Leaf Anatomy, Morphology and Photosynthesis of Three Tundra Shrubs after 7-Year Experimental Warming on Changbai Mountain
Abstract
:1. Introduction
2. Results
2.1. Environmental Measurements
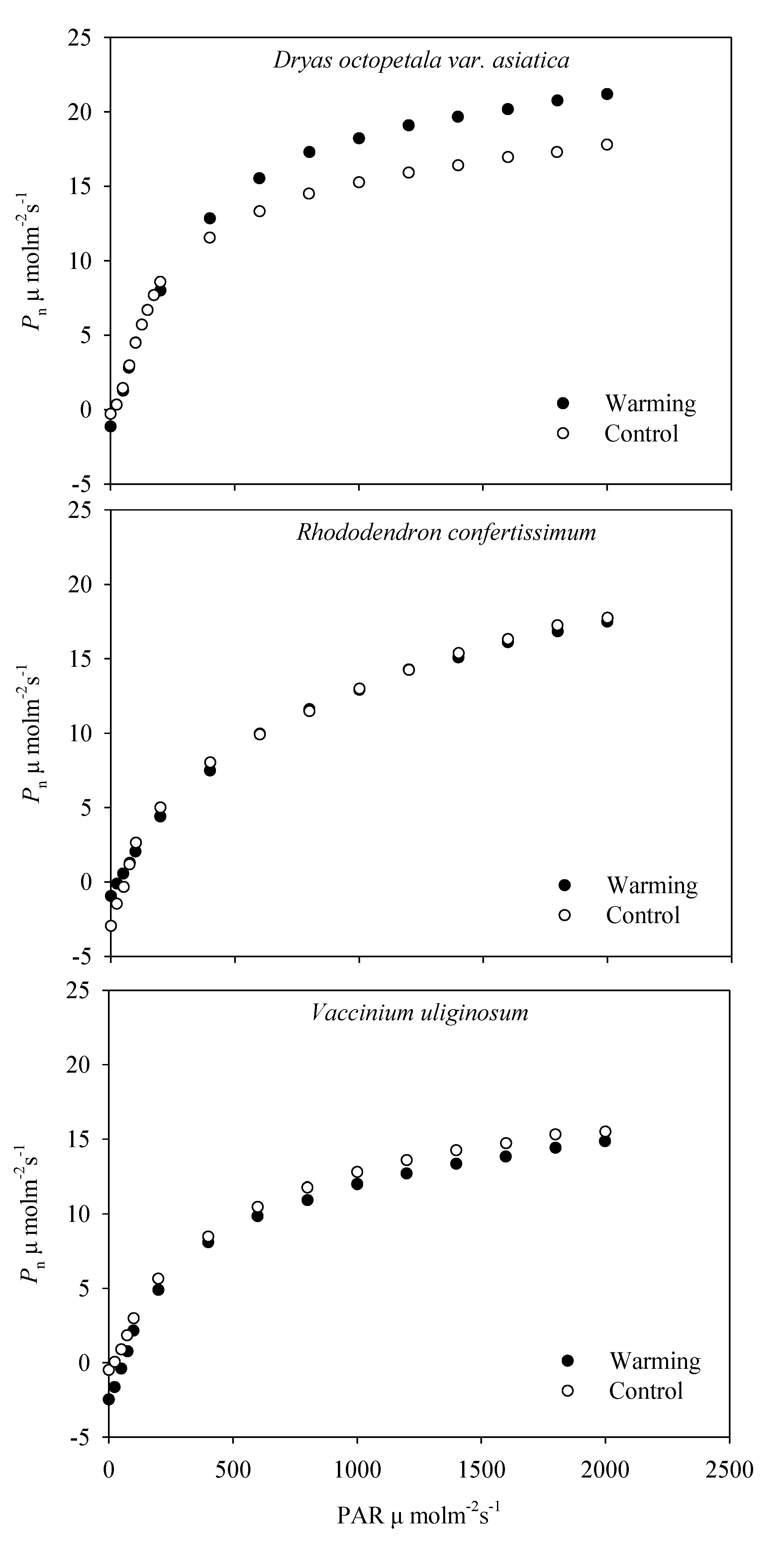
2.2. The Light Response Curves
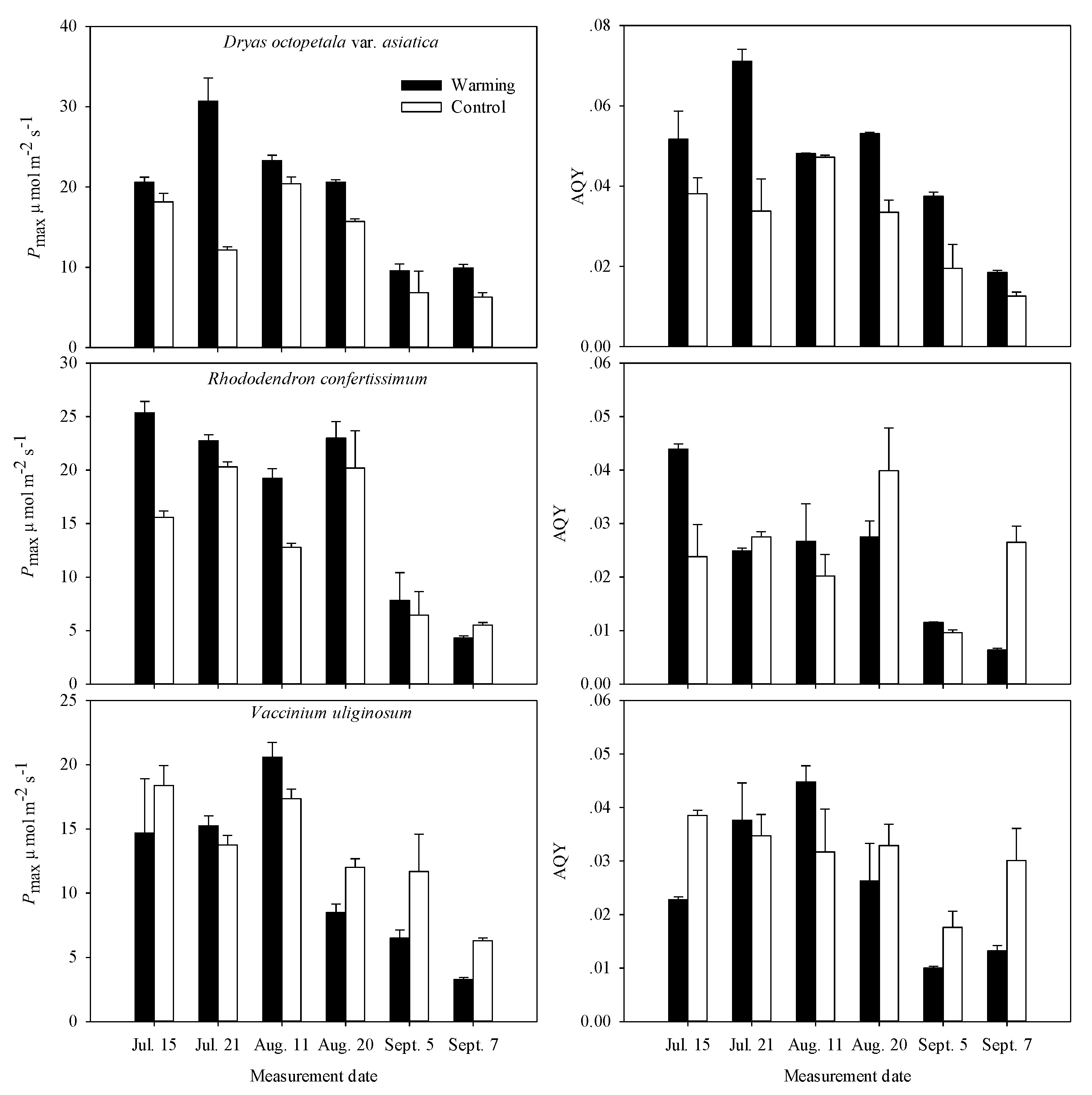
2.3. Photosynthetic Characteristics
2.4. Leaf Anatomy
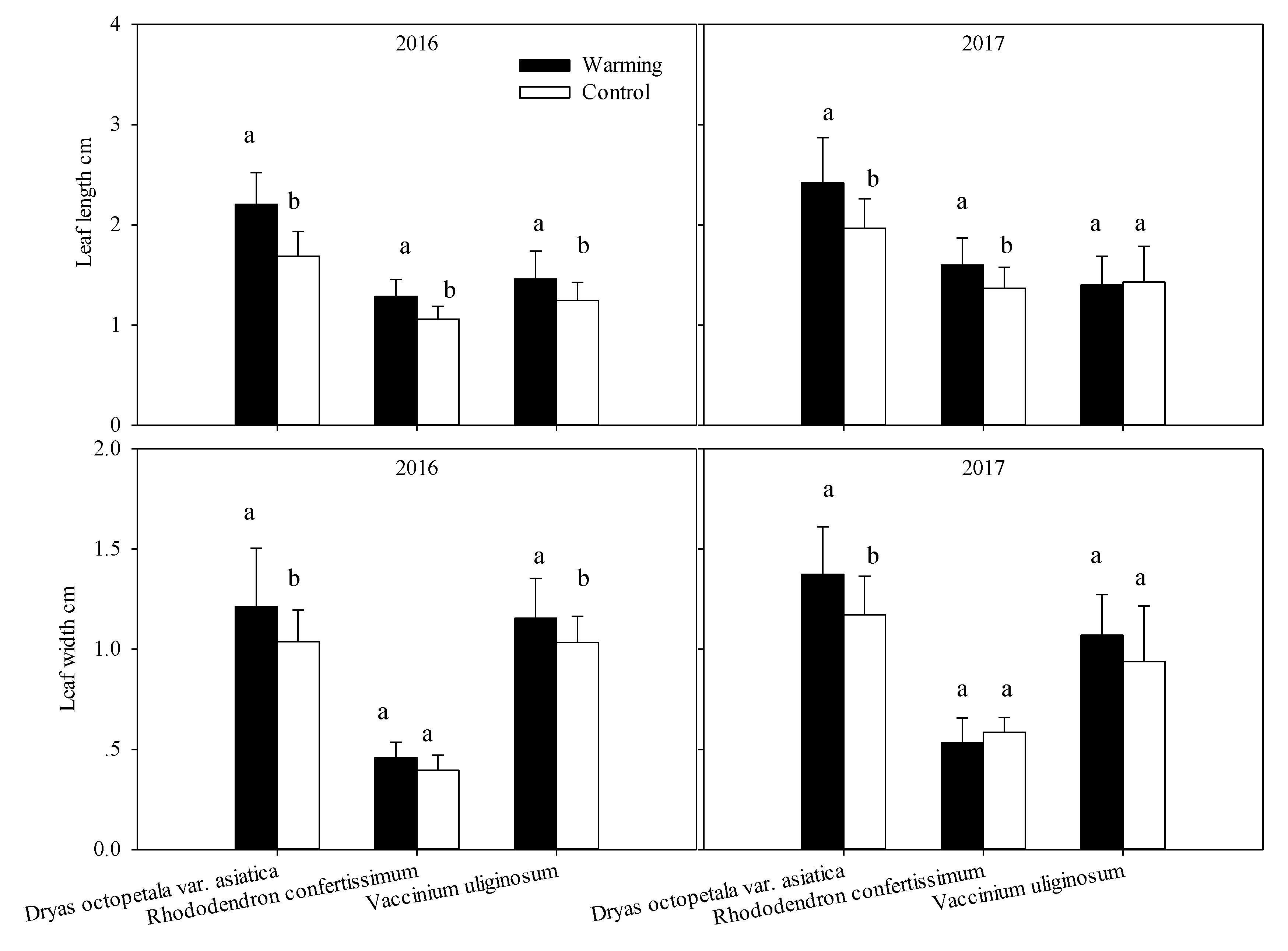
2.5. Leaf Morphology
3. Discussions
4. Materials and Methods
4.1. Study Site and Experimental Design
4.2. Photosynthetic Light Response Curves
4.3. Leaf Morphology Measurement
4.4. Leaf Anatomy Measurement
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Deslippe, J.R.; Simard, S.W. Below-ground carbon transfer among Betula nana may increase with warming in Arctic tundra. New Phytol. 2011, 192, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Shaver, G.R.; Canadell, J.; Chapin, F.S., III; Gurevitch, J.; Harte, J.; Henry, G.; Ineson, P.; Jonasson, S.; Melillo, J.; Pitelka, L.; et al. Global warming and terrestrial ecosystems: A conceptual framework for analysis. Bioscience 2000, 50, 871–882. [Google Scholar] [CrossRef]
- Henry, G.H.R.; Molau, U. Tundra plants and climate change: The International Tundra Experiment (ITEX). Glob. Chang. Biol. 1997, 3, 1–9. [Google Scholar] [CrossRef]
- Arft, A.M.; Walker, M.D.; Gurevitch, J.; Alatalo, J.M.; Bret-Harte, M.S.; Dale, M.; Diemer, M.; Gugerli, F.; Henry, G.H.R.; Jones, M.H.; et al. Responses of tundra plants to experimental warming: Meta-analysis of the International Tundra Experiment. Ecol. Monogr. 1999, 69, 491–511. [Google Scholar] [CrossRef]
- Starr, G.; Oberbauer, S.F.; Pop, E.W. Effects of lengthened growing season and soil warming on the phenology and physiology of Polygonum bistorta. Glob. Chang. Biol. 2000, 6, 357–369. [Google Scholar] [CrossRef]
