Identification of Key Regulators Mediating Gamma-Aminobutyric Acid (GABA) and Organic Acid Accumulation in Strawberry
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Determination of GABA, Anthocyanin, and AsA Content
2.3. Determination of Organic Acid Content
2.4. RNA-Seq and Transcriptome Analysis
2.5. WGCNA
2.6. Subcellular Localization of FaGAD4
2.7. Transient Expression of FaGAD4 in Strawberry Fruits
2.8. qRT-PCR Assays
2.9. Statistical Analysis
3. Results
3.1. Phenotype and Physiological Characteristics During Strawberry Fruit Ripening
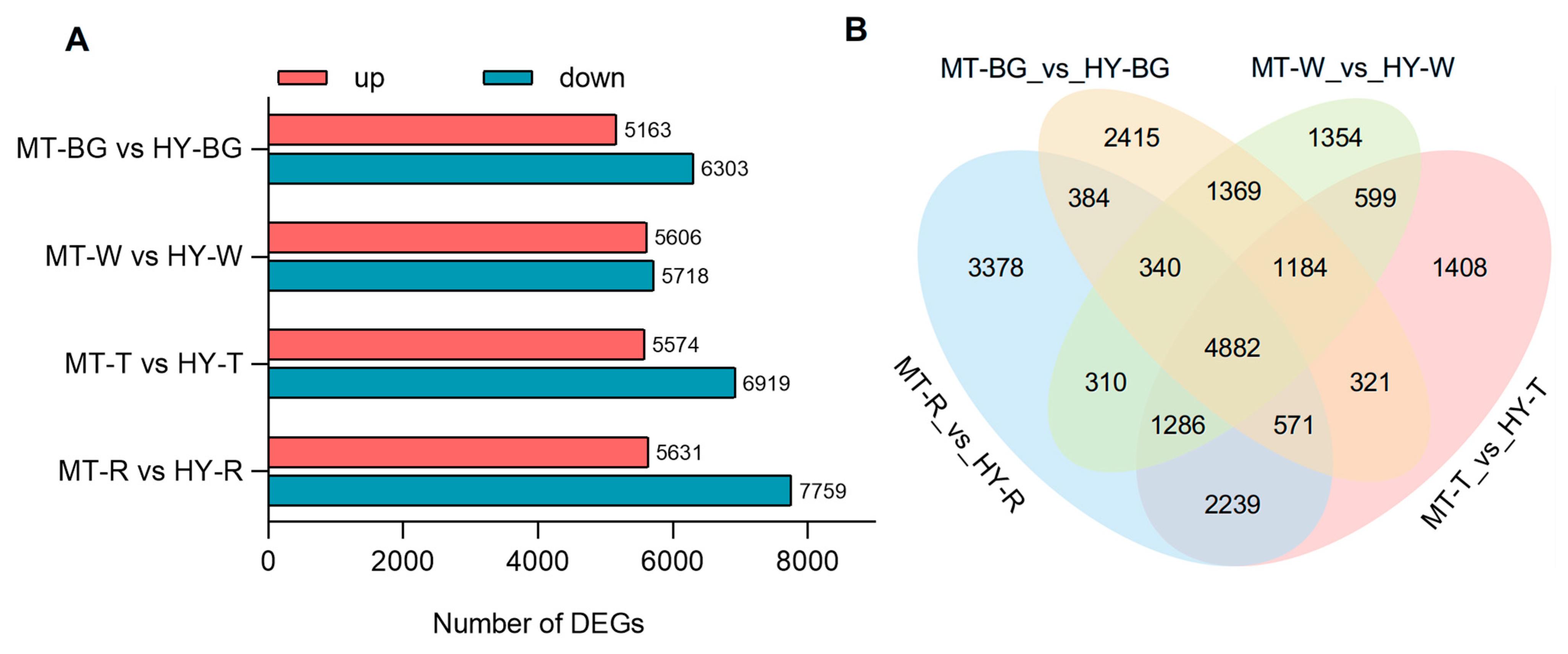
3.2. Transcriptome Analysis of MT and HY Fruits
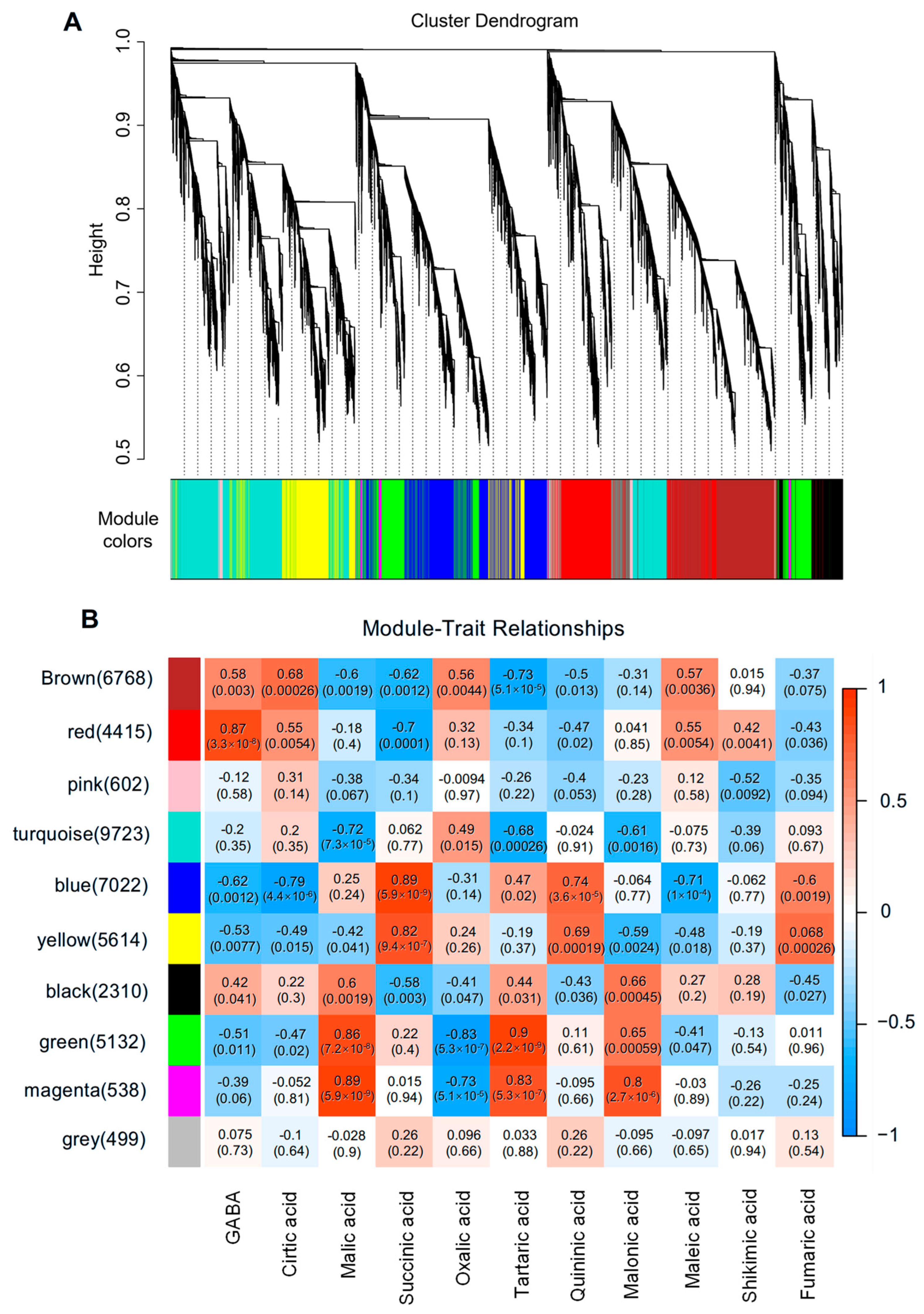
3.3. Establishment of ‘Metabolites vs. Gene’ Modules in Strawberry Fruit
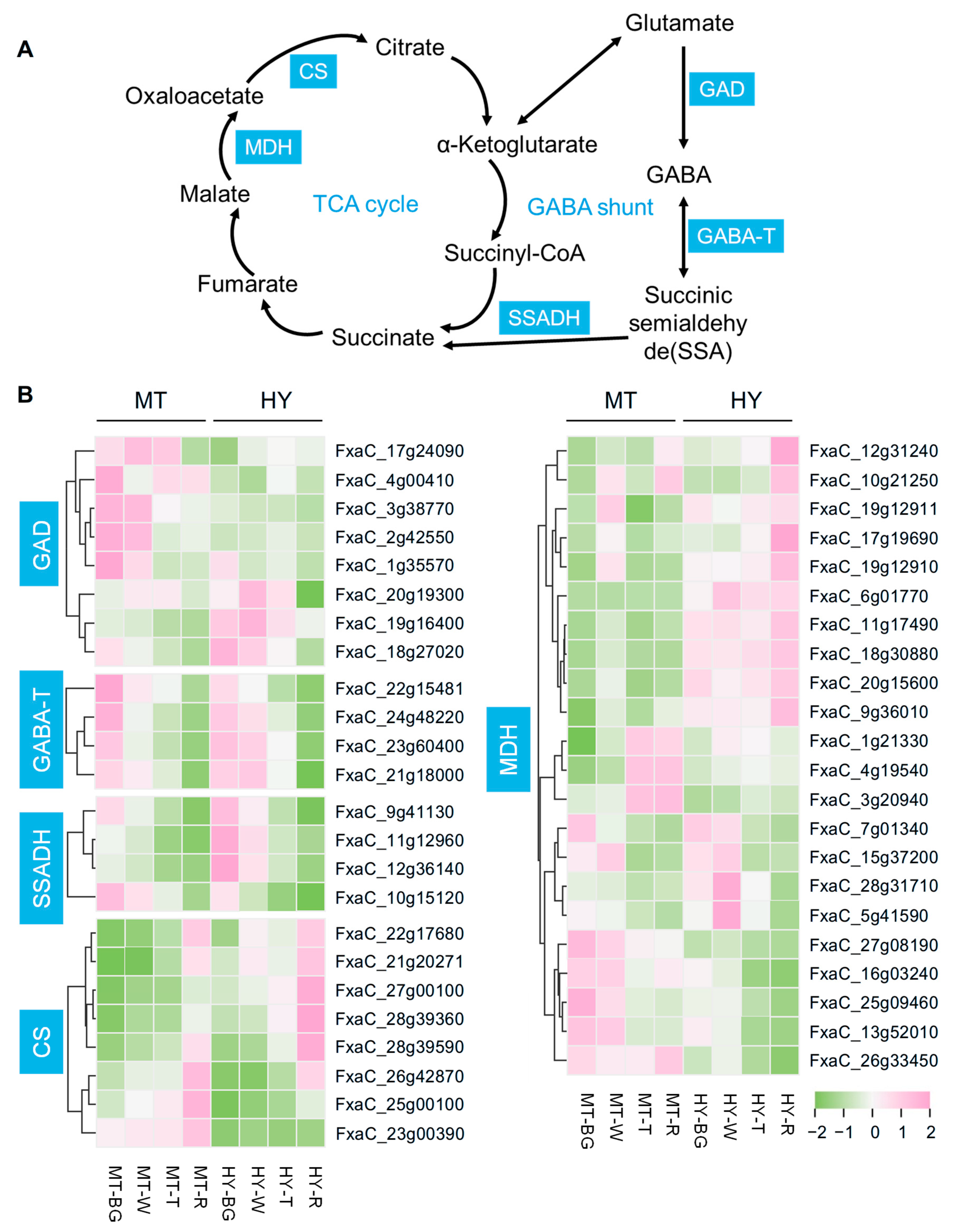
3.4. Identification of Key Structural Genes for GABA Shunt and TCA Cycle
3.5. Identification of Key Transcription Factors Regulating GABA Accumulation

3.6. FaGAD4 Promotes GABA, Anthocyanin, and AsA Accumulation in Strawberry Fruits
4. Discussion
4.1. Variation in GABA and Organic Acid Contents Between Two Strawberry Cultivars During Fruit Ripening
4.2. FaGAD4 Positively Regulates GABA Accumulation in Strawberry Fruits
4.3. GABA Metabolism Is Related to the TCA Cycle
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duan, M.Z.; Jiang, T.; Wang, X.; Song, K.J.; Xiao, X.; Fu, X.T.; He, S.J.; Feng, J.M.; Rao, M.J.; Guo, H.C. LC-MS/MS reveals variation in bioactive flavonoid profiles and MYB regulatory networks in wild vs. cultivated strawberries (white, pink, red). LWT 2025, 231, 118319. [Google Scholar] [CrossRef]
- Rao, M.J.; Zheng, B. The role of polyphenols in abiotic stress tolerance and their antioxidant properties to scavenge reactive oxygen species and free radicals. Antioxidants 2025, 14, 74. [Google Scholar] [CrossRef]
- Lei, Y.Y.; Nie, Y.X.; Wang, J.; Zhao, S.; Yao, J.X.; Zhang, J.X.; Dai, H.Y.; Zhang, Z.H. The FvMYB17 targets FvDHQS to promote ellagic acid biosynthesis in strawberry. Plant J. 2025, 122, e70224. [Google Scholar] [CrossRef]
