Production of White, Red and Black Quinoa (Chenopodium quinoa Willd Var. Real) Protein Isolates and Its Hydrolysates in Germinated and Non-Germinated Quinoa Samples and Antioxidant Activity Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production of Quinoa Protein Isolates (QPI) from Seeds not Germinated
2.3. Production of Quinoa Protein Isolates (QPI) from Seeds Germinated
2.4. Germinated and Non-Germinated QPI In Vitro Simulated Hydrolysis
2.5. Determination of the Degree of Hydrolysis by the Orthophthalaldehyde (OPA) Method
2.6. ABTS Assay
2.7. Oxygen Radical Absorbance Capacity-Fluorescein (ORAC-FL) Assay
2.8. DPPH Assay
2.9. Induction of Stress in Embryos Zebrafish Using AAPH
2.10. ROS Determination in Zebrafish Embryos
2.11. Statistical Analysis
3. Results and Discussion
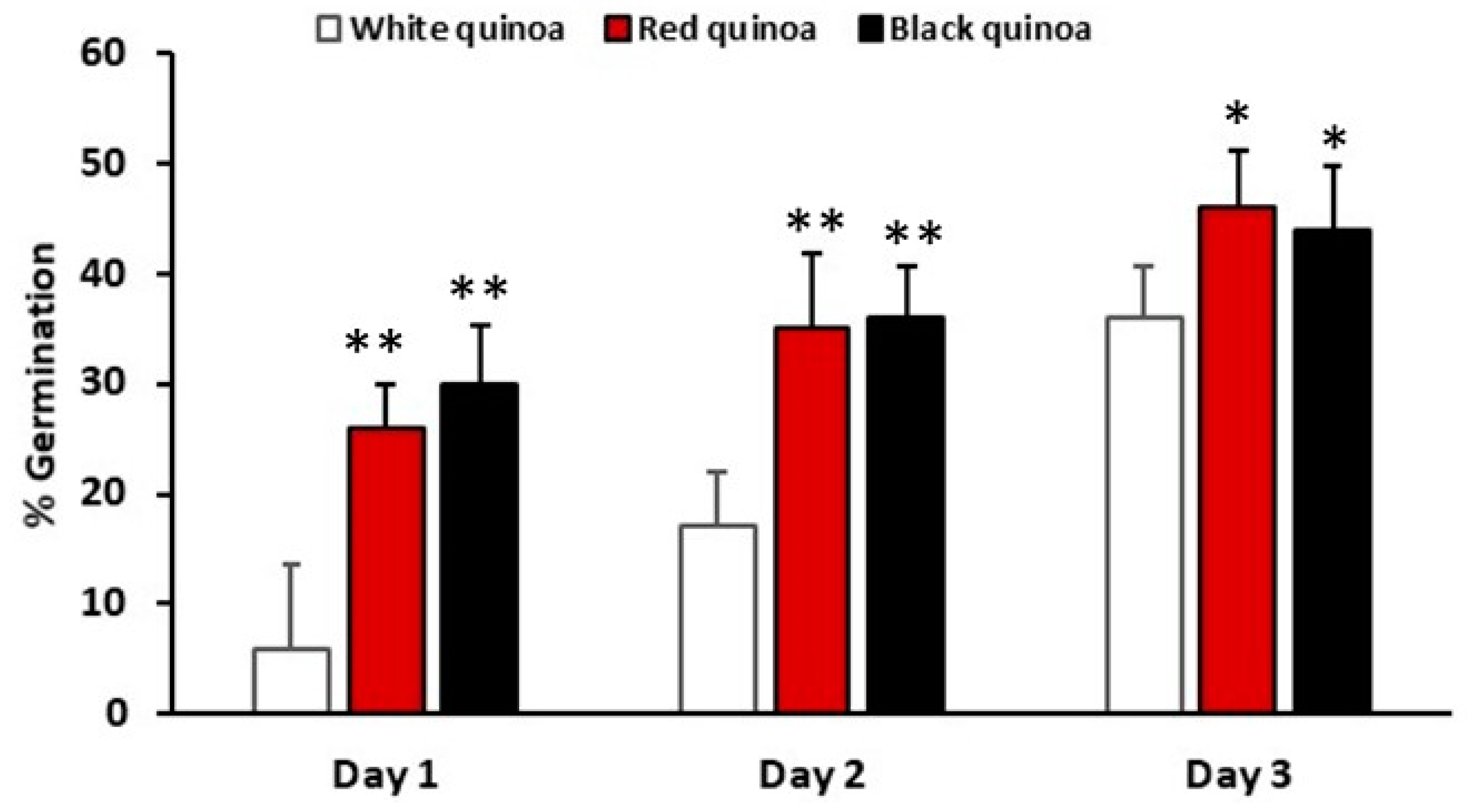
3.1. Percentage of Germination of Seeds Quinoas
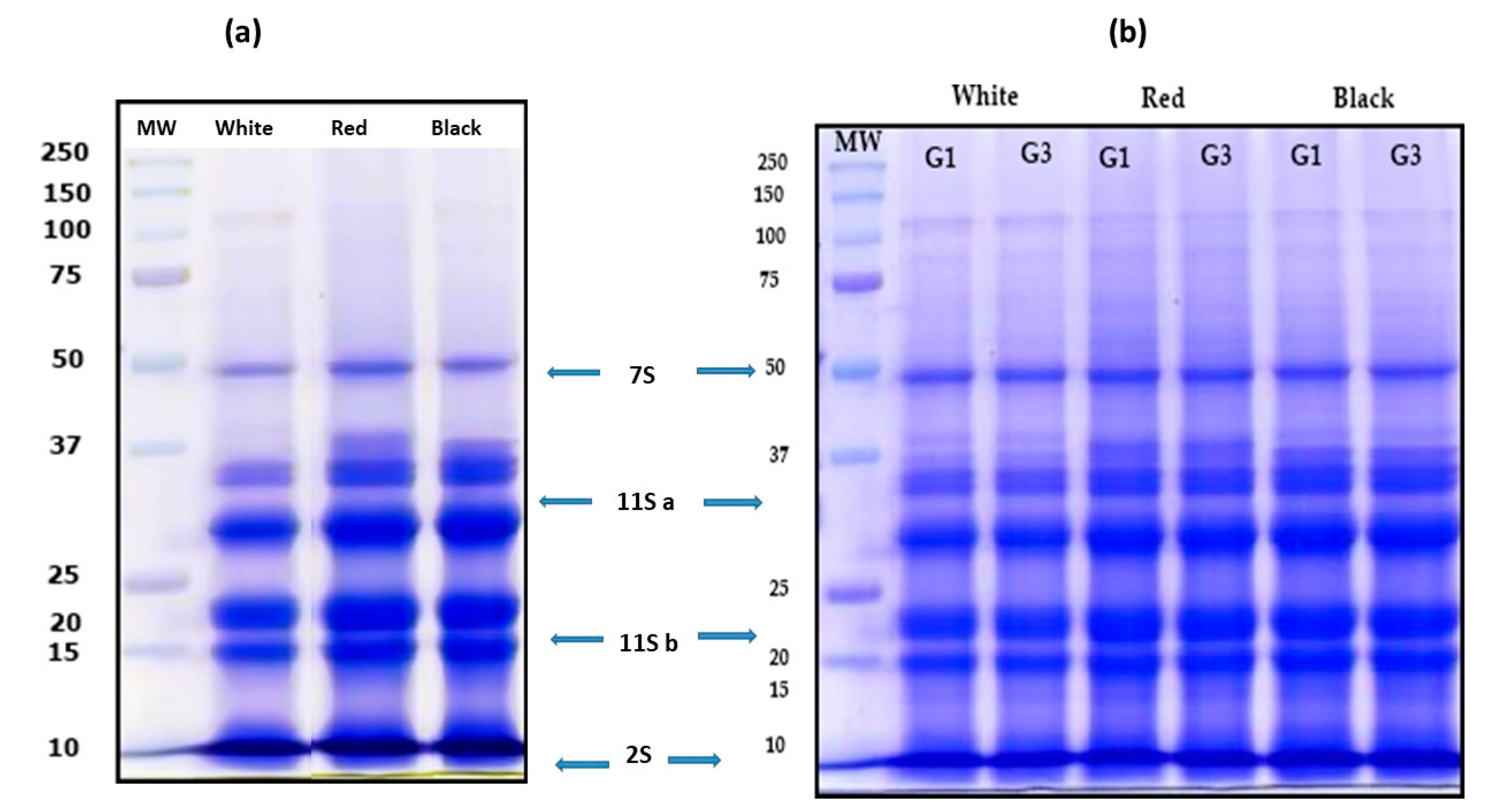
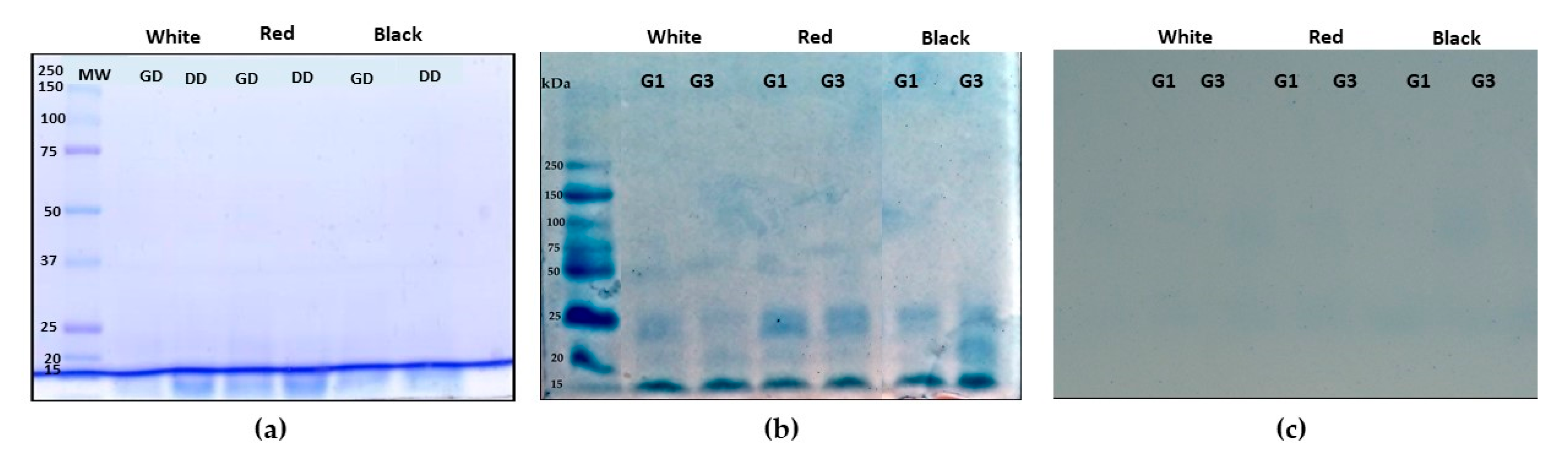
3.2. Characterization of QPI no Germinated and Germinated Using SDS-PAGE Electrophoresis Analysis
3.3. Antioxidant Activity of Non-Germinated and Germinated QPI and their Digest
3.3.1. Evaluation of Antioxidant Activity by the DPPH Method
3.3.2. Evaluation of Antioxidant Activity by the ABTS Method
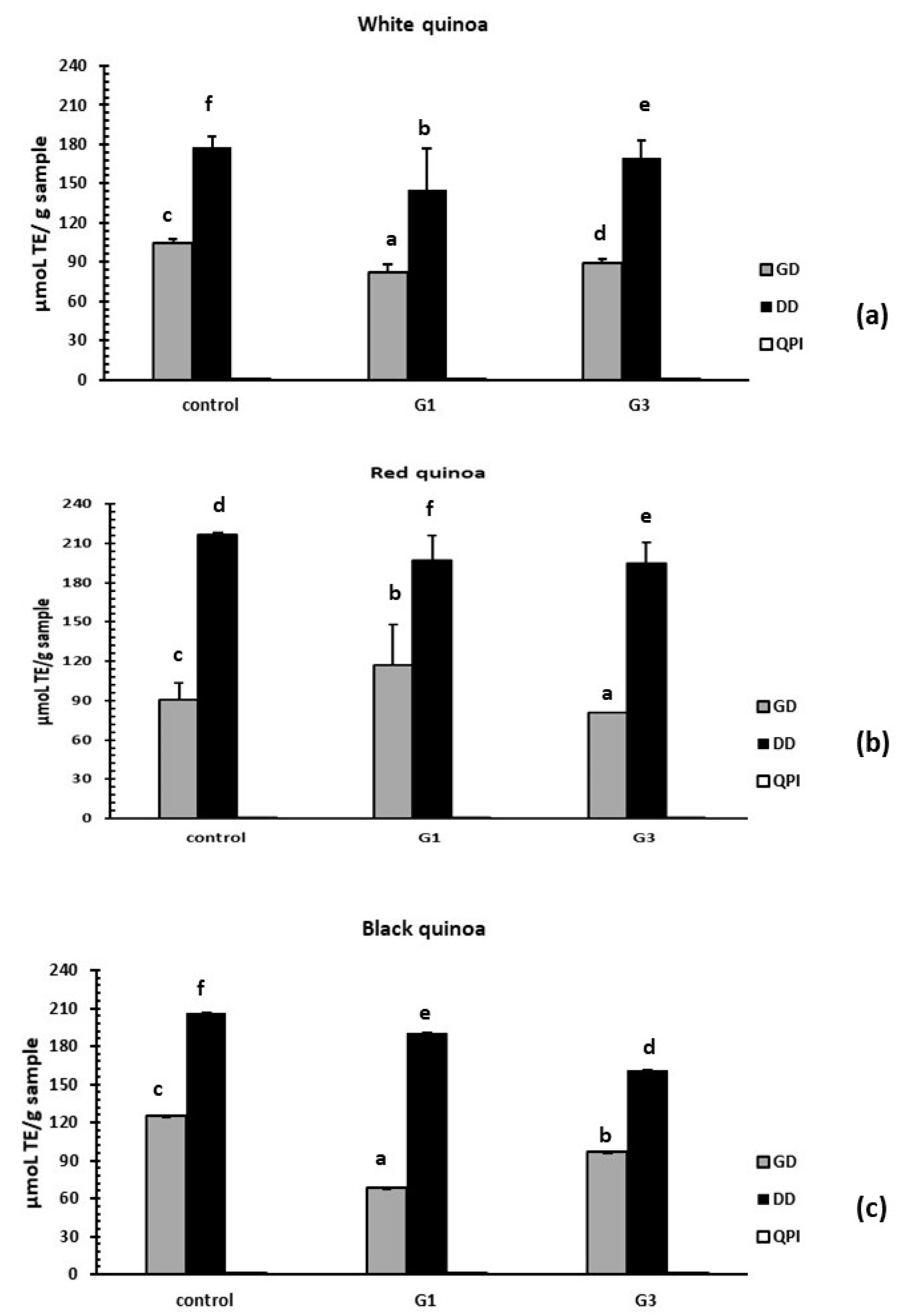
3.3.3. Evaluation of Antioxidant Activity by the ORAC-FL Method
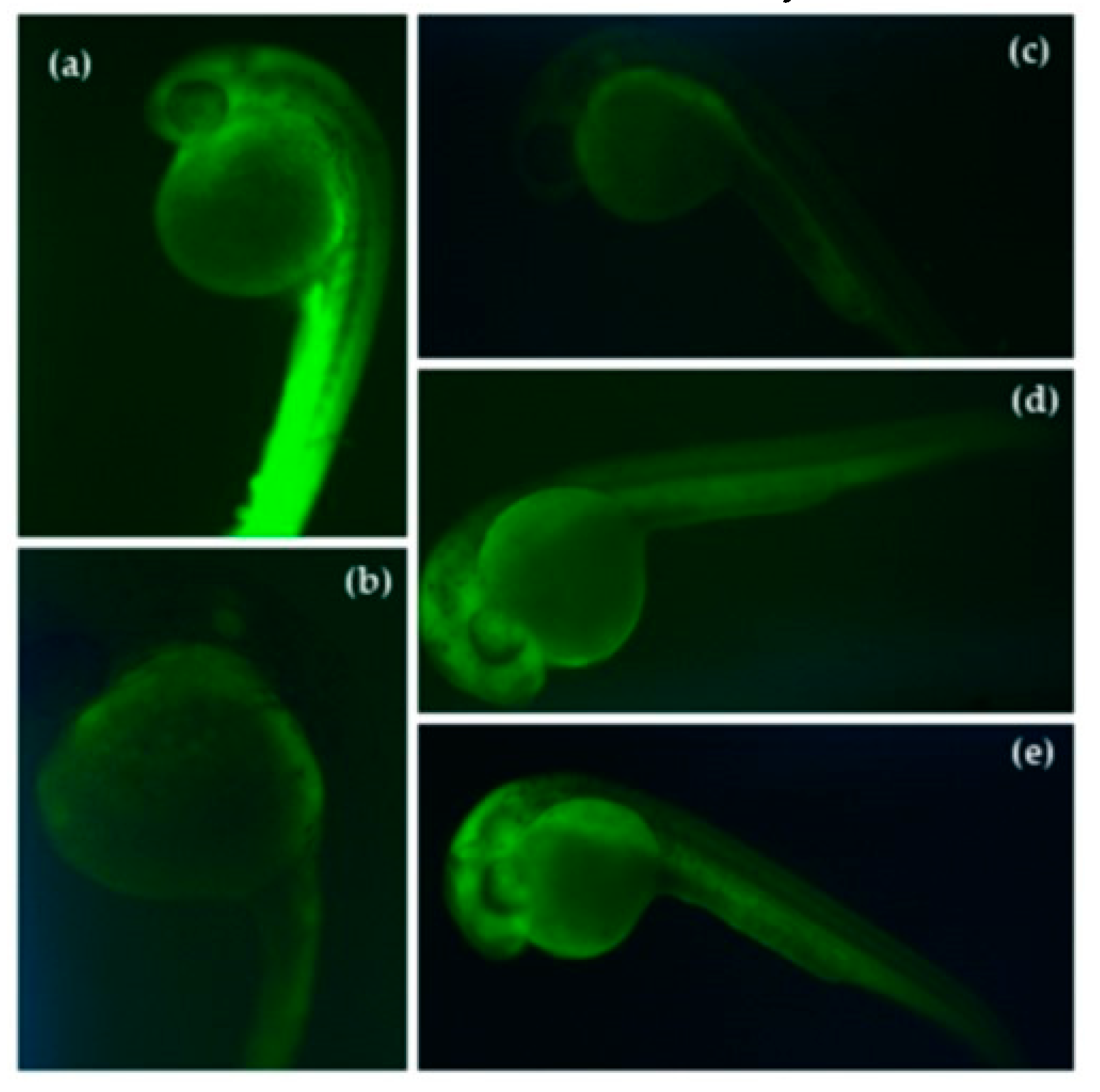
3.4. Reduction of Stress Oxidative ROS by Germinated QPI Digests
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vilcacundo, R.; Hernández-Ledesma, B. Nutritional and biological value of quinoa (Chenopodium quinoa Willd). Curr. Opin. Food Sci. 2017, 14, 1–6. [Google Scholar] [CrossRef]
- Pellegrini, M.; Lucas-Gonzales, R.; Ricci, A.; Fontecha, J.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Chemical, fatty acid, polyphenolic profile, techno-functional and antioxidant properties of flours obtained from quinoa (Chenopodium quinoa Willd) seeds. Ind. Crops Prod. 2018, 111, 38–46. [Google Scholar] [CrossRef]
- Vega-Gálvez, A.; Miranda, M.; Vergara, J.; Uribe, E.; Puente, L.; Martínez, E.A. Nutrition facts and functional potential of quinoa (Chenopodium quinoa Willd), an ancient Andean grain: A review. J. Sci. Food Agric. 2010, 90, 2541–2547. [Google Scholar] [CrossRef] [PubMed]