- Hallinger, M.; Manthey, M.; Wilmking, M. Establishing a missing link: Warm summers and winter snow cover promote shrub expansion into alpine tundra in Scandinavia. New Phytol. 2010, 186, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.D.; Wahren, C.H.; Hollister, R.D.; Henry, G.H.R.; Ahlquist, L.E.; Alatalo, J.M.; Bret-Harte, M.S.; Calef, M.P.; Callaghan, T.V.; Carroll, A.B.; et al. Plant community responses to experimental warming across the tundra biome. Prod. Nalt. Acad. Sci. USA 2006, 103, 1342–1346. [Google Scholar] [CrossRef] [Green Version]
- Weijers, S.; Buchwal, A.; Blok, D.; Löffler, J.; Elberling, B. High Arctic summer warming tracked by increased Cassiope tetragona growth in the world’s northernmost polar desert. Glob. Chang. Biol. 2017, 23, 5006–5020. [Google Scholar] [CrossRef]
- Welker, J.M.; Fahnestock, J.T.; Henry, G.H.R.; O’dea, K.W.; Chimner, R.A. CO2 exchange in three Canadian High Arctic ecosystems: Response to long-term experimental warming. Glob. Chang. Biol. 2004, 10, 1981–1995. [Google Scholar] [CrossRef]
- Björk, R.G.; Majdi, H.; Klemedtsson, L.; Lewis-Jonsson, L.; Molau, U. Long-term warming effects on root morphology, root mass distribution, and microbial activity in two dry tundra plant communities in northern Sweden. New Phytol. 2007, 176, 862–873. [Google Scholar] [CrossRef]
- Schollert, M.; Kivimäenpää, M.; Valolahti, H.M.; Rinnan, R. Climate change alters leaf anatomy, but has no effects on volatile emissions from arctic plants. Plant Cell Environ. 2015, 38, 2048–2060. [Google Scholar] [CrossRef]
- Slot, M.; Winter, K. In Situ temperature response of photosynthesis of 42 tree and liana species in the canopy of two Panamanian lowland tropical forests with contrasting rainfall regimes. New Phytol. 2017, 214, 1103–1117. [Google Scholar] [CrossRef]
