Meloidogyne incognita-Induced Giant Cells in Tomato and the Impact of Acetic Acid
Abstract
1. Introduction
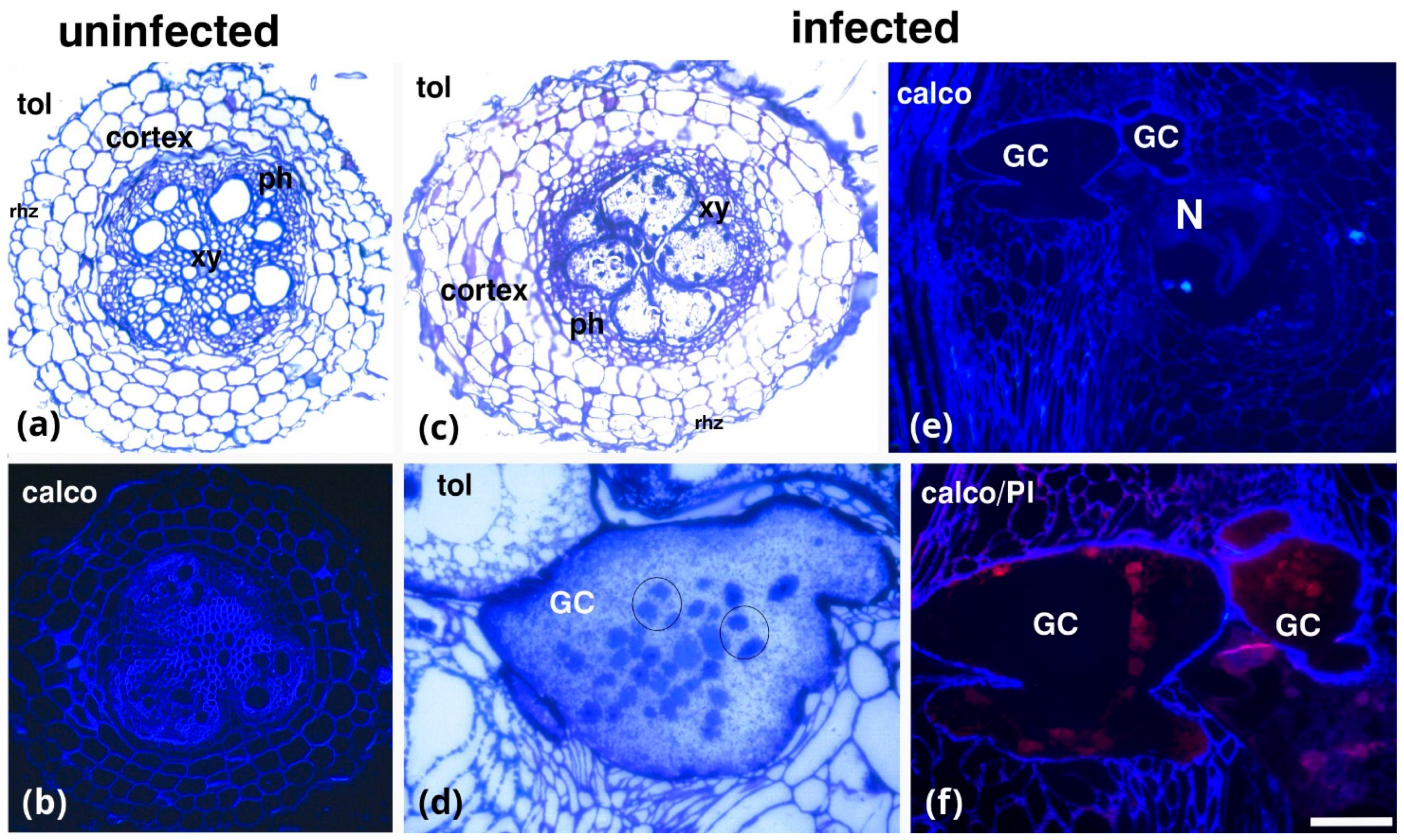
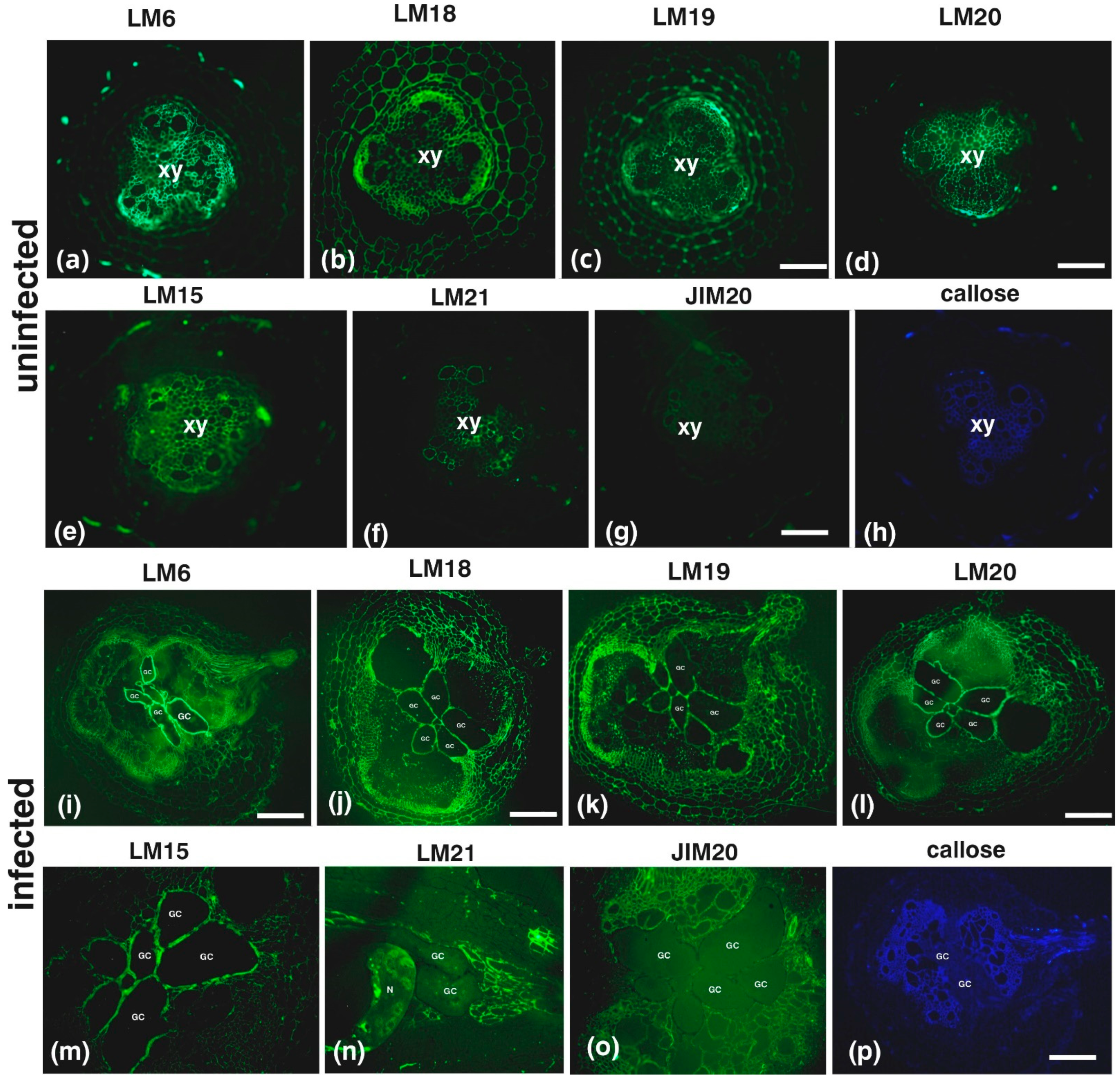
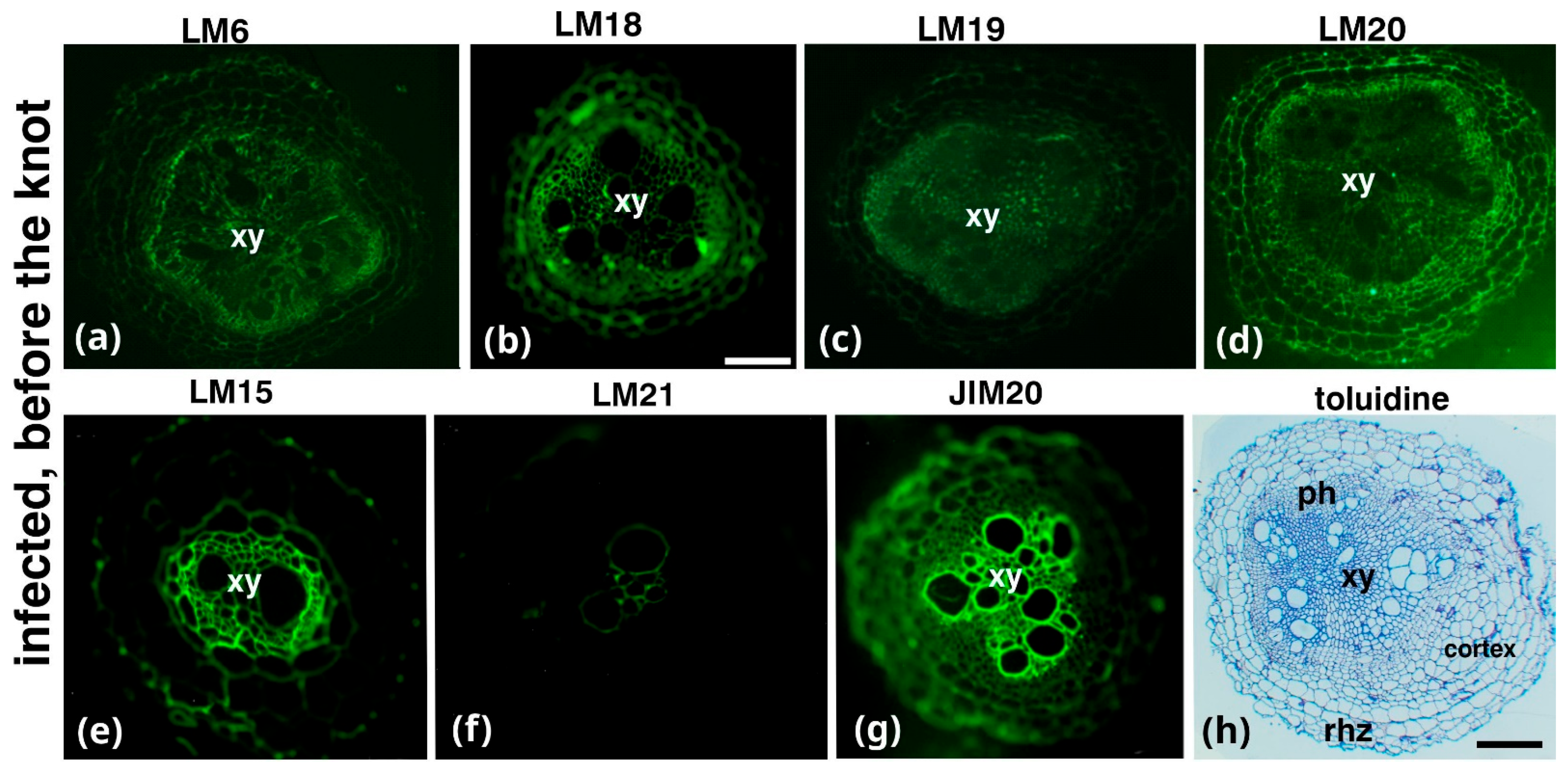
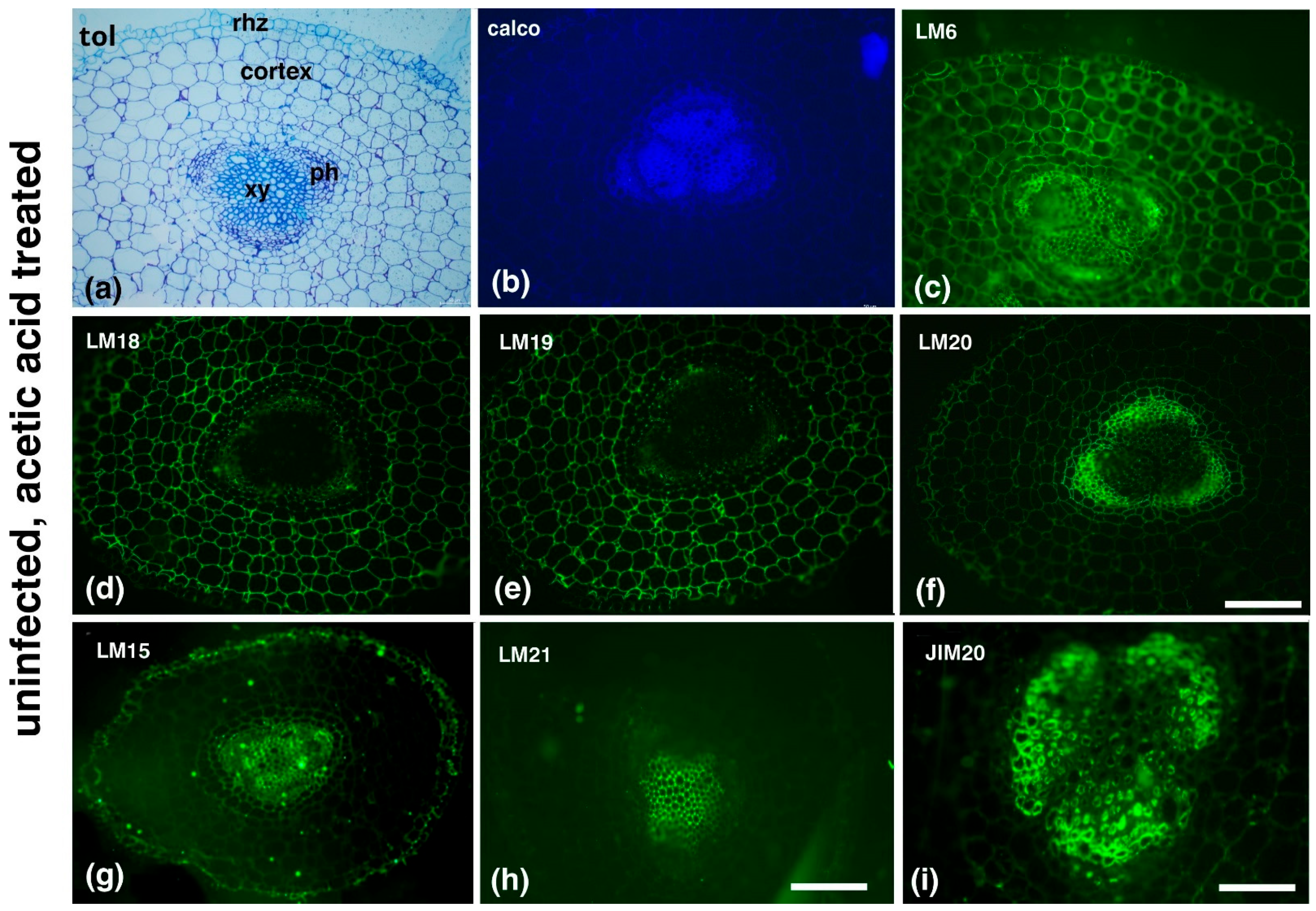
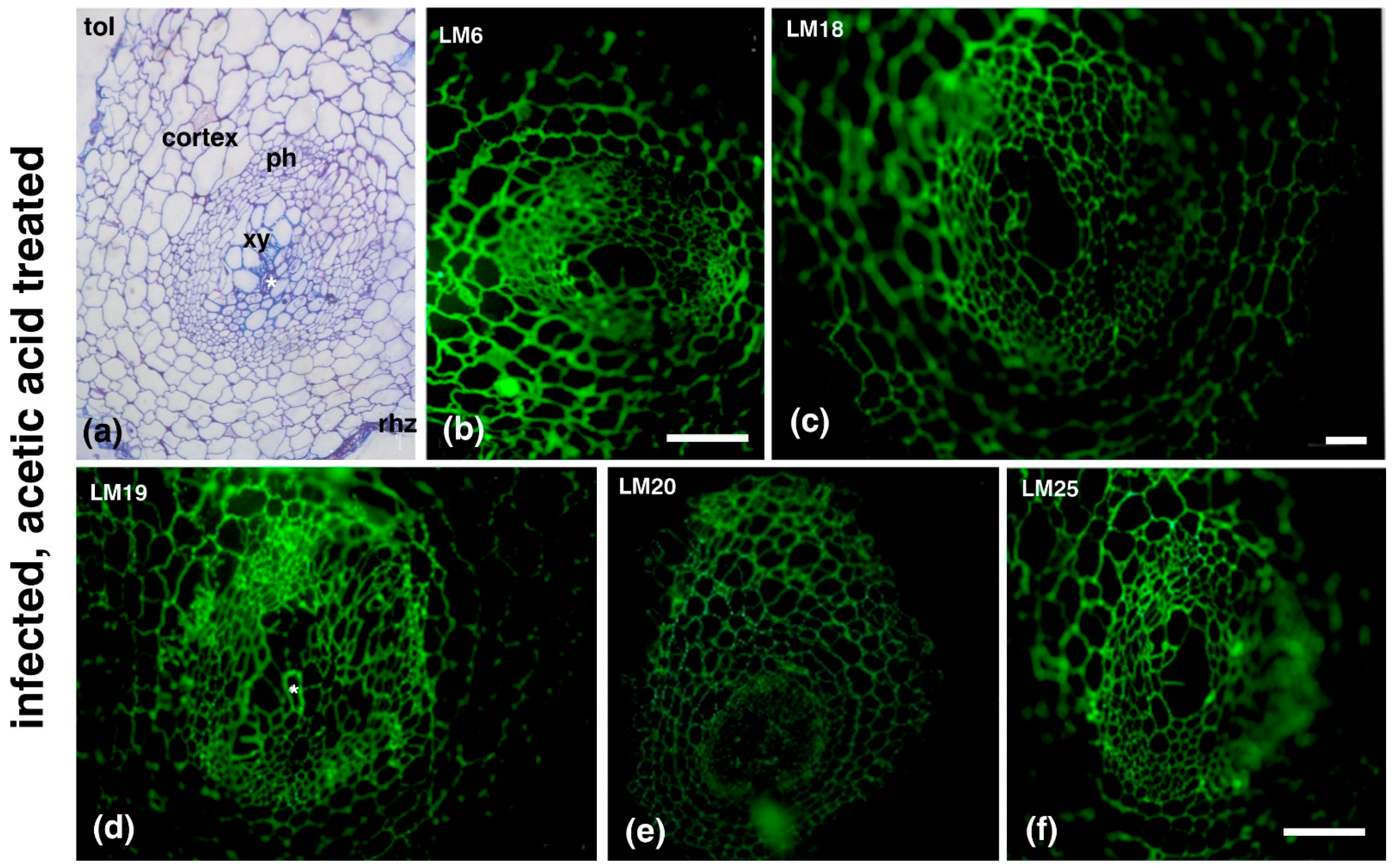
2. Results
3. Discussion
4. Materials and Methods
4.1. Nematode Rearing and Collection
4.2. Plant Infestation, Acetic Acid Treatments, and Root Chemical Fixation
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jones, J.T.; Haegeman, A.; Danchin, E.G.J.; Gaur, H.S.; Helder, J.; Jones, M.G.K.; Kikuchi, T.; Manzanilla-López, R.; Palomares-Rius, J.E.; Wesemael, W.I.M.M.L.; et al. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef] [PubMed]
- Koenning, S.R.; Wrather, J.A.; Kirkpatrick, T.L.; Walker, N.R.; Starr, J.L.; Mueller, J.D. Plant-parasitic nematodes attacking cotton in the United States: Old and emerging production challenges. Plant Dis. 2004, 88, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Kyndt, T.; Vieira, P.; Gheysen, G.; Engler, J.D.A. Nematode feeding sites: Unique organs in plant roots. Planta 2013, 238, 807–818. [Google Scholar] [CrossRef]
- Escobar, C.; Barcala, M.; Cabrera, J.; Fenoll, C. Overview of root-knot nematodes and giant cells. In Advances in Botanical Research; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; Volume 73, pp. 1–32. [Google Scholar]
- Vieira, P.; Gleason, C. Plant-parasitic nematode effectors—Insights into their diversity and new tools for their identification. Curr. Opin. Plant Biol. 2019, 50, 37–43. [Google Scholar] [CrossRef]