- Jaikishun, S.; Li, W.; Yang, Z.; Song, S. Quinoa: In perspective of global challenges. Agronomy 2019, 9, 176. [Google Scholar] [CrossRef]
- Toapanta, A.; Carpio, C.; Vilcacundo, R.; Carrillo, W. Analysis of protein isolate from quinoa (Chenopodium quinoa Willd). Asian J. Pharm. Clin. Res. 2016, 9, 332–334. [Google Scholar]
- Abugoch, L.E.; Romero, N.; Tapia, C.A.; Silva, J.; Rivera, M. Study of some physicochemical and functional properties of quinoa (Chenopodium quinoa Willd) protein isolates. J. Agric. Food Chem. 2008, 56, 4745–4750. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, J.; Zou, L.; Fu, C.; Li, P.; Zhao, G. Chemical characterization, antioxidant, immune-regulating and anticancer activities of a novel bioactive polysaccharide from Chenopodium quinoa seeds. Int. J. Biol. Macromol. 2017, 99, 622–629. [Google Scholar] [CrossRef]
- Vilcacundo, R.; Martínez-Villaluenga, C.; Hernández-Ledesma, B. Release of dipeptidyl peptidase IV. α-amylase and α-glucosidase inhibitory peptides from quinoa (Chenopodium quinoa Willd.) during in vitro simulated gastrointestinal digestion. J. Funct. Foods 2017, 35, 531–539. [Google Scholar] [CrossRef]
- Vilcacundo, R.; Miralles, B.; Carrillo, W. Hernández-Ledesma B in vitro chemopreventive properties of peptides released from quinoa (Chenopodium quinoa Willd) protein under simulated gastrointestinal digestion. Food Res. Int. 2018, 105, 403–411. [Google Scholar] [CrossRef]
- Saito, M.; Sakagami, H.; Fujisawa, S. Cytotoxicity and apoptosis induction by butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT). Anticancer Res. 2003, 23, 4693–4701. [Google Scholar]
- Nieva-Echevarría, B.; Manzanos, M.J.; Goicoechea, E.; Guillén, M.D. 2, 6-Di-tert-butyl-hydroxytoluene and its metabolites in foods. Compr. Rev. Food Sci. Food Saf. 2015, 14, 67–80. [Google Scholar] [CrossRef]
- Carrillo, W.; Ramos, M. Identification of antimicrobial peptides of native and heated hydrolysates from hen egg white lysozyme. J. Med. Food 2018, 21, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, W.; Gómez-Ruiz, J.A.; Miralles, B.; Ramos, M.; Barrio, D.; Recio, I. Identification of antioxidant peptides of hen egg-white lysozyme and evaluation of inhibition of lipid peroxidation and cytotoxicity in the Zebrafish model. Eur. Food Res. Technol. 2016, 242, 1777–1785. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez Saint-Jean, S.; De las Heras, A.; Carrillo, W.; Recio, I.; Ortiz-Delgado, J.B.; Ramos, M.; Pérez-Prieto, S.I. Antiviral activity of casein and αs2 casein hydrolysates against the infectious hematopoietic necrosis virus. a rhabdovirus from salmonid fish. J. Fish Dis. 2013, 36, 467–481. [Google Scholar] [CrossRef] [PubMed]