- Oberbauer, S.F.; Tweedie, C.E.; Welker, J.M.; Fahnestock, J.T.; Henry, G.H.R.; Webber, P.J.; Hollister, R.D.; Walker, M.D.; Kuchy, A.; Elmore, E.; et al. Tundra CO2 fluxes in response to experimental warming across latitudinal and moisture gradients. Ecol. Monogr. 2007, 77, 221–238. [Google Scholar] [CrossRef]
- Huemmrich, K.F.; Kinoshita, G.; Gamon, J.A.; Houston, S.; Kwon, H.; Oechel, W.C. Tundra carbon balance under varying temperature and moisture regimes. J. Geophys. Res. 2010, 115, G00I02. [Google Scholar] [CrossRef]
- Natali, S.M.; Schuur, E.A.G.; Trucco, C.; Pries, C.E.H.; Crummer, K.G.; Lopez, A.F.B. Effects of experimental warming of air, soil and permafrost on carbon balance in Alaskan tundra. Glob. Chang. Biol. 2011, 17, 1394–1407. [Google Scholar] [CrossRef]
- Natali, S.M.; Schuur, E.A.G.; Webb, E.E.; Pries, C.E.H.; Crummer, K.G. Permafrost degradation stimulates carbon loss from experimentally warmed tundra. Ecology 2014, 95, 602–608. [Google Scholar] [CrossRef] [Green Version]
- Sáez, P.L.; Cavieres, L.A.; Galmés, J.; Gil-Pelegrín, E.; Peguero-Pina, J.J.; Sancho-Knapik, D.; Vivas, M.; Sanhueza, C.; Ramírez, C.F.; Rivera, B.K.; et al. In Situ warming in the Antarctic: Effects on growth and photosynthesis in Antarctic vascular plants. New Phytol. 2018, 218, 1406–1418. [Google Scholar] [CrossRef]
- Danby, R.K.; Hik, D.S. Responses of white spruce (Picea glauca) to experimental warming at a subarctic alpine treeline. Glob. Chang. Biol. 2007, 13, 437–451. [Google Scholar] [CrossRef]
- Hudson, J.M.G.; Henry, G.H.R.; Cornwell, W.K. Taller and larger: Shifts in Arctic tundra leaf traits after 16 years of experimental warming. Glob. Chang. Biol. 2011, 17, 1013–1021. [Google Scholar] [CrossRef]
- Hartikainen, K.; Nerg, A.-M.; Kivimäenpää, M.; Kontunen-Soppela, S.; Mäenpää, M.; Oksanen, E.; Rousi, M.; Holopainen, T. Emissions of volatile organic compounds and leaf structural characteristics of European aspen (Populus tremula) grown under elevated ozone and temperature. Tree Physiol. 2009, 29, 1163–1173. [Google Scholar] [CrossRef]
