Mitigating Children’s Pain and Anxiety during Blood Draw Using Social Robots
Abstract
:1. Introduction
2. Research Method
2.1. Participants
2.1.1. Participants of the Focus Group Sessions (Phase A)
2.1.2. Participating Children and Their Parents (Phase B)
2.2. Measures and Materials
2.2.1. Focus Group Sessions (Phase A)
2.2.2. Measurement Instruments for Pain and Anxiety (Phase B)
2.3. Procedure and Data Collection
2.3.1. Procedure and Data Collection Regarding the Focus Group Sessions (Phase A)
2.3.2. Procedure and Data Collection Related to Children’s Pain and Anxiety (Phase B)
3. Data Analysis
3.1. The Design of the Robot (Phase A)
3.2. Children’s Pain and Anxiety (Phase B)
4. Results
4.1. Phase A: The Design of the Robot
4.2. Phase B: The Impact of the Robot on Children’s Pain and Anxiety
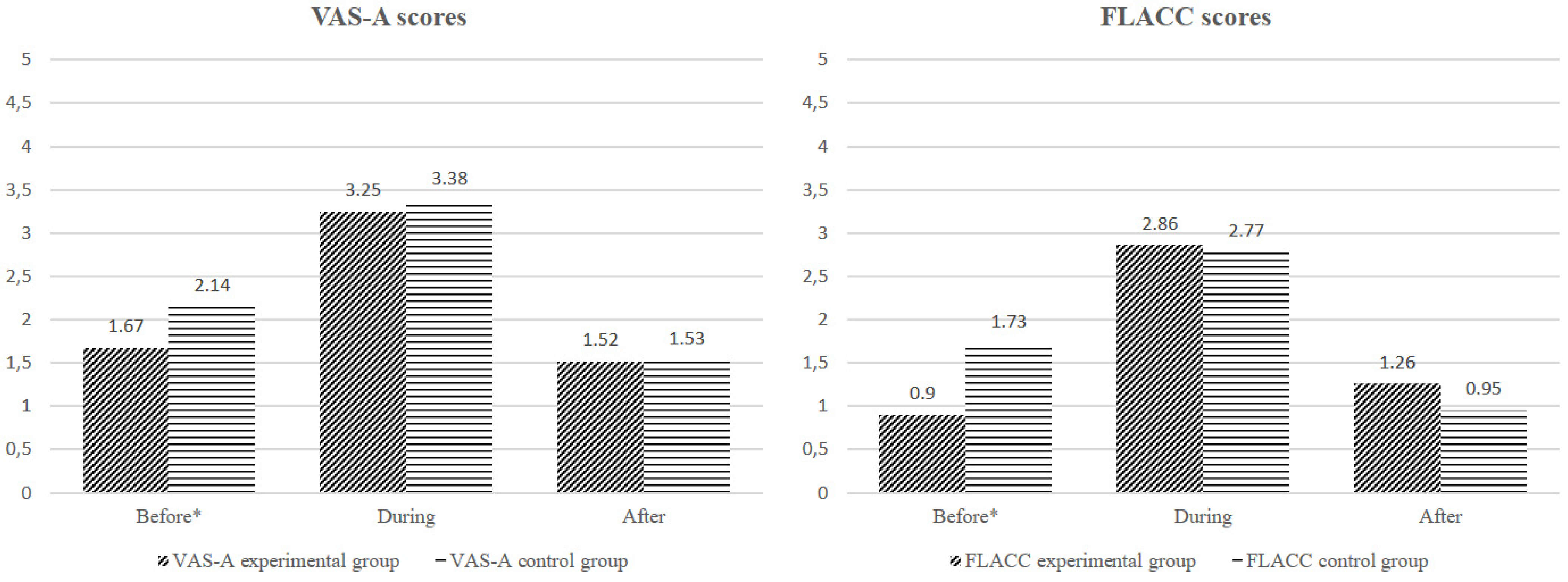
4.2.1. FLACC and VAS Scores
4.2.2. Experimental Group versus the Control Group
4.2.3. Children’s Recordings of VAS-A Score
4.2.4. Parents’ Attitudes towards the Use of Robots during Blood Collection
5. Discussion
5.1. Design of the Robot
5.2. Effectiveness of the Robot for Mitigating Pain and Anxiety
5.3. Strengths and Limitations
6. Conclusions and Future Research
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethical Statement
References
- Kennedy, R.M.; Luhmann, J.; Zempsky, W.T. Clinical Implications of Unmanaged Needle-Insertion Pain and Distress in Children. Pediatrics 2008, 122, S130–S133. [Google Scholar] [CrossRef] [Green Version]
- Uman, L.S.; Chambers, C.T.; McGrath, P.J.; Kisely, S.R. Psychological Interventions for Needle-related Procedural Pain and Distress in Children and Adolescents. Cochrane Database Syst. Rev. 2006, 4. [Google Scholar] [CrossRef] [Green Version]
- Sokolowski, C.J.; Giovannitti, J.A.; Boynes, S.G. Needle Phobia: Etiology, Adverse Consequences, and Patient Management. Dent. Clin. N. Am. 2010, 54, 731–744. [Google Scholar] [CrossRef]
- Willemsen, H.; Chowdhury, U.; Briscall, L. Needle Phobia in Children: A Discussion of Aetiology and Treatment Options—Hessel Willemsen, Uttom Chowdhury, Louise Briscall, 2002. Clin. Child Psychol. Psychiatry 2002, 7, 609–619. [Google Scholar] [CrossRef]
- Inal, S.; Kelleci, M. Distracting Children during Blood Draw: Looking through Distraction Cards Is Effective in Pain Relief of Children during Blood Draw. Int. J. Nurs. Pract. 2012, 18, 210–219. [Google Scholar] [CrossRef]
- Birnie, K.A.; Noel, M.; Parker, J.A.; Chambers, C.T.; Uman, L.S.; Kisely, S.R.; McGrath, P.J. Systematic Review and Meta-Analysis of Distraction and Hypnosis for Needle-Related Pain and Distress in Children and Adolescents. J. Pediatr. Psychol. 2014, 39, 783–808. [Google Scholar] [CrossRef] [Green Version]