- Deewatthanawong, R.; Nock, J.F.; Watkins, C.B. γ-Aminobutyric acid (GABA) accumulation in four strawberry cultivars in response to elevated CO2 storage. Postharvest Biol. Technol. 2010, 57, 92–96. [Google Scholar] [CrossRef]
- Matin, M.; Hrg, D.; Litvinova, O.; Łysek-Gładysinska, M.; Wierzbicka, A.; Horbańczuk, J.O.; Jóźwik, A.; Atanasov, A.G. The global patent landscape of functional food innovation. Nat. Biotechnol. 2024, 42, 1493–1497. [Google Scholar] [CrossRef]
- Owens, D.F.; Kriegstein, A.R. Is there more to GABA than synaptic inhibition? Nat. Rev. Neurosci. 2002, 3, 715–727. [Google Scholar] [CrossRef]
- Ahmad, S.; Fariduddin, Q. Deciphering the enigmatic role of gamma-aminobutyric acid (GABA) in plants: Synthesis, transport, regulation, signaling, and biological roles in interaction with growth regulators and abiotic stresses. Plant Physiol. Biochem. 2024, 208, 108502. [Google Scholar] [CrossRef] [PubMed]
- Bor, M.; Turkan, I. Is there a room for GABA in ROS and RNS signalling? Environ. Exp. Bot. 2019, 161, 67–73. [Google Scholar] [CrossRef]
- Sheng, L.; Shen, D.D.; Luo, Y.; Sun, X.H.; Wang, J.Q.; Luo, T.; Zeng, Y.; Xu, J.; Deng, X.X.; Cheng, Y.J. Exogenous γ-aminobutyric acid treatment affects citrate and amino acid accumulation to improve fruit quality and storage performance of postharvest citrus fruit. Food Chem. 2017, 216, 138–145. [Google Scholar] [CrossRef]
- Cheng, P.D.; Yue, Q.Y.; Zhang, Y.T.; Zhao, S.; Khan, A.; Yang, X.Y.; He, J.Q.; Wang, S.C.; Shen, W.Y.; Qian, Q.; et al. Application of γ-aminobutyric acid (GABA) improves fruit quality and rootstock drought tolerance in apple. J. Plant Physiol. 2023, 280, 153890. [Google Scholar] [CrossRef] [PubMed]
- Shoffe, Y.A.; Nock, J.F.; Zhang, Y.Y.; Watkins, C.B. Pre- and post-harvest γ-aminobutyric acid application in relation to fruit quality and physiological disorder development in ‘Honeycrisp’ apples. Sci. Hortic. 2021, 289, 110431. [Google Scholar] [CrossRef]