- Schollert, M.; Kivimäenpää, M.; Michelsen, A.; Blok, D.; Rinnan, R. Leaf anatomy, BVOC emission and CO2 exchange of arctic plants following snow addition and summer warming. Ann. Bot. 2017, 119, 433–445. [Google Scholar] [CrossRef]
- Cavieres, L.A.; Sáez, P.; Sanhueza, C.; Sierra-Almeida, A.; Rabert, C.; Corcuera, L.J.; Alberdi, M.; Bravo, L.A. Ecophysiological traits of Antarctic vascular plants: Their importance in the responses to climate change. Plant Ecol. 2016, 217, 343–358. [Google Scholar] [CrossRef]
- Hernández-Fuentes, C.; Bravo, L.A.; Cavieres, L.A. Photosynthetic responses and photoprotection strategies of Phacelia secunda plants exposed to experimental warming at different elevations in the central Chilean Andes. Alp. Bot. 2015, 125, 87–99. [Google Scholar] [CrossRef]
- Sharp, E.D.; Sullivan, P.F.; Steltzer, H.; Csank, A.Z.; Welker, J.M. Complex carbon cycle responses to multi-level warming and supplemental summer rain in the high Arctic. Glob. Chang. Biol. 2013, 19, 1780–1792. [Google Scholar] [CrossRef]
- Carroll, C.J.W.; Knapp, A.K.; Martin, P.H. Dominant tree species of the Colorado Rockies have divergent physiological and morphological responses to warming. For. Ecol. Manag. 2017, 402, 234–240. [Google Scholar] [CrossRef]
- Berry, J.; Björkman, O. Photosynthetic response and adaptation to temperature in higher plants. Annu. Rev. Plant Physiol. 1980, 31, 491–543. [Google Scholar] [CrossRef]
- Yamori, W.; Hikosaka, K.; Way, D.A. Temperature response of photosynthesis in C3, C4, and CAM plants: Temperature acclimation and temperature adaptation. Photosynth. Res. 2014, 119, 101–117. [Google Scholar] [CrossRef]
- Bacelar, E.A.; Correia, C.M.; Moutinho-Pereira, J.M.; Gonçalves, B.C.; Lopes, J.I.; Torres-Pereira, J.M.G. Sclerophylly and leaf anatomical traits of five field-grown olive cultivars growing under drought conditions. Tree Physiol. 2004, 24, 233–239. [Google Scholar] [CrossRef]
