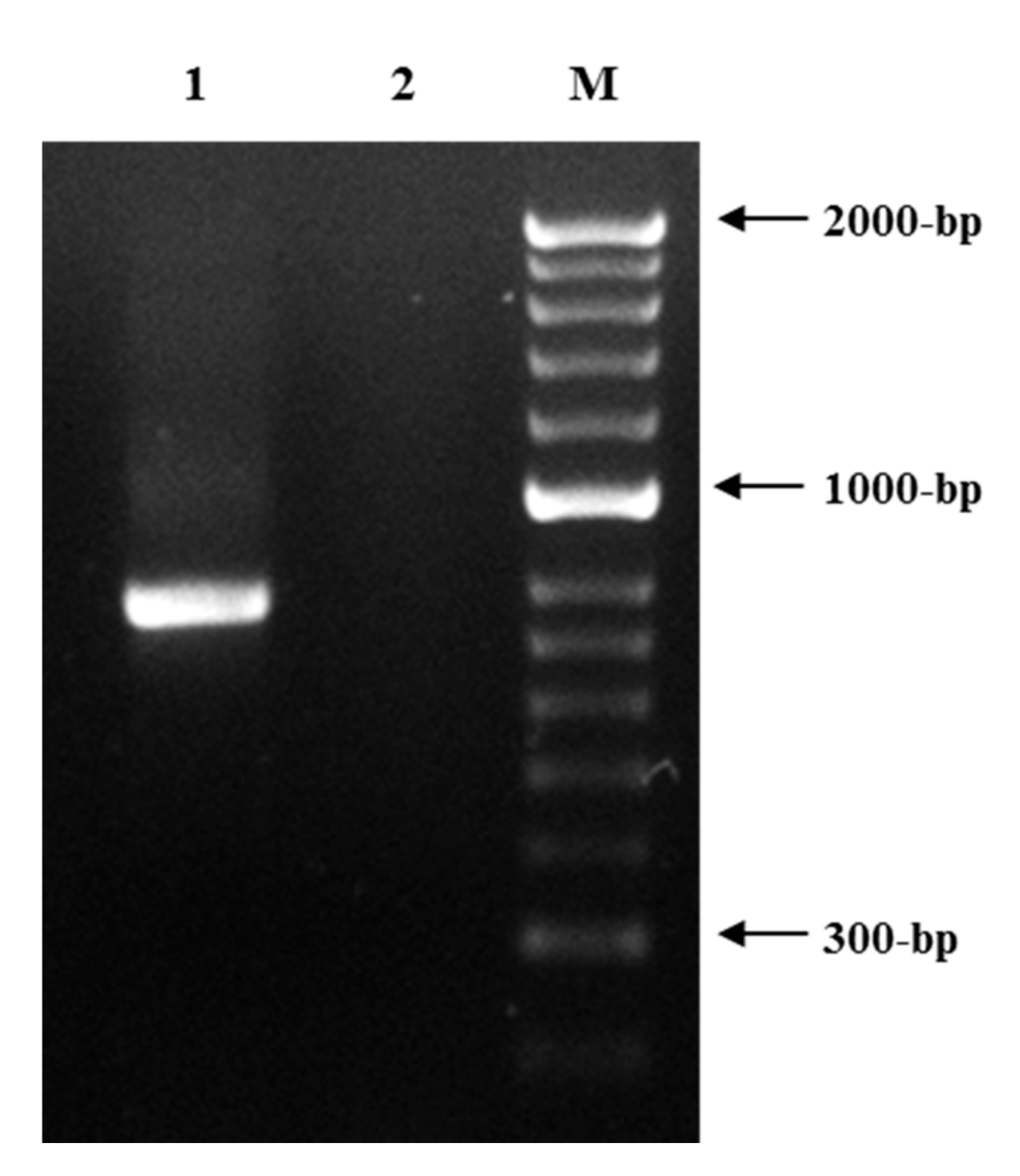
3.1. Root Knot Nematode Characterization/Identification
3.2. Spiral Nematode Characterization/Identification
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Eschen, R.; Roques, A.; Santini, A. Taxonomic dissimilarity in patterns of interception and establishment of alien arthropods, nematodes and pathogens affecting woody plants in Europe. Divers. Distrib. 2015, 21, 36–45. [Google Scholar] [CrossRef]
- Roques, A.; Blackburn, M.A.T.M.; David, R.; Garnas, J.; Pys, P.; Wingfield, M.J.; Liebhold, A.M.; Duncan, R.P. Temporal and interspecific variation in rates of spread for insect species invading Europe during the last 200 years. Biol. Invasions 2016, 18, 907–920. [Google Scholar] [CrossRef]
- Vovlas, N.; Trisciuzzi, N.; Troccoli, A.; De Luca, F.; Cantalapiedra-Navarrete, C.; Castillo, P. Integrative diagnosis and parasitic habits of Cryphodera brinkmani a non-cyst forming heteroderid nematode intercepted on Japanese white pine bonsai trees imported into Italy. Eur. J. Plant Pathol. 2013, 135, 717–726. [Google Scholar] [CrossRef] [Green Version]
- Blackburn, T.M.; Essl, F.; Evans, T.; Hulme, P.E.; Jeschke, J.M.; Kühn, I.; Kumschick, S.; Marková, Z.; Mrugała, A.; Nentwig, W.; et al. A unified classification of alien species based on the magnitude of their environmental impacts. PLoS Biol. 2014, 12, e1001850. [Google Scholar] [CrossRef] [Green Version]
- IFN6. 6º Inventário Florestal Nacional. Instituto da Conservação da Natureza e das Florestas. Lisboa, Portugal. Available online: http://www2.icnf.pt/portal/florestas/ifn/ifn6 (accessed on 2 July 2020).