- Arts, S.E.; Abu-Saad, H.H.; Champion, G.D.; Crawford, M.R.; Fisher, R.J.; Juniper, K.H.; Ziegler, J.B. Age-Related Response to Lidocaine-Prilocaine (EMLA) Emulsion and Effect of Music Distraction on the Pain of Intravenous Cannulation. Pediatrics 1994, 93, 797–801. [Google Scholar]
- Cassidy, K.-L.; Reid, G.J.; McGrath, P.J.; Finley, G.A.; Smith, D.J.; Morley, C.; Szudek, E.A.; Morton, B. Watch Needle, Watch TV: Audiovisual Distraction in Preschool Immunization. Pain Med. 2002, 3, 108–118. [Google Scholar] [CrossRef] [Green Version]
- Megel, M.E.; Houser, C.W.; Gleaves, L.S. Children’s Responses to Immunizations: Lullabies as a Distraction. Issues Compr. Pediatric Nurs. 1998, 21, 129–145. [Google Scholar] [CrossRef] [PubMed]
- Cohen, L.L.; Blount, R.L.; Panopoulos, G. Nurse Coaching and Cartoon Distraction: An Efective and Practical Intervention to Reduce Child, Parent, and Nurse Distress During Immunizations1. J. Pediatr. Psychol. 1997, 22, 355–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manne, S.L.; Redd, W.H.; Jacobsen, P.B.; Gorfinkle, K.; Schorr, O.; Rapkin, B. Behavioral Intervention to Reduce Child and Parent Distress during Venipuncture. J. Consult. Clin. Psychol. 1990, 58, 565–572. [Google Scholar] [CrossRef]
- Cifuentes, C.A.; Pinto, M.J.; Céspedes, N.; Múnera, M. Social Robots in Therapy and Care. Curr. Robot Rep. 2020, 1, 59–74. [Google Scholar] [CrossRef]
- Hegel, F.; Muhl, C.; Wrede, B.; Hielscher-Fastabend, M.; Sagerer, G. Understanding Social Robots. In Proceedings of the 2009 Second International Conferences on Advances in Computer-Human Interactions, Cancun, Mexico, 1–7 February 2009; pp. 169–174. [Google Scholar]
- Belpaeme, T.; Kennedy, J.; Ramachandran, A.; Scassellati, B.; Tanaka, F. Social Robots for Education: A Review. Sci. Robot. 2018, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konijn, E.A.; Hoorn, J.F. Robot Tutor and Pupils’ Educational Ability: Teaching the Times Tables. Comput. Educ. 2020, 157, 103970. [Google Scholar] [CrossRef]
- Vogt, P.; de Haas, M.; de Jong, C.; Baxter, P.; Krahmer, E. Child-Robot Interactions for Second Language Tutoring to Preschool Children. Front. Hum. Neurosci. 2017, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alemi, M.; Meghdari, A.; Ghazisaedy, M. The Impact of Social Robotics on L2 Learners’ Anxiety and Attitude in English Vocabulary Acquisition. Int. J. Soc. Robot. 2015, 7, 523–535. [Google Scholar] [CrossRef]