- Luomala, E.M.; Laitinen, K.; Sutinen, S.; Kellomäki, S.; Vapaavuori, E. Stomatal density, anatomy and nutrient concentrations of Scots pine needles are affected by elevated CO2 and temperature. Plant Cell Environ. 2005, 28, 733–749. [Google Scholar] [CrossRef]
- Chartzoulakis, K.; Bosabalidis, A.; Patakas, A.; Vemmos, S. Effects of water stress on water relations, gas exchange and leaf structure of olive tree. Acta Hortic. 2000, 537, 241–247. [Google Scholar] [CrossRef]
- Liu, Q.J.; Zhang, G.C.; Xu, Q.Q.; Wang, Y.D.; Wang, H.M. Simulation of soil respiration in response to temperature under snowpacks in the Changbai Mountain, China. Chinese J. Plant Ecol. 2010, 34, 477–487. [Google Scholar]
- Zhou, Y.M.; Frank, H.; Zhou, C.L.; Jiang, X.J.; Wang, X.X.; Li, M.-H. Experimental warming of a mountain tundra increases soil CO2 effluxes and enhances CH4 and N2O uptake at Changbai Mountain, China. Sci. Rep. 2016, 6, 21108. [Google Scholar] [CrossRef]
- Zong, S.; Jin, Y.; Xu, J.; Wu, Z.; He, H.; Du, H.; Wang, L. Nitrogen deposition but not climate warming promotes Deyeuxia angustifolia encroachment in alpine tundra of the Changbai Mountains, Northeast China. Sci. Total Environ. 2016, 544, 85–93. [Google Scholar] [CrossRef]
- Prioul, J.L.; Chartier, P. Partitioning of transfer and carboxylation components of intracellular resistance to photosynthetic CO2 fixation: A critical analysis of the methods used. Ann. Bot. 1977, 41, 789–800. [Google Scholar] [CrossRef]
| Parameter | 2016 | Significance | 2017 | Significance | ||||
|---|---|---|---|---|---|---|---|---|
| OTCs | Controls | Difference | OTCs | Controls | Difference | |||
| Daily air temperature (°C) | 15.3 | 13.7 | +1.6 | ** | 13.4 | 12.0 | +1.4 | ** |