- Bozbuga, R.; Lilley, C.J.; Knox, J.P.; Urwin, P.E. Host-specific signatures of the cell wall changes induced by the plant parasitic nematode, Meloidogyne incognita. Sci. Rep. 2018, 8, 35529. [Google Scholar] [CrossRef]
- Meidani, C.; Ntalli, N.G.; Giannoutsou, E.; Adamakis, I.-D.S. Cell wall modifications in giant cells induced by the plant parasitic nematode Meloidogyne incognita in wild-type (Col-0) and the fra2 Arabidopsis thaliana katanin mutant. Int. J. Mol. Sci. 2019, 20, 5465. [Google Scholar] [CrossRef]
- Veronico, P.; Paciolla, C.; Pomar, F.; De Leonardis, S.; García-Ulloa, A.; Melillo, M.T. Changes in lignin biosynthesis and monomer composition in response to benzothiadiazole and root-knot nematode Meloidogyne incognita infection in tomato. J. Plant Physiol. 2018, 230, 40–50. [Google Scholar] [CrossRef]
- Ntalli, N.G.; Caboni, P. Botanical nematicides: A review. J. Agric. Food Chem. 2012, 60, 9929–9940. [Google Scholar] [CrossRef]
- Ntalli, N.G.; Ozalexandridou, E.X.; Kasiotis, K.M.; Samara, M.; Golfinopoulos, S.K. Nematicidal activity and phytochemistry of greek Lamiaceae species. Agronomy 2020, 10, 1119. [Google Scholar] [CrossRef]
- Ntalli, N.G.; Vargiu, S.; Menkissoglu-Spiroudi, U.; Caboni, P. Nematicidal carboxylic acids and aldehydes from Melia azedarach Fruits. J. Agric. Food Chem. 2010, 58, 11390–11394. [Google Scholar] [CrossRef]
- Ntalli, N.; Ratajczak, M.; Oplos, C.; Menkissoglu-Spiroudi, U.; Adamski, Z. Acetic Acid, 2-Undecanone, and (E)-2-Decenal ultrastructural malformations on Meloidogyne incognita. J. Nematol. 