- Logan, D.E.; Breazeal, C.; Goodwin, M.S.; Jeong, S.; O’Connell, B.; Smith-Freedman, D.; Heathers, J.; Weinstock, P. Social Robots for Hospitalized Children. Pediatrics 2019, 144. [Google Scholar] [CrossRef]
- Dawe, J.; Sutherland, C.; Barco, A.; Broadbent, E. Can Social Robots Help Children in Healthcare Contexts? A Scoping Review. BMJ Paediatr. Open 2019, 3. [Google Scholar] [CrossRef] [Green Version]
- Beran, T.N.; Ramirez-Serrano, A.; Vanderkooi, O.G.; Kuhn, S. Reducing Children’s Pain and Distress towards Flu Vaccinations: A Novel and Effective Application of Humanoid Robotics. Vaccine 2013, 31, 2772–2777. [Google Scholar] [CrossRef]
- Moerman, C.J.; Jansens, R.M. Using Social Robot PLEO to Enhance the Well-Being of Hospitalised Children. J. Child Health Care 2020, 1367493520947503. [Google Scholar] [CrossRef]
- Farrier, C.E.; Pearson, J.D.R.; Beran, T.N. Children’s Fear and Pain During Medical Procedures: A Quality Improvement Study with a Humanoid Robot. Can. J. Nurs. Res. 2019, 844562119862742. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Manaloor, R.; Ma, K.; Sivakumar, M.; Beran, T.; Scott, S.D.; Vandermeer, B.; Beirnes, N.; Graham, T.A.D.; Curtis, S.; et al. A Randomized Trial of Robot-Based Distraction to Reduce Children’s Distress and Pain during Intravenous Insertion in the Emergency Department. Can. J. Emerg. Med. 2021, 23, 85–93. [Google Scholar] [CrossRef]
- Rossi, S.; Larafa, M.; Ruocco, M. Emotional and Behavioural Distraction by a Social Robot for Children Anxiety Reduction During Vaccination. Int. J. Soc. Robot. 2020, 12, 765–777. [Google Scholar] [CrossRef]
- Moerman, C.J.; van der Heide, L.; Heerink, M. Social Robots to Support Children’s Well-Being under Medical Treatment: A Systematic State-of-the-Art Review. J. Child Health Care 2019, 23, 596–612. [Google Scholar] [CrossRef]
- Harbert, K.R. Venipuncture. In Essential Clinical Procedures, 2nd ed.; Dehn, R.W., Asprey, D.P., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2007; Chapter 5; pp. 47–61. ISBN 978-1-4160-3001-0. [Google Scholar]
- Krleza, J.L.; Dorotic, A.; Grzunov, A.; Maradin, M. Capillary Blood Sampling: National Recommendations on Behalf of the Croatian Society of Medical Biochemistry and Laboratory Medicine. Biochem. Med. 2015, 25, 335–358. [Google Scholar] [CrossRef]
- Corbin, J.; Strauss, A. Basics of Qualitative Research. In Techniques and Procedures for Developing Grounded Theory, 3rd ed.; SAGE Publications, Inc.: Thousand Oaks, CA, USA, 2008; ISBN 978-1-4129-0644-9. [Google Scholar]
- SoftBank Robotics NAO the Humanoid and Programmable Robot|SoftBank Robotics. Available online: https://www.softbankrobotics.com/emea/en/nao (accessed on 16 September 2020).