- De la Fuente, B.; Beck, P.S.A. Invasive species may disrupt protected area networks: Insights from the pine wood nematode spread in Portugal. Forests 2018, 9, 282. [Google Scholar] [CrossRef] [Green Version]
- EPPO. Bursaphelenchus xylophilus. EPPO Datasheets on Pests Recommended for Regulation; EPPO Global Database: Paris, France. Available online: https://gd.eppo.int (accessed on 2 July 2020).
- Huang, D.; Haack, R.A.; Zhang, R. Does global warming increase establishment rates of invasive alien species? A centurial time series analysis. PLoS ONE 2011, 6, e24733. [Google Scholar] [CrossRef] [Green Version]
- Fey, S.B.; Herren, C.M. Temperature-mediated biotic interactions influence enemy release of nonnative species in warming environments. Ecology 2014, 95, 2246–2256. [Google Scholar] [CrossRef]
- Santos, D.; Abrantes, I.; Maleita, C. The quarantine root knot nematode Meloidogyne enterolobii—A potential threat to Portugal and Europe. Plant Pathol. 2019, 68, 1607–1615. [Google Scholar] [CrossRef]
- Santos, D.; Correia, A.; Abrantes, I.; Maleita, C. New hosts and records in Portugal for the root-knot nematode Meloidogyne luci. J. Nematol. 2019, 51, e2019-03. [Google Scholar] [CrossRef] [Green Version]
- European Commission. Commission implementing regulation (EU) 2018/2019. ANNEX I-List of High Risk Plants, Plant Products and Other Objects; European Commission: Brussels, Belgium. Available online: http://data.europa.eu/eli/reg_impl/2018/2019/oj (accessed on 20 May 2020).