2016, 48, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Ntalli, N.; Menkissoglu-Spiroudi, U.; Doitsinis, K.; Kalomoiris, M.; Papadakis, E.N.; Boutsis, G.; Dimou, M.; Monokrousos, N. Mode of action and ecotoxicity of hexanoic and acetic acids on Meloidogyne javanica. J. Pest Sci. 2020, 93, 867–877. [Google Scholar] [CrossRef]
- Allen, M.M.; Allen, D.J. Biostimulant potential of acetic acid under drought stress is confounded by pH-dependent root growth inhibition. Front. Plant Sci. 2020, 11, 647. [Google Scholar] [CrossRef]
- Rahman, M.M.; Mostofa, M.G.; Rahman, M.A.; Islam, M.R.; Keya, S.S.; Das, A.K.; Miah, M.G.; Kawser, A.Q.M.R.; Ahsan, S.M.; Hashem, A.; et al. Acetic Acid: A cost-effective agent for mitigation of seawater-induced salt toxicity in mung bean. Sci. Rep. 2019, 9, 51178. [Google Scholar] [CrossRef]
- Bird, A.F. The ultrastructure and histochemistry of a nematode-induced giant cell. J. Cell Biol. 1961, 11, 701–715. [Google Scholar]
- Paulson, R.E.; Webster, J.M. Giant cell formation in tomato roots caused by Meloidogyne incognita and Meloidogyne hapla (Nematoda) infection: A light and electron microscope study. Can. J. Bot. 1970, 48, 271–276. [Google Scholar] [CrossRef]
- Vilela, R.M.I.F.; Kuster, V.C.; Magalhães, T.A.; Moraes, C.A.; de Paula Filho, A.C.; de Oliveira, D.C. Impact of Meloidogyne incognita (Nematode) infection on root tissues and cell wall composition of Okra (Abelmoschus esculentus L. Moench, Malvaceae). Protoplasma 2021, 258, 979–990. [Google Scholar] [CrossRef]
- Silva-Sanzana, C.; Celiz-Balboa, J.; Garzo, E.; Marcus, S.E.; Parra-Rojas, J.P.; Rojas, B.; Blanco-Herrera, F. Pectin methylesterases modulate plant homogalacturonan status in defenses against the Aphid Myzus persicae. Plant Cell 2019, 31, 1913–1929. [Google Scholar]