- Vilcacundo, R.; Méndez, P.; Reyes, W.; Romero, H.; Pinto, A.; Carrillo, W. Antibacterial activity of hen egg white lysozyme denatured by thermal and chemical treatments. Sci. Pharm. 2018, 86, 48. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.Q.; Cheng, L.; Long, Z.-Y.; Li, H.B.; Gunaratne, A.; Gan, R.-Y.; Corke, H. Comparison of the phenolic profiles of soaked and germinated peanut cultivars via UPLC-QTOF-MS. Antioxidants 2019, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- López-Amorós, M.L.; Hernández, T.; Estrella, I. Effect of germination on legume phenolic compounds and their antioxidant activity. J. Food Comp. Anal. 2006, 19, 277–283. [Google Scholar] [CrossRef]
- Shi, H.; Nam, P.K.; Ma, Y. Comprehensive profiling of isoflavones, phytosterols, tocopherols, minerals, crude protein, lipid, and sugar during soybean (Glycine max) germination. J. Agric. Food Chem. 2010, 58, 4970–4976. [Google Scholar] [CrossRef]
- Wang, K.H.; Lai, Y.H.; Chang, J.C.; Ko, T.F.; Shyu, S.L.; Chiou, R.Y.Y. Germination of peanut kernels to enhance resveratrol biosynthesis and prepare sprouts as a functional vegetable. J. Agric. Food Chem. 2005, 53, 242–246. [Google Scholar] [CrossRef]
- Aguilera, Y.; Díaz, M.F.; Jiménez, T.; Benítez, V.; Herrera, T.; Cuadrado, C.; Martín-Cabrejas, M.A. Changes in non-nutritional factors and antioxidant activity during germination of nonconventional legumes. J. Agric. Food Chem. 2013, 61, 8120–8125. [Google Scholar] [CrossRef]
- Gan, R.Y.; Wang, M.F.; Lui, W.Y.; Wu, K.; Corke, H. Dynamic changes in phytochemical composition and antioxidant capacity in green and black mung bean (Vigna radiata) sprouts. Int. J. Food Sci. Technol. 2016, 51, 2090–2098. [Google Scholar] [CrossRef]
- Carciochi, R.A.; Manrique, G.D.; Dimitrov, K. Changes in phenolic composition and antioxidant activity during germination of quinoa seeds (Chenopodium quinoa Willd). Int. Food Res. J. 2014, 21, 767–773. [Google Scholar]