- Morgan, D.L. Focus Groups. Annu. Rev. Sociol. 1996, 22, 129–152. [Google Scholar] [CrossRef]
- Morgan, D. Focus Groups as Qualitative Research; SAGE Publications, Inc.: Thousand Oaks, CA, USA, 1997; ISBN 978-0-7619-0343-7. [Google Scholar]
- McCormack, H.M.; Horne, D.J.d.L.; Sheather, S. Clinical Applications of Visual Analogue Scales: A Critical Review. Psychol. Med. 1988, 18, 1007–1019. [Google Scholar] [CrossRef]
- Bringuier, S.; Dadure, C.; Raux, O.; Dubois, A.; Picot, M.-C.; Capdevila, X. The Perioperative Validity of the Visual Analog Anxiety Scale in Children: A Discriminant and Useful Instrument in Routine Clinical Practice to Optimize Postoperative Pain Management. Anesth. Analg. 2009, 109, 737–744. [Google Scholar] [CrossRef]
- Merkel, S.I.; Voepel-Lewis, T.; Shayevitz, J.R.; Malviya, S. The FLACC: A Behavioral Scale for Scoring Postoperative Pain in Young Children. Pediatr. Nurs. 1997, 23, 293–297. [Google Scholar]
- Nemoto, T.; Beglar, D. Developing Likert-Scale Questionnaires Campus Reference Data. In JALT2013 Conference Proceedings; Sonda, N., Krause, A., Eds.; JALT: Tokyo, Japan, 2014. [Google Scholar]
- Bishop, G.F. Experiments with the Middle Response Alternative in Survey Questions. Public Opin. Q. 1987, 51, 220–232. [Google Scholar] [CrossRef]
- Presser, S.; Schuman, H. The Measurement of a Middle Position in Attitude Surveys. Public Opin. Q. 1980, 44, 70–85. [Google Scholar] [CrossRef]
- Olesen, V.; Droes, N.; Hatton, D.; Chico, N.; Schatzman, L. Analyzing Together: Recollections of a Team Approach, 1st ed.; Routledge: London, UK, 1994; pp. 125–142. ISBN 978-0-203-41308-1. [Google Scholar]
- van Eemeren, F.H.; Garssen, B.; Krabbe, E.C.W.; Snoeck Henkemans, A.F.; Verheij, B.; Wagemans, J.H.M. Toulmin’s Model of Argumentation. In Handbook of Argumentation Theory; Springer: Dordrecht, The Netherlands, 2014; pp. 203–256. ISBN 978-90-481-9473-5. [Google Scholar]
- Stewart, D.; Shamdasani, P.N.; Rook, D.W. Analyzing Focus Group Data. In Focus Groups; SAGE Publications, Ltd.: Thousand Oaks, CA, USA, 2007; ISBN 978-0-7619-2583-5. [Google Scholar]
- Namey, E.; Guest, G.; Thairu, L.; Johnson, L. Data reduction techniques for large qualitative data sets approaches to data analysis. In Handbook for Team-Based Qualitative Research; AltaMira Press: Lanham, MD, USA, 2008; Volume 2, pp. 137–161. [Google Scholar]
- Maciaszek, L.A.; Loucopoulos, P. (Eds.) Communications in Computer and Information Science. In Evaluation of Novel Approaches to Software Engineering: 5th International Conference, ENASE 2010, Athens, Greece, 22–24 July 2010, Revised Selected Papers; Springer: Berlin/Heidelberg, Germany, 2011; ISBN 978-3-642-23390-6. [Google Scholar]
- Aguinis, H.; Gottfredson, R.K.; Joo, H. Best-Practice Recommendations for Defining, Identifying, and Handling Outliers. Organ. Res. Methods 2013, 16, 270–301. [Google Scholar] [CrossRef]
- Yazici, B.; Yolacan, S. A Comparison of Various Tests of Normality. J. Stat. Comput. Simul. 2007, 77, 175–183. [Google Scholar] [CrossRef]
- Hecke, T.V. Power Study of Anova versus Kruskal-Wallis Test. J. Stat. Manag. Syst. 2012, 15, 241–247. [Google Scholar] [CrossRef]
- Nachar, N. The Mann-Whitney U: A Test for Assessing Whether Two Independent Samples Come from the Same Distribution. TQMP 2008, 4, 13–20. [Google Scholar] [CrossRef]
- Myers, L.; Sirois, M.J. Spearman Correlation Coefficients, Differences between. In Encyclopedia of Statistical Sciences; American Cancer Society: Atlanta, GA, USA, 2006; ISBN 978-0-471-66719-3. [Google Scholar]
