Novel Oleanolic Acid-Tryptamine and -Fluorotryptamine Amides: From Adaptogens to Agents Targeting In Vitro Cell Apoptosis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Designing the Synthesis and Synthetic Protocol

2.2. Cytotoxicity
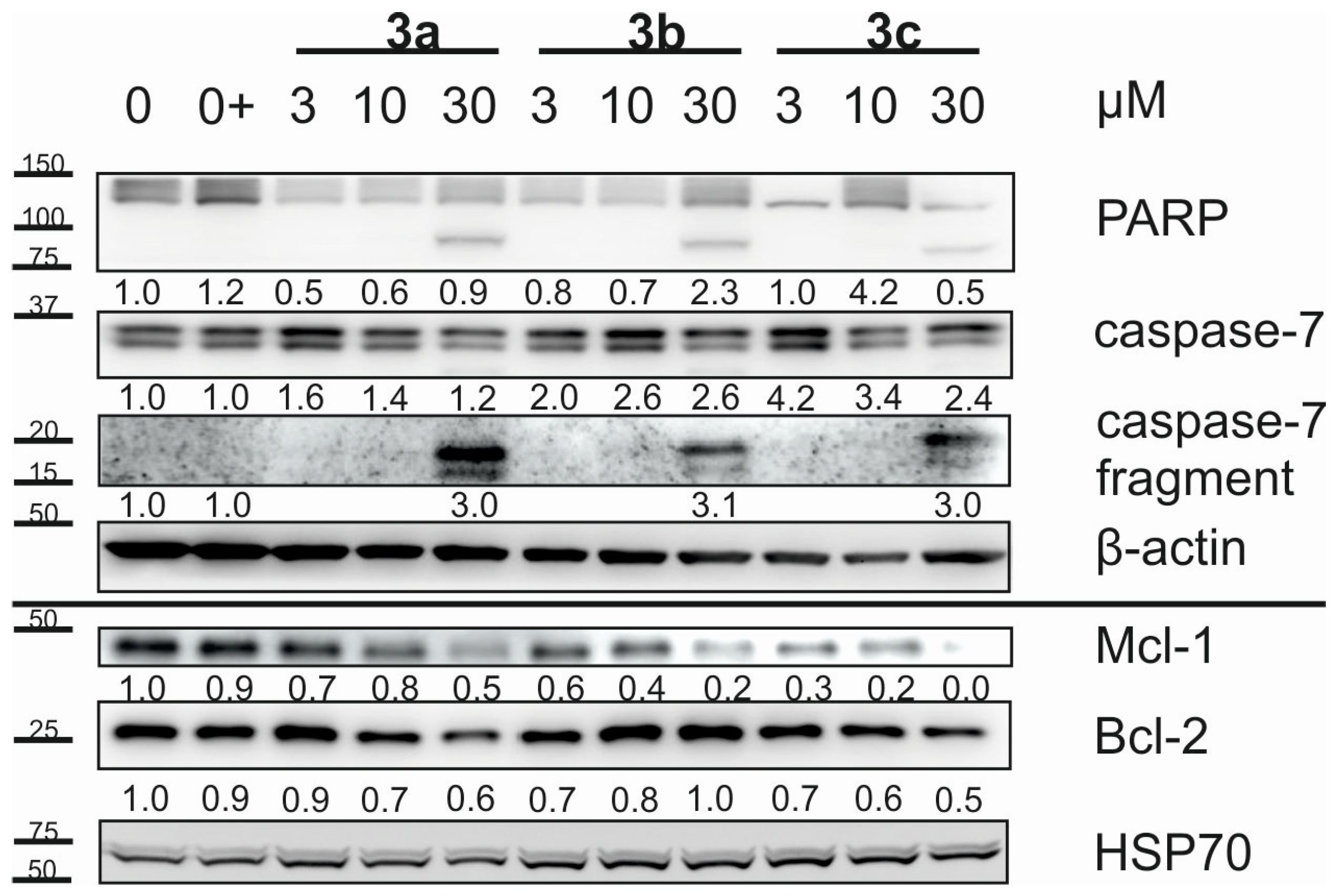
2.3. Cell Cycle and Apoptosis
2.4. In Silico Calculated Physico-Chemical and ADME Parameters
2.5. Investigation of Supramolecular Self-Assembly by UV Spectrometry
3. Materials and Methods
3.1. General
3.2. Synthesis of Compound 2
3.3. Synthesis of 3a–3c
3.4. Synthesis of 4a–4c
3.5. Cell Cultures and Cytotoxicity Screening Tests
3.6. Cell Cycle and Apoptosis
3.7. UV Spectrometry as a Tool for Supramolecular Self-Assembly Studies
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pollier, J.; Goossens, A. Oleanolic acid. Phytochemistry 2012, 77, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, E.O.; Seki, H.; Ohyama, K.; Ono, E.; Umemoto, N.; Mizutani, M.; Saito, K.; Muranaka, T. CYP716A subfamily members are multifunctional oxidases in triterpenoid biosynthesis. Plant Cell Physiol. 2011, 52, 2050–2061. [Google Scholar] [CrossRef] [PubMed]
- Fai, Y.M.; Tao, C.C. A review of presence of oleanolic acid in natural products. Nat. Proda Med. 2009, 2, 1–271. [Google Scholar]
- Özdemir, Z.; Bildziukevich, U.; Wimmerová, M.; Macůrková, A.; Lovecká, P.; Wimmer, Z. Plant adaptogens: Natural medicaments for 21st century? ChemistrySelect 2018, 3, 2196–2214. [Google Scholar] [CrossRef]
- Džubák, P.; Hajdúch, M.; Vydra, D.; Hustová, A.; Kvasnica, M.; Biedermann, D.; Marková, L.; Urban, M.; Šarek, J. Pharmacological activities of natural triterpenoids and their therapeutic implications. Nat. Prod. Rep. 2006, 23, 394–411. [Google Scholar] [CrossRef]
- Laszczyk, M.N. Pentacyclic triterpenes of the lupane, oleanane and ursane group as tools in cancer therapy. Planta Med. 2009, 75, 1549–1560. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Zhang, R.-H.; Wang, M.; Xu, G.-B.; Liao, S.-G. Prodrugs of triterpenoids and their derivatives. Eur. J. Med. Chem. 2017, 131, 222–236. [Google Scholar] [CrossRef]
- Garg, N.K.; Tandel, N.; Jadon, R.S.; Tyagi, R.K.; Katare, O.P. Lipid–polymer hybrid nanocarrier-mediated cancer therapeutics: Current status and future directions. Drug Discov. Today 2018, 23, 1610–1621. [Google Scholar] [CrossRef]
- Bildziukevich, U.; Özdemir, Z.; Wimmer, Z. Recent achievements in medicinal and supramolecular chemistry of betulinic acid and its derivatives. Molecules 2019, 24, 3546. [Google Scholar] [CrossRef] [Green Version]