- Barcala, M.; García, A.; Cabrera, J.; Casson, S.; Lindsey, K.; Favery, B.; Escobar, C. Early transcriptomic events in microdissected Arabidopsis nematode-induced giant cells. Plant J. 2010, 61, 698–712. [Google Scholar]
- Puthoff, D.P.; Ehrenfried, M.L.; Vinyard, B.T.; Tucker, M.L. GeneChip profiling of transcriptional responses to soybean cyst nematode, Heterodera glycines, colonization of soybean roots. J. Exp. Bot. 2007, 58, 3407–3418. [Google Scholar]
- Moolhuijzen, P.M.; Manzanilla-López, R.; Jones, J.T. Pectic polysaccharides in plant cell walls and their role in plant defense. Phytochemistry 2000, 54, 123–139. [Google Scholar]
- Narváez-Barragán, D.A.; Tovar-Herrera, O.E.; Guevara-García, A.; Serrano, M.; Martinez-Anaya, C. Mechanisms of plant cell wall surveillance in response to pathogens, cell wall-derived ligands, and the effect of expansins on infection resistance or susceptibility. Front. Plant Sci. 2022, 13, 969343. [Google Scholar] [CrossRef]
- Su, C. Pectin modifications at the symbiotic interface. New Phytol. 2023, 238, 25–32. [Google Scholar] [PubMed]
- Liu, Q.; Zhang, W.; Zhang, Y.; Li, J.; Li, Z.; Zhu, Y. The role of cell wall modifications in plant resistance to pathogens and environmental stress. Plants 2024, 13, 2813. [Google Scholar] [CrossRef]
- Rodríguez-Kessler, M.; Paredes, M.; León, R.; Díaz, J.; Rodríguez, H.; García-Bastidas, F.; Romero, J. The role of pectin methylesterases in plant defense against pathogens. Plant Pathol. 2000, 49, 189–195. [Google Scholar] [CrossRef]