- Mamilla, R.K. Effect of germination on antioxidant and ACE inhibitory activities of legumes. LWT-Food Sci. Technol. 2017, 75, 51–58. [Google Scholar] [CrossRef]
- De Souza Rocha, T.; Hernandez, L.M.R.; Mojica, L.; Johnson, M.H.; Chang, Y.K.; de Mejía, E.G. Germination of Phaseolus vulgaris and alcalase hydrolysis of its proteins produced bioactive peptides capable of improving markers related to type-2 diabetes in vitro. Food Res. Int. 2015, 76, 150–159. [Google Scholar] [CrossRef] [Green Version]
- Wilson, K.A.; Rightmire, B.R.; Chen, J.C.; Tan-Wilson, A.L. Differential proteolysis of glycinin and β-conglycinin polypeptides during soybean germination and seedling growth. Plant Physiol. 1986, 82, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Robles-Ramírez, M.C.; Ramón-Gallegos, E.; Mora-Escobedo, R.; Torres-Torres, N. A peptide fraction from germinated soybean protein down-regulates PTTG1 and TOP2A mRNA expression, inducing apoptosis in cervical cancer cells. J. Exp. Ther. Oncol. 2012, 9, 255–263. [Google Scholar]
- Vilcacundo, R.; Barrio, D.; Carpio, C.; García-Ruiz, A.; Rúales, J.; Hernández-Ledesma, B.; Carrillo, W. Digestibility of quinoa (Chenopodium quinoa Willd) protein concentrate and its potential to inhibit lipid peroxidation in the Zebrafish larvae model. Plant Foods Hum. Nutr. 2017, 72, 294–300. [Google Scholar] [CrossRef]
- Vilcacundo, R.; Barrio, D.A.; Piñuel, L.; Boeri, P.; Tombari, A.; Welbaum, J.; Pinto, A.; Hernández-Ledesma, B.; Carrillo, W. Inhibition of lipid peroxidation of kiwicha (Amaranthus caudatus) hydrolyzed protein using zebrafish larvae and embryos. Plants 2018, 7, 69. [Google Scholar] [CrossRef]
- Acosta, C.; Carpio, C.; Vilcacundo, R.; Carrillo, W. Identification of proteins isolate from amaranth (Amaranthus caudatus) by sodium dodecyl sulfate-polyacrylamide gel electrophoresis with water and NaCl 0.1 m solvents. Asian J. Pharm. Clin. Res. 2016, 9, 331–333. [Google Scholar]
- González-Montoya, M.; Hernández-Ledesma, B.; Silván, J.M.; Mora-Escobedo, R.; Martínez-Villaluenga, C. Peptides derived from in vitro gastrointestinal digestion of germinated soybean proteins inhibit human colon cancer cells proliferation and inflammation. Food Chem. 2018, 242, 75–82. [Google Scholar] [CrossRef] [Green Version]