- Asgarian, Z.S.; Karimi, R.; Ghabooli, M.; Maleki, M. Biochemical changes and quality characterization of cold-stored ‘Sahebi’ grape in response to postharvest application of GABA. Food Chem. 2022, 373, 131401. [Google Scholar] [CrossRef]
- Zheng, Y.; Han, X.H.; Zhang, Y.; Qiu, W.W.; Tao, T.; Xu, Y.T.; Li, M.H.; Xie, X.B.; Sun, P.P.; Zheng, G.H.; et al. Preharvest and postharvest γ-aminobutyric acid treatment enhance quality and shelf life in strawberry (Fragaria × ananassa) fruits. J. Plant Growth Regul. 2025, 44, 3900–3915. [Google Scholar] [CrossRef]
- Li, L.; Dou, N.; Zhang, H.; Wu, C.X. The versatile GABA in plants. Plant Signal. Behav. 2021, 16, 1862565. [Google Scholar] [CrossRef]
- Fait, A.; Fromm, H.; Walter, D.; Galili, G.; Fernie, A.R. Highway or byway: The metabolic role of the GABA shunt in plants. Trends Plant Sci. 2008, 13, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Bouché, N.; Fromm, H. GABA in plants: Just a metabolite? Trends Plant Sci. 2004, 9, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Shelp, B.J.; Bozzo, G.G.; Trobacher, C.P.; Zarei, A.; Deyman, K.L.; Brikis, C.J. Hypothesis/review: Contribution of putrescine to 4-aminobutyrate (GABA) production in response to abiotic stress. Plant Sci. 2012, 193–194, 130–135. [Google Scholar] [CrossRef]
- Chen, D.; Shao, Q.; Yin, L.; Younis, A.; Zheng, B. Polyamine function in plants: Metabolism, regulation on development, and roles in abiotic stress responses. Front. Plant Sci. 2019, 9, 1945. [Google Scholar] [CrossRef]
- Baum, G.; Lev-Yadun, S.; Fridmann, Y.; Arazi, T.; Katsnelson, H.; Zik, M.; Fromm, H. Calmodulin binding to glutamate decarboxylase is required for regulation of glutamate and GABA metabolism and normal development in plants. EMBO J. 1996, 15, 2988–2996. [Google Scholar] [CrossRef]
- Sakthivel, K.; Balasubramanian, R.; Sampathrajan, V.; Veerasamy, R.; Appachi, S.V.; Kumar, K.K. Transforming tomatoes into GABA-rich functional foods through genome editing: A modern biotechnological approach. Funct. Integr. Genom. 2025, 25, 27. [Google Scholar] [CrossRef]