- Saito, T.; Matsushima, N.; Nagai, K.; Koga, H.; Koike, M.; Ohshima, K. Pectin methylesterase activities and their role in the modulation of plant defense responses. Plant Cell Physiol. 2024, 65, 1224–1235. [Google Scholar] [CrossRef]
- Komarova, T.V.; Sheshukova, E.V.; Dorokhov, Y.L. Cell wall methanol as a signal in plant immunity. Front. Plant Sci. 2014, 5, 101. [Google Scholar] [CrossRef]
- Stranne, M.; Sakuragi, Y. Arabinan: Biosynthesis and a role in host-pathogen interactions. Plant Pathogen Resist. Biotechnol. 2016, 91, 91–107. [Google Scholar]
- Wieczorek, K. Cell wall alterations in nematode-infected roots. Adv. Bot. Res. 2015, 73, 61–90. [Google Scholar]
- Kieliszewski, M.J.; Lamport, D.T.A.; Tan, L.; Cannon, M.C. Hydroxyproline-rich glycoproteins: Form and function. Annu. Plant Rev. Online 2018, 41, 321–342. [Google Scholar] [CrossRef]
- Showalter, A.M. Structure and function of plant cell wall proteins. Annu. Rev. Plant Biol. 1996, 47, 205–230. [Google Scholar] [CrossRef]
- Guo, W.; Shirsat, A.H. Extensin over-expression in Arabidopsis limits pathogen invasiveness. Mol. Plant Pathol. 2006, 7, 579–592. [Google Scholar]
- Castilleux, R.; Plancot, B.; Vicré, M.; Nguema-Ona, E.; Driouich, A. Extensin, an underestimated key component of cell wall defence? Ann. Bot. 2021, 127, 709–713. [Google Scholar] [PubMed]
- Ali, S.; Magne, M.; Chen, S.; Cote, O.; Stare, B.G.; Obradovic, N.; Moffett, P. Analysis of putative apoplastic effectors from the nematode, Globodera rostochiensis, and identification of an expansin-like protein that can induce and suppress host defenses. PLoS ONE 2015, 10, e0115042. [Google Scholar]