- Piñuel, L.; Vilcacundo, E.; Boeri, P.; Barrio, D.A.; Morales, D.; Pinto, A.; Morán, R.; Samaniego, I.; Carrillo, W. Extraction of protein concentrate from red bean (Phaseolus vulgaris L.): Antioxidant activity and inhibition of lipid peroxidation. J. Appl. Pharm. Sci. 2019, 9. [Google Scholar] [CrossRef]
- Morais, H.A.; Silvestre, M.P.C.; Silveira, J.N.; Silva, V.D.M.; Silva, M.R. Action of a pancreatin and an Aspergillus oryzae protease on whey proteins: Correlation among the methods of analysis of the enzymatic hydrolysates. Braz. Arch. Biol. Technol. 2013, 56, 985–995. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Acosta, M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Moore, J.; Hao, Z.; Zhou, K.; Luther, M.; Costa, J.; Yu, L. Carotenoid, tocopherol, phenolic acid, and antioxidant properties of Maryland-grown soft wheat. J. Agric. Food Chem. 2005, 53, 6649–6657. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L.W.T. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Cunliffe, V.T. Zebrafish: A practical approach. Edited by Nüsslein-Volhard C and Dahm R Oxford university press. Genet. Res. Camb. 2003, 82, 79–83. [Google Scholar] [CrossRef]
- Rosenkranz, A.R.; Schmaldienst, S.; Stuhlmeier, K.M.; Chen, W.; Knapp, W.; Zlabinger, G.P. A microplate assay for the detection of oxidative product using 2%, 7%-dichlorofluorescein-diacetate. J. Immunol. Met. 1992, 156, 39–45. [Google Scholar] [CrossRef]
- D’ambrosio, T.; Amodio, M.L.; Pastore, D.; De Santis, G.; Colelli, G. Chemical, physical and sensorial characterization of fresh Quinoa Sprouts (Chenopodium quinoa Willd) and effects of modified atmosphere packaging on quality during cold storage. Food Packag. Shelf. 2017, 14, 52–58. [Google Scholar] [CrossRef]
- Shi, Z.; Hao, Y.; Teng, C.; Yao, Y.; Ren, G. Functional properties and adipogenesis inhibitory activity of protein hydrolysates from quinoa (Chenopodium quinoa Willd). Food Sci. Nutr. 2019, 7, 2103–2112. [Google Scholar] [CrossRef]
- Ruiz, G.A.; Opazo-Navarrete, M.; Meurs, M.; Minor, M.; Sala, G.; van Boekel, M.; Janssen, A.E. Denaturation and in vitro gastric digestion of heat-treated quinoa protein isolates obtained at various extraction pH. Food Biophys. 2016, 11, 184–197. [Google Scholar] [CrossRef]
- Opazo-Navarrete, M.; Freire, D.T.; Boom, R.M.; Janssen, A E. The Influence of starch and fibre on in vitro protein digestibility of dry fractionated quinoa seed (Riobamba Variety). Food Biophys. 2019, 14, 49–59. [Google Scholar] [CrossRef]