- Takayama, M.; Koike, S.; Kusano, M.; Matsukura, C.; Saito, K.; Ariizumi, T.; Ezura, H. Tomato glutamate decarboxylase genes SlGAD2 and SlGAD3 play key roles in regulating γ-aminobutyric acid levels in tomato (Solanum lycopersicum). Plant Cell Physiol. 2015, 56, 1533–1545. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Jalil, S.U.; Chopra, P.; Chhillar, H.; Ferrante, A.; Khan, N.A.; Ansari, M.I. Role of GABA in plant growth, development and senescence. Plant Gene 2021, 26, 100283. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Fernie, A. The role of TCA cycle enzymes in plants. Adv. Biol. 2023, 7, e2200238. [Google Scholar] [CrossRef] [PubMed]
- Fait, A.; Hanhineva, K.; Beleggia, R.; Dai, N.; Rogachev, I.; Nikiforova, V.J.; Fernie, A.R.; Aharoni, A. Reconfiguration of the achene and receptacle metabolic networks during strawberry fruit development. Plant Physiol. 2008, 148, 730–750. [Google Scholar] [CrossRef]
- Dong, S.Q.; Liu, H.T.; Duan, K.; Zou, X.H.; Yang, J.; Zhou, Y.K.; Ni, D.A.; Gao, Q.H. Study on dynamic patterns and diversity of organic acids accumulation in strawberry fruits of different germplasms. Acta Agric. Shanghai 2023, 39, 26–31. [Google Scholar]
- Vallarino, J.G.; Pott, D.M.; Cruz-Rus, E.; Miranda, L.; Medina-Minguez, J.J.; Valpuesta, V.; Fernie, A.R.; Sánchez-Sevilla, J.F.; Osorio, S.; Amaya, I. Identification of quantitative trait loci and candidate genes for primary metabolite content in strawberry fruit. Hortic. Res. 2019, 6, 4. [Google Scholar] [CrossRef]
- Zhao, J.; Zhou, D.N.; Zhu, H.L.; Zhang, Y.; Xie, X.B.; Fang, C.B. R2R3-MYB transcription factor FaMYB73 involved in malic acid accumulation of strawberry fruits. Acta Bot. Boreali-Occident. Sin. 2020, 40, 1638–1645. [Google Scholar]
- Yang, M.; Hou, G.Y.; Peng, Y.T.; Wang, L.X.; Liu, X.Y.; Jiang, Y.Y.; He, C.X.; She, M.S.; Zhao, M.T.; Chen, Q.; et al. FaGAPC2/FaPKc2.2 and FaPEPCK reveal differential citric acid metabolism regulation in late development of strawberry fruit. Front. Plant Sci. 2023, 14, 1138865. [Google Scholar] [CrossRef]