- Anthoine, G.; Niere, B.; den Nijs, L.; Prior, T.; Pylypenco, L.; Viaene, N. Nematode interceptions in international trade of plants for planting. J. Nematol. 2014, 46, 134–135. [Google Scholar]
- Harrison, R.D. Figs and the diversity of tropical rainforests. BioScience 2005, 55, 1053–1064. [Google Scholar] [CrossRef]
- McSorley, R. Plant Parasitic Nematodes Associated with Tropical and Subtropical Fruits; Technical Bulletin no. 823; Florida Agricultural Experiment Station: Gainesville, FL, USA, 1981; Volume 823, pp. 1–49. [Google Scholar]
- McSorley, R. Nematological problems in tropical and subtropical fruit tree crops. Nematropica 1992, 22, 103–116. [Google Scholar]
- Cohn, E.; Duncan, L.W. Nematode parasites of subtropical and tropical fruit trees. In Plant Parasitic Nematodes in Subtropical and Tropical Agriculture; Luc, M., Sikora, R.A., Bridge, J., Eds.; CABI Publishing: Wallingford, UK, 1990; pp. 347–362. [Google Scholar]
- Campos, V.P. Nematóides na cultura da figueira. Inf. Agropecu. (Belo Horizonte) 1997, 18, 33–38. [Google Scholar]
- Krnjaic, D.; Krnjaic, S.; Bacic, J. Distribution and population density of fig cyst nematode (Heterodera fici Kirjanova) in the region of SR Yugoslavia. Zast. Bilja 1997, 48, 245–251. [Google Scholar]
- Li, H.; Xu, J.; Shen, P.; Cheng, H. Distribution and seasonal dynamic changes of nematode parasites in fig main growing areas in Jiangsu Province. J. Nanjing Agric. Univ. 1999, 22, 38–41. [Google Scholar]
- Abrantes, I.M.d.O.; Vieira dos Santos, M.C.; da Conceição, I.L.P.M.; Santos, M.S.N.d.A.; Vovlas, N. Root-knot and other plant-parasitic nematodes associated with fig trees in Portugal. Nematol. Mediterr. 2008, 36, 131–136. [Google Scholar]
- Wohlfarter, M.; Giliomee, J.H.; Venter, E.; Storey, S. A survey of the arthropod pests and plant parasitic nematodes associated with commercial figs, Ficus carica (Moraceae), in South Africa. Afr. Entomol. 2011, 19, 165–172. [Google Scholar] [CrossRef]
- Mokbel, A. Nematodes and their associated host plants cultivated in Jazan province, southwest Saudi Arabia. Egypt. J. Exp. Biol. (Zool) 2014, 10, 35–39. [Google Scholar]
- Fanelli, E.; Vovlas, A.; Santoro, S.; Troccoli, A.; Lucarelli, G.; Trisciuzzi, N.; De Luca, F. Integrative diagnosis, biological observations, and histopathology of the fig cyst nematode Heterodera fici Kirjanova (1954) associated with Ficus carica L. in southern Italy. ZooKeys 2019, 823, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Karssen, G.; Wesemael, W.M.L.; Moens, M. Root-knot nematodes. In Plant Nematology; Perry, R.N., Moens, M., Eds.; CABI Publishing: Wallingford, UK, 2013; pp. 73–108. [Google Scholar]
- Quénéhervé, P.; Topart, P.; Poliakoff, F. Interception of nematodes on imported bonsai in Martinique. Nematropica 1998, 28, 101–105. [Google Scholar]
- EPPO. Study on the Risk of Imports of Plants for Planting; EPPO Technical Document; EPPO Global Database: Paris, France, 2012; Volume 1061. [Google Scholar]
- Chan, E.W.C.; Tangah, J.; Inoue, T.; Kainuma, M.; Baba, K.; Oshiro, N.; Kezuka, M.; Kimura, N. Botany, uses, chemistry and pharmacology of Ficus microcarpa: A short review. Syst. Rev. Pharm. 2017, 8, 103–111. [Google Scholar] [CrossRef]
- Hodel, D.R. New pests of landscape Ficus in California. Calif. Assoc. Pest Control Adv. (CAPCA) 2017, 5, 58–62. [Google Scholar]
- Churchill, R.C., Jr.; Ruehle, J.L. Occurrence, parasitism, and pathogenicity of nematodes associated with sycamore (Platanus occidentalis L.). J. Nematol. 1971, 3, 189–196. [Google Scholar] [PubMed]