- Bildziukevich, U.; Malík, M.; Özdemir, Z.; Rárová, L.; Janovská, L.; Šlouf, M.; Šaman, D.; Šarek, J.; Nonappa; Wimmer, Z. Spermine amides of selected triterpenoid acids: Dynamic supramolecular systems formation influences cytotoxicity of the drugs. J. Mater. Chem. B 2020, 8, 484–491. [Google Scholar] [CrossRef]
- Guo, Z.; Xu, Y.; Peng, Y.; Rashid, H.; Quan, W.; Xie, P.; Wu, L.; Jiang, J.; Wang, L.; Liu, X. Design, synthesis and evaluation of novel (S)-tryptamine derivatives containing an allyl group and an aryl sulfonamide unit as anticancer agents. Bioorg. Med. Chem. Lett. 2019, 29, 1133–1137. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Green, D.; Ho, A.; Klein, U.D.; Taylor, R.; Turner, S. Designing selective, high affinity ligands of 5-HT1D receptor by covalent dimerization of 5-HT1F ligands derived from 4-fluoro-N-[3-(1-methyl-4-piperidinyl)-1H-indol-5-yl]benzamide. J. Med. Chem. 2008, 51, 3609–3616. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, K.C.; Hurley, K.A.; Weibel, D.B. 5-Alkyloxytryptamines are membrane-targeting, broad-spectrum antibiotics. Bioorg. Med. Chem. Lett. 2016, 26, 5539–5544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minrovich, B.M.; Hubble, V.B.; Barker, W.T.; Jania, L.A.; Melander, R.J.; Koller, B.H.; Mellander, C. Second-generation tryptamine derivatives potently sensitize colistin resistant bacteria to colistin. ACS Med. Chem. Lett. 2019, 10, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Huang, T.; Xia, M.; Deng, L.; Hao, X.; Wang, Y.; Mu, S. Design and synthesis of novel monoterpenoid indole alkaloid-like analogues and their antitumour activities in vitro. Org. Biomol. Chem. 2018, 16, 3026–3037. [Google Scholar] [CrossRef] [PubMed]
- Jose, J.; Tavares, C.D.J.; Ebelt, N.D.; Lodi, A.; Edupuganti, R.; Xie, X.; Devkota, A.K.; Kaoud, T.S.; Van Den Berg, C.L.; Anslyn, E.V.; et al. Serotonin analogues as inhibitors of breast cancer cell growth. ACS Med. Chem. Lett. 2017, 8, 1072–1076. [Google Scholar] [CrossRef]
- Xiong, R.; He, D.; Deng, X.; Liu, J.; Lei, X.; Xie, Z.; Cao, X.; Chen, Y.; Peng, J.; Tang, G. Design, synthesis and biological evaluation of tryptamine salicylic acid derivatives as potential antitumor agents. Med. Chem. Commun. 2019, 10, 573–583. [Google Scholar] [CrossRef]
- Araujo, A.M.; Carvalho, F.; de Lourdes Bastos, M.; de Pinho, P.G.; Carvalho, M. The hallucinogenic world of tryptamines: An updated review. Arch. Toxicol. 2015, 89, 1151–1173. [Google Scholar] [CrossRef]
- Amin, N.; Shafabakhsh, R.; Reiter, R.J.; Asemi, Z. Melatonin is an appropriate candidate for breast cancer treatment: Based on known molecular mechanisms. J. Cell. Biochem. 2019, 120, 12208–12215. [Google Scholar] [CrossRef]