- Jia, H.F.; Lu, D.; Sun, J.H.; Li, C.L.; Xing, Y.; Qin, L.; Shen, Y.Y. Type 2C protein phosphatase ABI1 is a negative regulator of strawberry fruit ripening. J. Exp. Bot. 2013, 64, 1677–1687. [Google Scholar] [CrossRef]
- Deng, X.X.; Xu, X.W.; Liu, Y.; Zhang, Y.; Yang, L.Y.; Zhang, S.Q.; Xu, J. Induction of γ-aminobutyric acid plays a positive role to Arabidopsis resistance against Pseudomonas syringae. J. Integr. Plant Biol. 2020, 62, 1797–1812. [Google Scholar] [CrossRef]
- Zheng, B.; Zhao, L.; Jiang, X.; Cherono, S.; Liu, J.; Ogutu, C.; Ntini, C.; Zhang, X.; Han, Y. Assessment of organic acid accumulation and its related genes in peach. Food Chem. 2021, 334, 127567. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Pi, M.T.; Gao, Q.; Kang, C.Y. Transient expression assay in strawberry fruits. Bio-Protocol 2019, 9, e3249. [Google Scholar] [CrossRef] [PubMed]
- Clancy, M.A.; Rosli, H.G.; Chamala, S.; Barbazuk, W.B.; Civello, P.M.; Folta, K.M. Validation of reference transcripts in strawberry (Fragaria spp.). Mol. Genet. Genom. 2013, 288, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; He, C.X.; Hou, G.Y.; She, M.S.; Zhao, M.T.; Hu, R.X.; Xiao, W.F.; Yu, H.; Lin, Y.X.; Zhang, Y.T.; et al. Combining transcriptomics and HPLC to uncover variations in quality formation between ‘Benihoppe’ and ‘Fenyu No.1’ strawberries. Plant Physiol. Biochem. 2024, 215, 109043. [Google Scholar] [CrossRef]
- Ramos-Ruiz, R.; Martinez, F.; Knauf-Beiter, G. The effects of GABA in plants. Cogent Food Agric. 2019, 5, 1670553. [Google Scholar] [CrossRef]
- Yoshimura, M.; Toyoshi, T.; Sano, A.; Izumi, T.; Fujii, T.; Konishi, C.; Inai, S.; Matsukura, C.; Fukuda, N.; Ezura, H.; et al. Antihypertensive effect of a gamma-aminobutyric acid rich tomato cultivar ‘DG03-9’ in spontaneously hypertensive rats. J. Agric. Food Chem. 2010, 58, 615–619. [Google Scholar] [CrossRef]