| Daily relative humidity (%) | 85 | 84 | +1 | ns | 79 | 77 | +2 | ns |
| Daily soil temperature at 10 cm depth (°C) | 13.7 | 13.1 | +0.6 | ns | 13.9 | 12.9 | +1.0 | ns |
| Daily soil water content (m3 m−3) | 0.314 | 0.358 | −0.044 | *** | 0.257 | 0.309 | −0.052 | *** |
| Daytime air temperature (°C) | 18.7 | 16.2 | +2.5 | *** | 16.0 | 13.1 | + 2.9 | *** |
| Daytime relative humidity (%) | 78 | 81 | 3 | ns | 72 | 73 | 1 | ns |
| Daytime soil temperature at 10 cm depth (°C) | 17.5 | 14.6 | +2.9 | *** | 17.8 | 14.5 | −3.3 | *** |
| Daytime soil water content (m3 m−3) | 0.313 | 0.358 | −0.045 | *** | 0.257 | 0.309 | −0.052 | *** |
| Nighttime air temperature (°C) | 12.1 | 11.2 | +0.9 | * | 10.9 | 10.6 | +0.3 | * |
| Nighttime relative humidity (%) | 91 | 86 | +5 | ns | 86 | 80 | +6 | ns |
| Nighttime soil temperature at 10 cm depth (°C) | 11.3 | 11.3 | 0 | ns | 11.0 | 11.2 | −0.2 | ns |
| Nighttime soil water content (m3 m−3) | 0.315 | 0.358 | −0.043 | *** | 0.257 | 0.309 | −0.052 | *** |
| Daytime PAR (μmol m−2 s−1) | 655 | 676 | 21 | ns | 722 | 768 | −46 | ns |
| Dr. octopetala var. asiatica | Rh. confertissimum | V. uliginosum | ||||
|---|---|---|---|---|---|---|
| Pmax | AQY | Pmax | AQY | Pmax | AQY | |
| Treatment | ** | * | *** | ns | ns | * |
| Measurement date | * | * | ns | ns | ns | ns |
| Interaction | ns | ns | ns | ns | ns | ns |
| July | August | September | Whole Growing Season | |||||
|---|---|---|---|---|---|---|---|---|
| Warming | Control | Warming | Control | Warming | Control | Warming | Control | |
| Dryas octopetala var. asiatica | ||||||||
| Pmax (μmol m−2 s−1) | 25.6 | 15.2 | 21.9 | 18.1 | 9.7 | 6.6 | 19.1 | 13.3 |
| AQY | 0.061 | 0.036 | 0.051 | 0.040 | 0.028 | 0.016 | 0.047 | 0.031 |
| Rhododendron confertissimum | ||||||||
| Pmax (μmol m−2 s−1) | 24.0 | 18.0 | 21.1 | 16.5 | 6.1 | 6.0 | 17.1 | 13.5 |
| AQY | 0.034 | 0.026 | 0.027 | 0.030 | 0.009 | 0.018 | 0.023 | 0.025 |