- Maleita, C.M.; Simões, M.J.; Egas, C.; Curtis, R.H.C.; Abrantes, I.M.d.O. Biometrical, biochemical, and molecular diagnosis of Portuguese Meloidogyne hispanica isolates. Plant Dis. 2012, 96, 865–874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lima-Medina, I.; Somavilla, L.; Carneiro, R.M.D.G.; Gomes, C.B. Espécies de Meloidogyne em figueira (Ficus carica) e em plantas infestantes. Nematropica 2013, 43, 56–62. [Google Scholar]
- Hunt, J. List of Intercepted Plant Pests; Bureau of Entomology and Plant Quarantine, United States Department of Agriculture: Washington, DC, USA, 1956. [Google Scholar]
- Santos, M.S.N.d.A. Identificação de populações portuguesas de Meloidogyne spp. pelas reações induzidas em plantas diferenciadoras-I. In I Congresso Português de Fitiatria e Fitofarmacologia II; Instituto Superior de Agronomia: Lisboa, Portugal, 1980; pp. 147–150. [Google Scholar]
- Santos, M.S.N.d.A.; Abrantes, I.M.d.O. Root-knot nematodes in Portugal. In Proceedings of the Second Research Planning Conference on Root-Knot Nematodes, Meloidogyne spp., Region VII. Athens, Greece, 22–26 March 1982; North Carolina State University Graphics: Raleigh, NC, USA, 1982; pp. 17–23. [Google Scholar]
- Reis, L.G.L. Prospeção Nematológica. Relatório das atividades da Estação Agronómica Nacional: Oeiras, Portugal, 1983. [Google Scholar]
- Santos, M.S.N.d.A.; Abrantes, I.M.d.O.; Fernandes, M.F.M. Identificação de populações portuguesas de Meloidogyne spp. (Nematoda: Meloidogynidae) pelas reações induzidas em plantas diferenciadoras-III. Ciênc. Biol. Ecol. Syst. (Portugal) 1987, 7, 37–42. [Google Scholar]
- da Conceição, I.L.P.M.; da Cunha, M.J.M.; Feio, G.; Correia, M.; Vieira dos Santos, M.C.; Abrantes, I.M.d.O.; Santos, M.S.N.d.A. Root-knot nematodes, Meloidogyne spp., on potato in Portugal. Nematology 2009, 11, 311–313. [Google Scholar]
- Esteves, I.; Maleita, C.; Abrantes, I. Root-lesion and root-knot nematodes parasitizing potato. Eur. J. Plant Pathol. 2015, 141, 397–406. [Google Scholar] [CrossRef] [Green Version]
- Rusinque, L.; Inácio, M.L.; Mota, M.; Nóbrega, F. Morphological, biochemical and molecular characterisation of Meloidogyne javanica, from North Portugal, in tomato. Rev. Ciênc. Agrár. 2018, 41, 201–210. [Google Scholar] [CrossRef]
- Fortuner, R.; Merny, G.; Roux, C. Morphometrical variability in Helicotylenchus Steiner, 1945. 3: Observations on African populations of Helicotylenchus dihystera and considerations on related species. Rev. Nématol. 1981, 4, 235–260. [Google Scholar]
- Benson, D.M.; Barker, K.R.; Aycock, R. Effects of density of Helicotylenchus dihystera and Pratylenchus vulnus on American Boxwood growing in microplots. J. Nematol. 1976, 8, 322–326. [Google Scholar] [PubMed]
- Willers, P.; Grech, N.M. Pathogenicity of the spiral nematode Helicotylenchus dihystera to Guava. Plant Dis. 1986, 70, 352. [Google Scholar] [CrossRef]
- Pedersen, J.F.; Rodriguez-Kabana, R. Nematode response to cool season annual graminaceous species and cultivars. Ann. Nematol. 1987, 1, 116–118. [Google Scholar]
- Siddiqi, M.R. Helicotylenchus dihystera. C.I.H. Descriptions of Plant-Parasitic Nematodes. Set 1–9; Commonwealth Agricultural Bureaux: Farnham Royal, UK, 1972. [Google Scholar]
- CABI/EPPO. Helicotylenchus dihystera. Distribution map. In Distribution Maps of Plant Diseases, 1st ed.; CABI Publishing: Wallingford, UK, 2010. [Google Scholar]
- Plantwise Knowledge Bank. Common Spiral Nematode Helicotylenchus Dihystera; CABI Publishing: Wallingford, UK, 1956; Available online: https://www.plantwise.org/knowledgebank/datasheet/26824 (accessed on 30 March 2020).