- Roze, E.; Hanse, B.; Mitreva, M.; Vanholme, B.; Bakker, J.; Smant, G. Mining the secretome of the root-knot nematode Meloidogyne chitwoodi for candidate parasitism genes. Mol. Plant Pathol. 2008, 9, 1–10. [Google Scholar] [CrossRef]
- Niebel, A.; De Almeida Engler, J.; Tire, C.; Engler, G.; Van Montagu, M.; Gheysen, G. Induction patterns of an extensin gene in tobacco upon nematode infection. Plant Cell 1993, 5, 1697–1710. [Google Scholar]
- Seo, Y.; Kim, Y.H. Control of Meloidogyne incognita using mixtures of organic acids. Plant Pathol. J. 2014, 30, 450. [Google Scholar]
- Sharma, S.; Singh, R. Acetic acid treatment enhances plant resistance to abiotic stress. Front. Plant Sci. 2020, 11, 575–586. [Google Scholar] [CrossRef]
- Rahman, M.M.; Keya, S.S.; Sahu, A.; Gupta, A.; Dhingra, A.; Tran, L.-S.P.; Mostofa, M.G. Acetic acid: A cheap but chief metabolic regulator for abiotic stress tolerance in plants. Stress Biol. 2024, 4, 34. [Google Scholar] [CrossRef]
- Sgherri, C.; Zolla, L.; Barbagallo, R.; Navazio, L.; Collins, D.; Di Toppi, L.S.; Paolocci, F.; Casini, F.; Marabottini, R.; Massai, R.; et al. Acetic acid as a biostimulant in plant stress management. J. Plant Growth Regul. 2020, 27, 1–9. [Google Scholar]
- Panchal, P.; Miller, A.J.; Giri, J. Organic acids: Versatile stress-response roles in plants. J. Exp. Bot. 2021, 72, 4038–4052. [Google Scholar] [PubMed]
- Jamiołkowska, A. Natural compounds as elicitors of plant resistance against diseases and new biocontrol strategies. Agronomy 2020, 10, 173. [Google Scholar] [CrossRef]