- Li, Z.; Yu, J.J.; Peng, Y.; Huang, B.R. Metabolic pathways regulated by abscisic acid, salicylic acid and γ-aminobutyric acid in association with improved drought tolerance in creeping bentgrass (Agrostis stolonifera). Physiol. Plant. 2017, 159, 42–58. [Google Scholar] [CrossRef]
- Shang, H.T.; Cao, S.F.; Yang, Z.F.; Cai, Y.T.; Zheng, Y.H. Effect of exogenous γ-aminobutyric acid treatment on proline accumulation and chilling injury in peach fruit after long-term cold storage. J. Agric. Food Chem. 2011, 59, 1264–1268. [Google Scholar] [CrossRef]
- Saito, T.; Matsukura, C.; Sugiyama, M.; Watahiki, A.; Ohshima, I.; Iijima, Y.; Konishi, C.; Fujii, T.; Inai, S.; Fukuda, N.; et al. Screening for γ-aminobutyric acid (GABA)-rich tomato varieties. J. Jpn. Soc. Hortic. Sci. 2008, 77, 242–250. [Google Scholar] [CrossRef]
- Pu, Y.F.; Sinclair, A.J.; Zhong, J.H.; Liu, D.H.; Song, L.J. Determination of γ-aminobutyric acid (GABA) in jujube fruit (Ziziphus jujuba Mill.). CyTA-J. Food 2019, 17, 158–162. [Google Scholar] [CrossRef]
- Yang, B.M.; Yao, L.X.; Li, G.L.; Zhou, C.M.; He, Z.H.; Tu, S.H. Analysis of amino acids and aromatic components of pulps for different litchi variety. Chin. J. Trop. Crops 2014, 35, 1228–1234. [Google Scholar]
- Wei, W.; Zhang, T.; Chen, Y.; Zhou, Z.; Su, W.; Xu, Q.; Zhang, Y.; Zheng, S.; Jiang, J.; Deng, C. Characterization of the glutamate decarboxylase (GAD) gene and functional analysis of DlGAD3 in the accumulation of γ-aminobutyric acid in longan (Dimocarpus longan Lour.) pulp. Horticulturae 2025, 11, 686. [Google Scholar] [CrossRef]
- Gramazio, P.; Takayama, M.; Ezura, H. Challenges and prospects of new plant breeding techniques for GABA improvement in crops: Tomato as an example. Front. Plant Sci. 2020, 11, 577980. [Google Scholar] [CrossRef]
- Liu, X.; Ma, H.; Liu, J.; Liu, D.H.; Wang, C.L. The γ-aminobutyric Acid (GABA) synthesis gene regulates the resistance to water core-induced hypoxia stress for pear fruits. Agronomy 2023, 13, 1062. [Google Scholar] [CrossRef]