| Vaccinium uliginosum | ||||||||
| Pmax (μmol m−2 s−1) | 15.0 | 16.1 | 14.5 | 14.7 | 4.9 | 9.0 | 11.5 | 13.2 |
| AQY | 0.030 | 0.037 | 0.036 | 0.032 | 0.012 | 0.024 | 0.026 | 0.031 |
| Warming | Control | |||
|---|---|---|---|---|
| July | September | July | September | |
| Dryas octopetala var. asiatica | ||||
| Leaf thickness (μm) | 147.2 ± 3.033 a | 125.4 ± 2.560 b | 150.0 ± 3.131 a | 122.5 ± 3.401 b |
| Palisade thickness (μm) | 67.7 ± 2.413 a | 65.7 ± 2.528 a | 69.2 ± 1.475 a | 62.6 ± 1.341 a |
| Palisade: leaf thickness | 0.459 ± 0.012 b | 0.525 ± 0.019 a | 0.463 ± 0.007 b | 0.513 ± 0.011 a |
| Palisade: spongy parenchyma | 1.343 ± 0.114 b | 1.790 ± 0.195 a | 1.241 ± 0.044 b | 1.532 ± 0.101 ab |
| Adaxial epidermis length (μm) | 21.0 ± 0.712 a | 17.3 ± 0.609 b | 21.3 ± 0.638 a | 16.9 ± 1.009 b |
| Adaxial epidermis thickness (μm) | 13.8 ± 0.520 a | 11.2 ± 0.647 b | 12.9 ± 0.473 a | 9.7 ± 0.537 b |
| Abaxial epidermis length (μm) | 13.5 ± 0.461 a | 12.3 ± 0.856 ab | 13.0 ± 0.387 ab | 11.3 ± 0.931 b |
| Abaxial epidermis thickness (μm) | 10.2 ± 0.315 a | 8.2 ± 0.508 b | 9.9 ± 0.326 a | 6.9 ± 0.222 c |
| Rhododendron confertissimum | ||||
| Leaf thickness (μm) | 254.3 ± 10.335 b | 222.8 ± 11.513 c | 280.9 ± 6.895 a | 244.5 ± 7.164 bc |
| Palisade thickness (μm) | 63.5 ± 2.933 a | 65.9 ± 2.796 a | 69.9 ± 2.890 a | 70.8 ± 2.164 a |
| Palisade: leaf thickness | 0.252 ± 0.007 b | 0.301 ± 0.012 a | 0.247 ± 0.006 b | 0.293 ± 0.008 a |
| Palisade: spongy parenchyma | 0.404 ± 0.020 b | 0.482 ± 0.031 a | 0.372 ± 0.012 b | 0.478 ± 0.022 a |
| Adaxial epidermis length (μm) | 20.9 ± 0.583 a | 17.0 ± 0.545 b | 18.1 ± 0.494 b | 17.8 ± 0.680 b |
| Adaxial epidermis thickness (μm) | 14.2 ± 0.465 a | 9.3 ± 0.429 d | 12.7 ± 0.309 b | 10.7 ± 0.397 c |
| Abaxial epidermis length (μm) | 16.0 ± 0.557 a | 12.6 ± 0.863 b | 13.6 ± 0.389 b | 12.4 ± 0.431 b |