- Zakki, S.A.; Muhammad, J.S.; Li, J.-L.; Sun, L.; Li, M.-L.; Feng, Q.-W.; Li, Y.-L.; Cui, Z.-G.; Inadera, H. Melatonin triggers the anticancer potential of phenylarsine oxide via induction of apoptosis through ROS generation and JNK activation. Metallomics 2020, 12, 396–407. [Google Scholar] [CrossRef]
- Blair, J.B.; Kurrash-Orbaugh, D.; Marona-Lewicka, D.; Cumbay, M.G.; Watts, V.J.; Barker, E.L.; Nichols, D.E. Effect of ring fluorination on the pharmacology of hallucinogenic tryptamines. J. Med. Chem. 2000, 43, 4701–4710. [Google Scholar] [CrossRef] [PubMed]
- Rowland, R.S.; Taylor, R. Intermolecular nonbonded contact distances in organic crystal structures: Comparison with distances expected from van der Waals radii. J. Phys. Chem. 1996, 100, 7384–7391. [Google Scholar] [CrossRef]
- Böhm, H.-J.; Banner, D.; Bendels, S.; Kansy, M.; Kuhn, B.; Müller, K.; Obst-Sander, U.; Stahl, M. Fluorine in Medicinal Chemistry. ChemBioChem 2004, 5, 637–643. [Google Scholar] [CrossRef]
- Shah, P.; Westwell, A.D. The role of fluorine in medicinal chemistry. J. Enzyme Inhib. Med. Chem. 2007, 22, 527–540. [Google Scholar] [CrossRef] [Green Version]
- Campbell, I.W.; Ewing, D.J.; Clarke, B.F. Therapeutic experience with fluorocortisone in diabetic postural hypotension. Br. Med. J. 1976, 1, 872–874. [Google Scholar] [CrossRef] [Green Version]
- Isanbor, C.; O’Hagan, D. Fluorine in medicinal chemistry: A review of anti-cancer agents. J. Fluor. Chem. 2006, 127, 303–319. [Google Scholar] [CrossRef]
- Sun, S.; Adejare, A. Fluorinated molecules as drugs and imaging agents in the CNS. Curr. Top. Med. Chem. 2006, 6, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Seca, A.M.L.; Pinto, D.C.G.A. Plant secondary metabolites as anticancer agents: Successes in clinical trials and therapeutic application. Int. J. Mol. Sci. 2018, 19, 263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoenke, S.; Serbian, I.; Deigner, H.-P.; Csuk, R. Mitocanic di- and triterpenoid rhodamine B conjugates. Molecules 2020, 25, 5443. [Google Scholar] [CrossRef] [PubMed]
- Khwaza, V.; Mlala, S.; Oyedeji, O.O.; Aderibigbe, B.A. Pentacyclic triterpenoids with nitrogen-containing heterocyclic moiety, privileged hybrids in anticancer drug discovery. Molecules 2021, 26, 2401. [Google Scholar] [CrossRef] [PubMed]
- Malík, M.; Velechovský, J.; Tlustoš, P. Natural pentacyclic triterpenoid acids potentially useful as biocompatible nanocarriers. Fitoterapia 2021, 151, 104845. [Google Scholar] [CrossRef]
- Gudoityte, E.; Arandarcikaite, O.; Mazeikiene, I.; Bendokas, V.; Liobikas, J. Ursolic and oleanolic acids: Plant metabolites with neuroprotective potential. Int. J. Mol. Sci. 2021, 22, 4599. [Google Scholar] [CrossRef]