- Kim, J.Y.; Jung, Y.J.; Kim, D.H.; Kang, K.K. Transcriptomic Insights into GABA Accumulation in Tomato via CRISPR/Cas9-Based Editing of SlGAD2 and SlGAD3. Genes 2025, 16, 744. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, S.; Arai, C.; Takayama, M.; Matsukura, C.; Ezura, H. Efficient increase of γ-aminobutyric acid (GABA) content in tomato fruits by targeted mutagenesis. Sci. Rep. 2017, 7, 7057. [Google Scholar] [CrossRef]
- Waltz, E. GABA-enriched tomato is first CRISPR-edited food to enter market. Nat. Biotechnol. 2022, 40, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.F.; Li, Y.X.; Shi, Y.J.; Ma, J.J.; Peng, Y.X.; Tian, X.C.; Zhang, N.Q.; Ma, F.W.; Li, C.Y. γ-Aminobutyric acid mediated by MdCBF3-MdGAD1 mitigates low temperature damage in apple. Int. J. Biol. Macromol. 2024, 279, 135331. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Y.S.; Ji, T.; Wang, C.Q.; Shi, Q.H.; Li, C.Y.; Wei, J.W.; Gong, B. High-nitrogen-induced γ-aminobutyric acid triggers host immunity and pathogen oxidative stress tolerance in tomato and Ralstonia solanacearum interaction. New Phytol. 2024, 244, 1537–1551. [Google Scholar] [CrossRef]
- Li, Y.X.; Tian, X.C.; Liu, T.F.; Shi, Y.J.; Li, Y.H.; Wang, H.T.; Cui, Y.L.; Lu, S.Y.; Gong, X.Q.; Mao, K.; et al. MdSINA2-MdNAC104 module regulates apple alkaline resistance by affecting γ-aminobutyric acid synthesis and transport. Adv. Sci. 2024, 11, e2400930. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.R.; Zhang, Y.; Wang, J.Z.; Khan, A.; Kang, Z.; Ma, Y.B.; Zhang, J.R.; Dang, H.R.; Li, T.L.; Hu, X.H. SlGAD2 is the target of SlTHM27, positively regulates cold tolerance by mediating anthocyanin biosynthesis in tomato. Hortic. Res. 2024, 11, uhae096. [Google Scholar] [CrossRef] [PubMed]
- Michaeli, S.; Fromm, H. Closing the loop on the GABA shunt in plants: Are GABA metabolism and signaling entwined? Front. Plant Sci. 2015, 6, 419. [Google Scholar] [CrossRef]