| Abaxial epidermis thickness (μm) | 12.2 ± 0.626 a | 8.6 ± 0.368 c | 10.5 ± 0.301 b | 8.9 ± 0.352 c |
| Cuticula thickness (μm) | 7.1 ± 0.269 b | 8.5 ± 0.542 a | 6.8 ± 0.225 b | 7.5 ± 0.250 b |
| Vaccinium uliginosum | ||||
| Leaf thickness (μm) | 174.8 ± 2.766 a | 149.5 ± 4.412 b | 180.8 ± 4.218 a | 171.2 ± 4.445 a |
| Palisade thickness (μm) | 66.3 ± 1.451 ab | 67.0 ± 3.013 ab | 70.5 ± 2.153 a | 64.0 ± 2.233 b |
| Palisade: leaf thickness | 0.381 ± 0.007 b | 0.448 ± 0.014 a | 0.391 ± 0.009 b | 0.375 ± 0.011 b |
| Palisade: spongy parenchyma | 0.793 ± 0.029 b | 1.008 ± 0.095 a | 0.886 ± 0.036 ab | 0.773 ± 0.059 b |
| Adaxial epidermis length (μm) | 21.8 ± 0.710 b | 24.0 ± 1.104 b | 29.2 ± 0.980 a | 29.0 ± 1.113 a |
| Adaxial epidermis thickness (μm) | 12.3 ± 0.475 b | 8.1 ± 0.646 c | 16.0 ± 0.576 a | 12.3 ± 0.594 b |
| Abaxial epidermis length (μm) | 17.8 ± 0.640 b | 16.3 ± 1.087 b | 22.0 ± 0.738 a | 23.2 ± 0.703 a |
| Abaxial epidermis thickness (μm) | 9.8 ± 0.280 b | 6.4 ± 0.618 c | 12.5 ± 0.515 a | 10.6 ± 0.611 b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Deng, J.; Tai, Z.; Jiang, L.; Han, J.; Meng, G.; Li, M.-H. Leaf Anatomy, Morphology and Photosynthesis of Three Tundra Shrubs after 7-Year Experimental Warming on Changbai Mountain. Plants 2019, 8, 271. https://doi.org/10.3390/plants8080271
Zhou Y, Deng J, Tai Z, Jiang L, Han J, Meng G, Li M-H. Leaf Anatomy, Morphology and Photosynthesis of Three Tundra Shrubs after 7-Year Experimental Warming on Changbai Mountain. Plants. 2019; 8(8):271. https://doi.org/10.3390/plants8080271
Chicago/Turabian StyleZhou, Yumei, Jifeng Deng, Zhijuan Tai, Lifen Jiang, Jianqiu Han, Gelei Meng, and Mai-He Li. 2019. "Leaf Anatomy, Morphology and Photosynthesis of Three Tundra Shrubs after 7-Year Experimental Warming on Changbai Mountain" Plants 8, no. 8: 271. https://doi.org/10.3390/plants8080271