- Aluko, R.E.; Monu, E. Functional and bioactive properties of quinoa seed protein hydrolysates. J. Food Sci. 2003, 68, 1254–1258. [Google Scholar] [CrossRef]
- Mudgil, P.; Omar, L.S.; Kamal, H.; Kilari, B.P.; Maqsood, S. Multi-functional bioactive properties of intact and enzymatically hydrolysed quinoa and amaranth proteins. LWT Food Sci. Technol. 2019, 110, 207–213. [Google Scholar] [CrossRef]
- Tang, Y.; Li, X.; Chen, P.X.; Zhang, B.; Hernandez, M.; Zhang, H.; Tsao, R. Characterisation of fatty acid, carotenoid, tocopherol/tocotrienol compositions and antioxidant activities in seeds of three Chenopodium quinoa Willd genotypes. Food Chem. 2015, 174, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Likang, Q.; Qingnan, S.; Anyan, W. Effect of quinoa saponins extraction and sprouting on saponins content. J. Chin. Cereals Oils Assoc. 2017, 11, 8. [Google Scholar]
- Paucar-Menacho, L.M.; Martínez-Villaluenga, C.; Dueñas, M.; Frias, J.; Peñas, E. Response surface optimisation of germination conditions to improve the accumulation of bioactive compounds and the antioxidant activity in quinoa. Int. J. Food Sci. Technol. 2018, 53, 516–524. [Google Scholar] [CrossRef]
- Li, X.; Da, S.; Li, C.; Xue, F.; Zang, T. Effects of high-intensity ultrasound pretreatment with different levels of power output on the antioxidant properties of alcalase hydrolyzates from Quinoa (Chenopodium quinoa Willd) protein isolate. Cereal Chem. 2018, 95, 518–526. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; Le Maux, S.; Dubrulle, C.; Barre, C.; FitzGerald, R.J. Quinoa (Chenopodium quinoa Willd.) protein hydrolysates with in vitro dipeptidyl peptidase IV (DPP-IV) inhibitory and antioxidant properties. J. Cereal Sci. 2015, 65, 112–118. [Google Scholar] [CrossRef]
- Engeszer, R.E.; Patterson, L.B.; Rao, A.A.; Parichy, D.M. Zebrafish in the wild: A review of natural history and new notes from the field. Zebrafish 2007, 4, 21–40. [Google Scholar] [CrossRef]
- De Esch, C.; Slieker, R.; Wolterbeek, A.; Woutersen, R.; de Groot, D. Zebrafish as potential model for developmental neurotoxicity testing: A mini review. Neurotoxicol. Teratol. 2012, 34, 545–553. [Google Scholar] [CrossRef]