- Jia, D.F.; Xu, Z.Y.; Chen, L.; Huang, Q.; Huang, C.H.; Tao, J.J.; Qu, X.Y.; Xu, X.B. Analysis of organic acid metabolism reveals citric acid and malic acid play major roles in determining acid quality during the development of kiwifruit (Actinidia eriantha). J. Sci. Food Agric. 2023, 103, 6055–6069. [Google Scholar] [CrossRef]
- Yu, J.Q.; Gu, K.D.; Sun, C.H.; Zhang, Q.Y.; Wang, J.H.; Ma, F.F.; You, C.X.; Hu, D.G.; Hao, Y.J. The apple bHLH transcription factor MdbHLH3 functions in determining the fruit carbohydrates and malate. Plant Biotechnol. J. 2020, 19, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.H.; Ma, B.Q.; Wang, C.Z.; Chen, X.Y.; Ruan, Y.L.; Yuan, Y.Y.; Ma, F.W.; Li, M.J. MdWRKY126 modulates malate accumulation in apple fruit by regulating cytosolic malate dehydrogenase (MdMDH5). Plant Physiol. 2022, 188, 2059–2072. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, L.; Li, S.; Sun, R.; Wei, Y.; Zhang, H.; Chang, L.; Zhong, C.; Dong, J.; Wang, G.; Sun, J. Identification of Key Regulators Mediating Gamma-Aminobutyric Acid (GABA) and Organic Acid Accumulation in Strawberry. Plants 2025, 14, 3437. https://doi.org/10.3390/plants14223437
Wei L, Li S, Sun R, Wei Y, Zhang H, Chang L, Zhong C, Dong J, Wang G, Sun J. Identification of Key Regulators Mediating Gamma-Aminobutyric Acid (GABA) and Organic Acid Accumulation in Strawberry. Plants. 2025; 14(22):3437. https://doi.org/10.3390/plants14223437
Chicago/Turabian StyleWei, Lingzhi, Shuangtao Li, Rui Sun, Yongqing Wei, Hongli Zhang, Linlin Chang, Chuanfei Zhong, Jing Dong, Guixia Wang, and Jian Sun. 2025. "Identification of Key Regulators Mediating Gamma-Aminobutyric Acid (GABA) and Organic Acid Accumulation in Strawberry" Plants 14, no. 22: 3437. https://doi.org/10.3390/plants14223437
APA StyleWei, L., Li, S., Sun, R., Wei, Y., Zhang, H., Chang, L., Zhong, C., Dong, J., Wang, G., & Sun, J. (2025). Identification of Key Regulators Mediating Gamma-Aminobutyric Acid (GABA) and Organic Acid Accumulation in Strawberry. Plants, 14(22), 3437. https://doi.org/10.3390/plants14223437