- Hoenke, S.; Christoph, M.A.; Friedrich, S.; Heise, N.; Brandes, B.; Deigner, H.-P.; Al-Harrasi, A.; Csuk, R. The presence of a cyclohexyldiamine moiety confers cytotoxicity to pentacyclic triterpenoids. Molecules 2021, 26, 2102. [Google Scholar] [CrossRef]
- Fan, J.-P.; Lai, X.-H.; Zhang, X.-H.; Yang, L.; Yuan, T.-T.; Chen, H.-P.; Liang, X. Synthesis and evaluation of the cancer cell growth inhibitory activity of the ionic derivatives of oleanolic acid and ursolic acid with improved solubility. J. Mol. Liquids 2021, 332, 115837. [Google Scholar] [CrossRef]
- Özdemir, Z.; Šaman, D.; Bertula, K.; Lahtinen, M.; Bednárová, L.; Pazderková, M.; Rárová, L.; Nonappa; Wimmer, Z. Rapid self-healing and thixotropic organogelation of amphiphilic oleanolic acid–spermine conjugates. Langmuir 2021, 37, 2693–2706. [Google Scholar] [CrossRef] [PubMed]
- Bildziukevich, U.; Rárová, L.; Šaman, D.; Wimmer, Z. Picolyl amides of betulinic acid as antitumor agents causing tumor cell apoptosis. Eur. J. Med. Chem. 2018, 145, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Advanced Chemistry Development, Software ACD/iLabs, Version 12.02. 2011. Available online: https://ilabs.eccouncil.org (accessed on 10 September 2021).
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and developmental settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Ghose, A.K.; Viswanadhan, V.N.; Wendoloski, J.J. A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases. J. Comb. Chem. 1999, 1, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Ha, W.; Zhao, X.-B.; Zhao, W.-H.; Tang, J.-J.; Shi, Y.P. A colon-targeted podophyllotoxin nanoprodrug: Synthesis, characterization, and supramolecular hydrogel formation for the drug combination. J. Mater. Chem. B 2021, 9, 3200–3209. [Google Scholar] [CrossRef]
- Keum, C.; Hong, J.; Kim, D.; Lee, S.-Y.; Kim, H. Lysozyme-instructed self-assembly of amino-acid-functionalized perylene diimide for multidrug-resistant cancer cells. ACS Appl. Mater. Interfaces 2021, 13, 14866–14874. [Google Scholar] [CrossRef]
- Bildziukevich, U.; Rárová, L.; Šaman, D.; Havlíček, L.; Drašar, P.; Wimmer, Z. Amides derived from heteroaromatic amines and selected steryl hemiesters. Steroids 2013, 78, 1347–1352. [Google Scholar] [CrossRef]
- Bildziukevich, U.; Vida, N.; Rárová, L.; Kolář, M.; Šaman, D.; Havlíček, L.; Drašar, P.; Wimmer, Z. Polyamine derivatives of betulinic acid and β-sitosterol: A comparative investigation. Steroids 2015, 100, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, Z.; Rybková, M.; Vlk, M.; Šaman, D.; Rárová, L.; Wimmer, Z. Synthesis and pharmacological effects of diosgenin–betulinic acid conjugates. Molecules 2020, 25, 3546. [Google Scholar] [CrossRef] [PubMed]


| Compound | MW a | Cytotoxicity (IC50 [µM], 72 h) b | Therapeutic Index (TI) | |||||