- Jensen, G.S.; Attridge, V.L.; Beaman, J.L.; Guthrie, J.; Ehmann, A.; Benson, K.F. Antioxidant and anti-inflammatory properties of an aqueous Cyanophyta extract derived from Arthrospira platensis: Contribution to bioactivities by the non-phycocyanin aqueous fraction. J. Med. Food. 2015, 18, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.C.; Kim, S.Y.; Kim, Y.T.; Kim, E.A.; Lee, S.H.; Ko, S.C.; Jang, H.S. In Vitro and in vivo antioxidant activities of polysaccharide purified from aloe vera (Aloe barbadensis) gel. Carbohydr. Polym. 2014, 99, 365–371. [Google Scholar] [CrossRef] [PubMed]


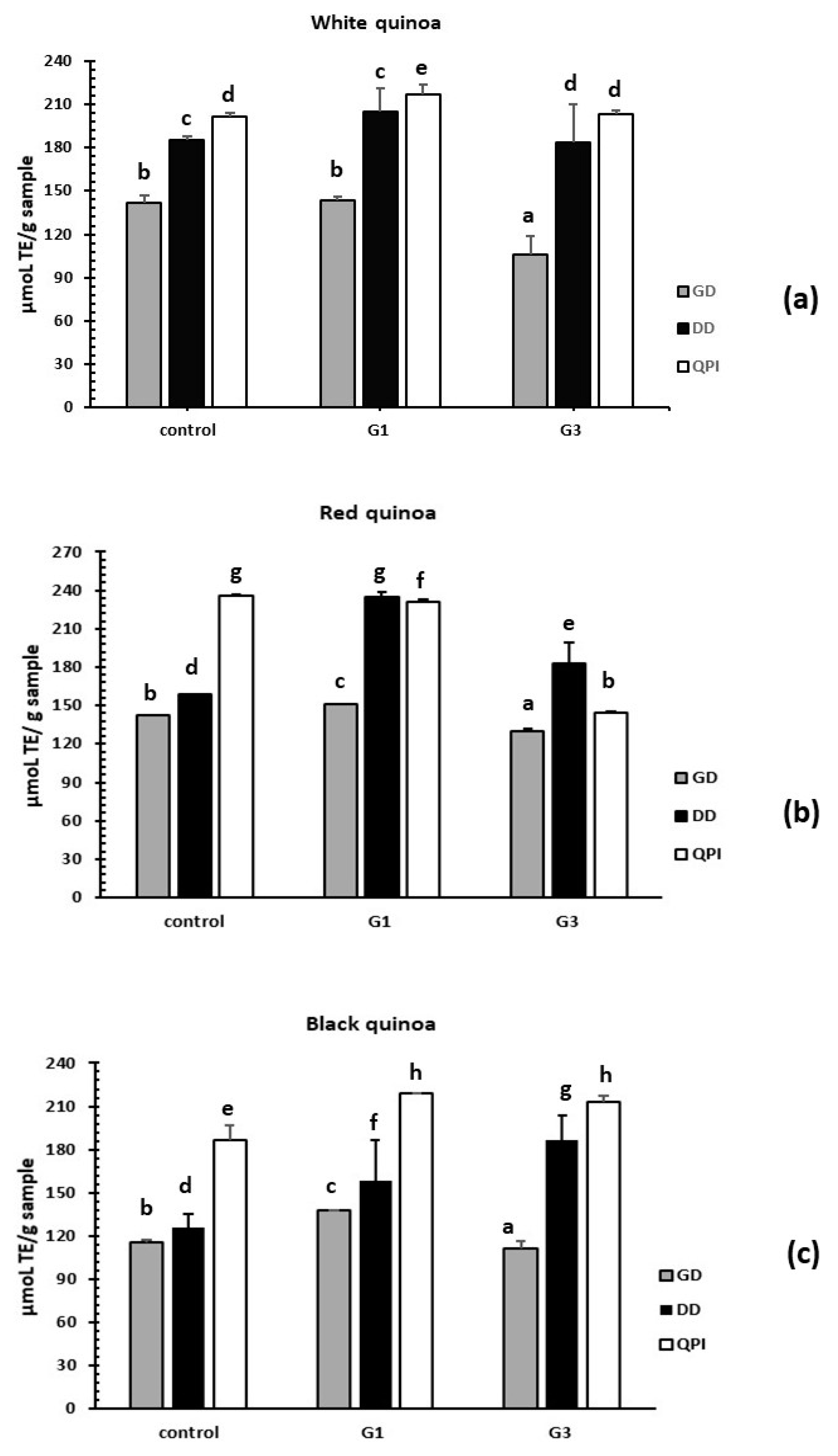
| % Protein Content | |||
|---|---|---|---|
| Sample | Not Germinated | 1 Day of Germination | 3 Days of Germination |
| QPI- white quinoa | 90.6 ± 2.55 a | 92.4 ± 1.76 a | 87.0 ± 0.59 a |
| QPI- red quinoa | 94.8 ± 0.43 b | 107.0 ± 1.85 b | 91.2 ± 0.75 b |
| QPI-black quinoa | 95.7 ± 0.21 b | 108.0 ± 3.68 b | 71.5 ± 0.92 c |
| Sample | % DH of Gastric Phase | % DH of Duodenal Phase |
|---|---|---|
| White quinoa | ||
| QPI-NG | 27.44 ± 0.21 e | 23.61 ± 107 a |
| QPI-G1 | 23.86 ± 1.44 d | 22.99 ± 0.80 a |
| QPI-G3 | 23.40 ± 0.25 d | 46.80 ± 5.10 e |
| Black quinoa | ||
| QPI-NG | 19.68 ± 0.29 b | 28.14 ± 5.36 b |
| QPI-G1 | 28.70 ± 2.31 e | 39.96 ± 2.58 d |
| QPI-G3 | 27.46 ± 2.14 a | 52.35 ± 0.26 f |
| Red quinoa | ||
| QPI-NG | 17.91 ± 1.04 a | 36.81 ± 0.65 c |
| QPI-G1 | 32.86 ± 1.87 d | 40.68 ± 2.47 d |
| QPI-G3 | 21.19 ± 0.60 c | 64.96 ± 3.19 g |
| ORAC (µmoL TE/g QPI) | |||
|---|---|---|---|
| Samples | 10 µmoL | 50 µmoL | 200 µmoL |
| White quinoa | |||
| QPI-NG | 112.80 ± 1.12 a | 151.56 ± 0.95 d | 175.15 ± 1.35 b |
| QPI-G1 | 110.01 ± 0.97 a | 147.80 ± 0.91 a | 170.82 ± 1.83 a |
| QPI-G3 | 112.33 ± 0.83 a | 150.93 ± 0.89 b | 174.43 ± 1.86 b |
| Black quinoa | |||
| QPI-NG | 111.18 ± 1.95 a | 149.38 ± 1.77 b | 172.64 ± 1.78 a |
| QPI-G1 | 111.05 ± 1.78 a | 149.21 ± 1.75 b | 172.44 ± 2.24 a |
| QPI-G3 | 111.31 ± 1.14 a | 149.55 ± 1.21 b | 172.84 ± 2.21 a |
| Red quinoa | |||
| QPI-NG | 112.35 ± 1.10 a | 152.21 ± 1.20 b | 175.91 ± 2.00 b |
| QPI-G1 | 113.29 ± 0.96 a | 152.21 ± 0.85 b | 175.91 ± 2.15 b |
| QPI-G3 | 113.47 ± 0.98 a | 152.35 ± 0.81 b | 175.66 ± 2.23 b |
| ORAC (µmoL TE/g Digests) | |||
|---|---|---|---|
| Samples | 10 µmoL | 100 µmoL | 200 µmoL |
| White quinoa | |||
| QPI-NG-GD | 181.62 ± 3.22 | 233.85 ± 3.00 | 280.10 ± 1.78 |