- Macara, A.M. Contribuição para o estudo de algumas espécies do género Heterodera Schmidt 1871 encontradas em Portugal-Relatório Final do Curso de Engenheiro Agrónomo; Instituto Superior de Agronomia, Universidade de Lisboa: Lisboa, Portugal, 1962. [Google Scholar]
- Sher, S.A. Revision of the Hoplolaiminae (Nematoda) VI Helicotylenchus Steiner, 1945. Nematologica 1966, 12, 1–56. [Google Scholar] [CrossRef]
- Siddiqi, M.R. On the genus Helicotylenchus Steiner, 1945 (Nematoda: Tylenchida), with descriptions of nine new species. Nematologica 1972, 18, 74–91. [Google Scholar] [CrossRef]
- Coyne, D.L.; Fourie, H.H.; Moens, M. Current and future management strategies in resource-poor farming. In Root-Knot Nematodes; Perry, R.N., Moens, M., Starr, J.L., Eds.; CABI Publishing: Wallingford, UK, 2009; pp. 444–475. [Google Scholar]
- Nicol, J.M.; Turner, S.J.; Coyne, D.L.; den Nijs, L.; Hockland, S.; Tahna Maafi, Z. Current nematode threats to world agriculture. In Genomics and Molecular Genetics of Plant-Nematode Interactions; Jones, J., Gheysen, G., Fenoll, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 21–44. [Google Scholar]
- Byrd, D.W.; Kirkpatrick, T.; Barker, K.R. An improved technique for clearing and staining plant tissues for detection of nematodes. J. Nematol. 1983, 15, 142–143. [Google Scholar]
- Whitehead, A.G.; Hemming, J.R. A comparison of some quantitative methods of extracting small vermiform nematodes from soil. Ann. Appl. Biol. 1965, 55, 25–38. [Google Scholar] [CrossRef]
- Pais, C.S.; Abrantes, I.M.d.O. Esterase and malate dehydrogenase phenotypes in Portuguese populations of Meloidogyne species. J. Nematol. 1989, 21, 342–346. [Google Scholar]
- Zijlstra, C.; Donkers-Venne, D.T.H.M.; Fargette, M. Identification of Meloidogyne incognita, M. javanica and M. arenaria using sequence characterised amplified region (SCAR) based PCR assays. Nematology 2000, 2, 847–853. [Google Scholar] [CrossRef] [Green Version]
- De Leij, O.; Félix, M.A.; Frisse, L.; Nadler, S.; Sternberg, P.; Thomas, W. Molecular and morphological characterisation of two reproductively isolated species with mirror image anatomy (Nematoda: Cephalobidae). Nematology 1999, 1, 591–612. [Google Scholar]
- Hall, T.A. BioEdit: A user friendly biological sequence alignment editor and analyses program for windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7. 0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, D.; Abrantes, I.; Maleita, C. Ficus microcarpa Bonsai “Tiger bark” Parasitized by the Root-Knot Nematode Meloidogyne javanica and the Spiral Nematode Helicotylenchus dihystera, a New Plant Host Record for Both Species. Plants 2020, 9, 1085. https://doi.org/10.3390/plants9091085
Santos D, Abrantes I, Maleita C. Ficus microcarpa Bonsai “Tiger bark” Parasitized by the Root-Knot Nematode Meloidogyne javanica and the Spiral Nematode Helicotylenchus dihystera, a New Plant Host Record for Both Species. Plants. 2020; 9(9):1085. https://doi.org/10.3390/plants9091085
Chicago/Turabian StyleSantos, Duarte, Isabel Abrantes, and Carla Maleita. 2020. "Ficus microcarpa Bonsai “Tiger bark” Parasitized by the Root-Knot Nematode Meloidogyne javanica and the Spiral Nematode Helicotylenchus dihystera, a New Plant Host Record for Both Species" Plants 9, no. 9: 1085. https://doi.org/10.3390/plants9091085