|---|---|---|---|---|---|---|---|---|
| CEM | MCF7 | HeLa | G-361 | BJ | TI (HeLa) | TI (G-361) | ||
| 1 | 456.70 | >50 | >50 | >50 | >50 | >50 | – | – |
| 2 | 498.74 | >50 | >50 | >50 | >50 | >50 | – | – |
| 3a | 640.94 | >50 | >50 | 8.7 ± 0.4 | 9.0 ± 0.4 | >50 | 5 | 5 |
| 3b | 658.93 | >50 | >50 | 6.7 ± 1.4 | 15.9 ± 6.6 | >50 | 7 | 3 |
| 3c | 658.93 | >50 | >50 | 12.2 ± 4.7 | >50 | >50 | 4 | – |
| 4a | 598.90 | >50 | >50 | >50 | >50 | >50 | – | – |
| 4b | 616.89 | >50 | >50 | 27.9 ± 0.8 | >50 | >50 | 2 | – |
| 4c | 616.89 | >50 | 13.5 ± 3.3 c | 20.3 ± 7.6 | >50 | >50 | 2.5 | – |
| CDDP d | 300.05 | 0.8 ± 0.1 | 7.7 ± 1.7 | 11.4 ± 3.8 | 4.5 ± 0.6 | 6.9 ± 0.9 | n.c. e | n.c. e |
| Compd. or Ref. No. | MW | Physico-Chemical and ADME Parameters a | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Log P | Log D (pH 7.4) | Log S (pH 7.4) | Hacc/Hdon/n.m.b. | Bioav. [%] | Log PS ∗ fu, brain | Log PS | Log PB | Log BB | PPB [%] | ||
| 1 | 456.70 | +7.84 | +5.35 | −3.76 | 3/2/1 | 30−70 | −5.5 | −3.7 | −0.41 | −0.19 | 99.39 |
| 2 | 498.74 | +8.55 | +6.05 | −4.64 | 4/1/3 | <30 | −6.0 | −4.2 | −0.48 | −0.19 | 99.49 |
| 3a | 640.94 | +10.67 | +10.67 | −9.07 | 5/2/6 | <30 | −6.9 | −4.6 | −0.70 | −0.70 | 99.90 |
| 3b | 658.93 | +10.68 | +10.68 | −9.50 | 5/2/6 | <30 | −7.0 | −4.6 | −0.62 | −0.62 | 99.88 |
| 3c | 658.93 | +10.68 | +10.68 | −9.40 | 5/2/6 | <30 | −7.0 | −4.6 | −0.62 | −0.62 | 99.88 |
| 4a | 598.90 | +9.89 | +9.89 | −8.20 | 4/3/4 | 30−70 | −6.4 | −4.1 | −0.58 | −0.58 | 99.87 |
| 4b | 616.89 | +10.09 | +10.09 | −8.70 | 4/3/4 | 30−70 | −6.6 | −4.3 | −0.55 | −0.55 | 99.86 |
| 4c | 616.89 | +10.09 | +10.09 | −8.60 | 4/3/4 | 30−70 | −6.6 | −4.3 | −0.55 | −0.55 | 99.86 |
| Recom. range | 180/500 | −0.4/+5.6 | - | −6.5/+0.5 | max.10/max. 5/− | − | − | − | −1.5/+1.5 | −3.0/+1.2 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bildziukevich, U.; Kvasnicová, M.; Šaman, D.; Rárová, L.; Wimmer, Z. Novel Oleanolic Acid-Tryptamine and -Fluorotryptamine Amides: From Adaptogens to Agents Targeting In Vitro Cell Apoptosis. Plants 2021, 10, 2082. https://doi.org/10.3390/plants10102082
Bildziukevich U, Kvasnicová M, Šaman D, Rárová L, Wimmer Z. Novel Oleanolic Acid-Tryptamine and -Fluorotryptamine Amides: From Adaptogens to Agents Targeting In Vitro Cell Apoptosis. Plants. 2021; 10(10):2082. https://doi.org/10.3390/plants10102082
Chicago/Turabian StyleBildziukevich, Uladzimir, Marie Kvasnicová, David Šaman, Lucie Rárová, and Zdeněk Wimmer. 2021. "Novel Oleanolic Acid-Tryptamine and -Fluorotryptamine Amides: From Adaptogens to Agents Targeting In Vitro Cell Apoptosis" Plants 10, no. 10: 2082. https://doi.org/10.3390/plants10102082