| QPI-NG-DD | 206.23 ± 4.15 | 260.36 ± 3.42 | 345.91 ± 1.02 |
| QPI-G1-GD | 190.01 ± 2.36 | 240.70 ± 2.08 | 305.22 ± 2.07 |
| QPI-G1-DD | 220.01 ± 0.57 | 275.01 ± 4.15 | 394.62 ± 3.19 |
| QPI-G3-GD | 195.08 ± 3.45 | 252.87 ± 2.09 | 310.13 ± 4.11 |
| QPI-G3-DD | 224.56 ± 2.13 | 272.87 ± 6.26 | 390.13 ± 5.03 |
| Black quinoa | |||
| QPI-NG-GD | 184.89 ± 1.09 | 237.94 ± 3.36 | 275.43 ± 5.47 |
| QPI-NG-DD | 200.14 ± 4.03 | 257.11 ± 1.09 | 352.67 ± 3.12 |
| QPI-G1-GD | 230.05 ± 3.18 | 278.22 ± 1.55 | 401.42 ± 2.36 |
| QPI-G1-DD | 230.05 ± 3.18 | 277.22 ± 3.44 | 412.51 ± 3.98 |
| QPI-G3-GD | 235.31 ± 2.00 | 279.24 ± 0.60 | 395.14 ± 4.023 |
| QPI-G3-DD | 228.35 ± 2.30 | 273.01 ± 0.31 | 385.91 ± 2.25 |
| Red quinoa | |||
| QPI-NG-GD | 180.02 ± 1.18 | 241.95 ± 0.01 | 272.11 ± 2.55 |
| QPI-NG-DD | 203.55 ± 1.10 | 263.08 ± 0.35 | 349.11 ± 1.00 |
| QPI-G1-GD | 232.05 ± 1.95 | 281.54 ± 0.94 | 396.21 ± 2.37 |
| QPI-G1-DD | 229.28 ± 3.24 | 295.63 ± 1.92 | 398.55 ± 2.60 |
| QPI-G3-GD | 233.35 ± 3.19 | 290.64 ± 1.11 | 385.04 ± 3.00 |
| QPI-G3-DD | 228.29 ± 2.65 | 275.49 ± 3.34 | 382.09 ± 3.14 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piñuel, L.; Boeri, P.; Zubillaga, F.; Barrio, D.A.; Torreta, J.; Cruz, A.; Vásquez, G.; Pinto, A.; Carrillo, W. Production of White, Red and Black Quinoa (Chenopodium quinoa Willd Var. Real) Protein Isolates and Its Hydrolysates in Germinated and Non-Germinated Quinoa Samples and Antioxidant Activity Evaluation. Plants 2019, 8, 257. https://doi.org/10.3390/plants8080257
Piñuel L, Boeri P, Zubillaga F, Barrio DA, Torreta J, Cruz A, Vásquez G, Pinto A, Carrillo W. Production of White, Red and Black Quinoa (Chenopodium quinoa Willd Var. Real) Protein Isolates and Its Hydrolysates in Germinated and Non-Germinated Quinoa Samples and Antioxidant Activity Evaluation. Plants. 2019; 8(8):257. https://doi.org/10.3390/plants8080257
Chicago/Turabian StylePiñuel, Lucrecia, Patricia Boeri, Fanny Zubillaga, Daniel Alejandro Barrio, Joaquin Torreta, Andrea Cruz, Grace Vásquez, Adelita Pinto, and Wilman Carrillo. 2019. "Production of White, Red and Black Quinoa (Chenopodium quinoa Willd Var. Real) Protein Isolates and Its Hydrolysates in Germinated and Non-Germinated Quinoa Samples and Antioxidant Activity Evaluation" Plants 8, no. 8: 257. https://doi.org/10.3390/plants8080257