- Swaminathan, S.; Lionetti, V.; Zabotina, O.A. Plant cell wall integrity perturbations and priming for defense. Plants 2022, 11, 3539. [Google Scholar] [CrossRef]
- Aranega-Bou, P.; de la O Leyva, M.; Finiti, I.; García-Agustín, P.; González-Bosch, C. Priming of plant resistance by natural compounds: Hexanoic acid as a model. Front. Plant Sci. 2014, 5, 488. [Google Scholar] [CrossRef]
- Blakstad, J.I.; Strimbeck, R.; Poveda, J.; Bones, A.M.; Kissen, R. Frass from yellow mealworm (Tenebrio molitor) as plant fertilizer and defense priming agent. Biocatal. Agric. Biotechnol. 2023, 53, 102862. [Google Scholar]
- Napoleão, T.A.; Soares, G.; Vital, C.E.; Bastos, C.; Castro, R.; Loureiro, M.E.; Giordano, A. Methyl jasmonate and salicylic acid are able to modify cell wall, but only salicylic acid alters biomass digestibility in the model grass Brachypodium distachyon. Plant Sci. 2017, 263, 46–54. [Google Scholar]
- Hussey, R.S.; Barker, K.R. A comparison of methods of collecting inocula of Meloidogyne spp., including a new technique. Plant Dis. Rep. 1973, 57, 1025–1028. [Google Scholar]
- Meidani, C.; Giannoutsou, E.; Telioglanidis, K.; Ntalli, N.G.; Adamakis, I.-D.S. PIN1 auxin efflux carrier absence in Meloidogyne incognita-induced root-knots of tomato plants. Eur. J. Plant Pathol. 2021, 161, 987–992. [Google Scholar] [CrossRef]
- Giannoutsou, E.; Galatis, B.; Apostolakos, P. De-esterified homogalacturonan enrichment of the cell wall region adjoining the preprophase cortical cytoplasmic zone in some protodermal cell types of three land plants. Int. J. Mol. Sci. 2020, 21, 81. [Google Scholar] [CrossRef]
| Antibody | Epitope Recognized |
|---|---|
| Hemicelluloses | |
| LM15/LM25 | XXXG/XXLG and XLLG motifs of xyloglucan |
| LM21 | β-(1–4)-manno-oligosaccharides from DP2 to DP5 |
| Pectins | |
| LM6 | (1→5)-α-L-arabinans |
| LM18 | Homogalacturonan (HG) domain of pectic polysaccharides (binds to both partially methyl-esterified and unesterified HGs) |
| LM19 | HG domain of pectic polysaccharides (binds strongly to unesterified HGs) |
| LM20 | HG domain of pectic polysaccharides (requires methyl-esters for HG recognition, does not bind to unesterified HGs) |
| Extensins (EXTs) | |
| JIM20 | Extensin glucoprotein |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meidani, C.; Telioglanidis, K.; Giannoutsou, E.; Ntalli, N.; Adamakis, I.D.S. Meloidogyne incognita-Induced Giant Cells in Tomato and the Impact of Acetic Acid. Plants 2025, 14, 1015. https://doi.org/10.3390/plants14071015
Meidani C, Telioglanidis K, Giannoutsou E, Ntalli N, Adamakis IDS. Meloidogyne incognita-Induced Giant Cells in Tomato and the Impact of Acetic Acid. Plants. 2025; 14(7):1015. https://doi.org/10.3390/plants14071015
Chicago/Turabian StyleMeidani, Christianna, Konstantinos Telioglanidis, Eleni Giannoutsou, Nikoleta Ntalli, and Ioannis Dimosthenis S. Adamakis. 2025. "Meloidogyne incognita-Induced Giant Cells in Tomato and the Impact of Acetic Acid" Plants 14, no. 7: 1015. https://doi.org/10.3390/plants14071015
APA StyleMeidani, C., Telioglanidis, K., Giannoutsou, E., Ntalli, N., & Adamakis, I. D. S. (2025). Meloidogyne incognita-Induced Giant Cells in Tomato and the Impact of Acetic Acid. Plants, 14(7), 1015. https://doi.org/10.3390/plants14071015