- Gautheir, T.D. Detecting Trends Using Spearman’s Rank Correlation Coefficient. Environ. Forensics 2001, 2, 359–362. [Google Scholar] [CrossRef]
- Trost, M.J.; Chrysilla, G.; Gold, J.I.; Matarić, M. Socially-Assistive Robots Using Empathy to Reduce Pain and Distress during Peripheral IV Placement in Children. Pain Res. Manag. 2020, 2020, e7935215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalmers, T.C.; Smith, H.; Blackburn, B.; Silverman, B.; Schroeder, B.; Reitman, D.; Ambroz, A. A Method for Assessing the Quality of a Randomized Control Trial. Control. Clin. Trials 1981, 2, 31–49. [Google Scholar] [CrossRef]
- Frey, B.B. Ecological Validity. The SAGE Encyclopedia of Educational Research, Measurement, and Evaluation; SAGE Publications, Ltd.: Thousand Oaks, CA, USA, 2018. [Google Scholar]

| Program | Category | Description of Program |
|---|---|---|
| A | Lower classes in primary education (aimed at children aged 4–6) | First, the robot introduced itself and told the child what the robot was about to do. Thereafter, the robot asked questions about the child’s favorite color and animal. The robot continued to sing a song from a Dutch series of picture books (Miffy). Then, the robot danced to the song “head, shoulders, knees and toes”. Finally, the robot told the children that they did well and said goodbye. |
| B | Middle classes in primary education (aimed at children aged 6–9) | First, the robot introduced itself and told the child what the robot was about to do. Thereafter, the robot asked questions about the child’s hobbies. The robot continued demonstrating its hobbies: Tai chi and playing saxophone. Afterward, the robot told some jokes and danced to “head, shoulders, knees and toes”. Finally, the robot told the children that they did well and said goodbye. |
| C | Higher classes in primary education (aimed at children aged 9–12) | First, the robot introduced itself and told the child what the robot was about to do. The robot then demonstrated its hobbies: telling jokes and playing saxophone. Thereafter, the robot danced to the song named Gangnam Style by Psy, and to a 1970s’ style dance. Finally, the robot told the children that they did well and said goodbye. |
| Name | Description | Rationale | Priority |
|---|---|---|---|
| Interaction | The robot must be able to interact with the child during blood draw. | The child must be distracted during the moment of blood draw. | Must-have |
| Word use | The words used in the interaction must differ for every age group. | The choice of wording must be understandable for all children participating. | Must-have |
| Behavior | The robot’s behavior must be based on the age group to which the child belongs to. | The behavior must be tailored to the child’s social age to be approachable. | Must-have |
| Response | The robot should clarify if it wants a response from the child. | The child must not be confused by the expected interactions of the robot. | Must-have |
| Movement | The movement of the robot must be minimal. | To allow for normal blood draw procedures, the child must sit still. It is important to make sure the child does not mimic the robot’s movements during blood draw. | Must-have |
| Movement | The robot must be able to sit, lay down, and be able to get on its feet again when it has fallen over. | To ensure distractions for the child, the robot must be able to recover to normal operation. | Must-have |
| Encouragement | The robot must be able to give supportive encouragement. | To allow for the robot to successfully support the child through the blood draw, the robot provides supportive encouragement. | Must-have |
| Games | The robot must be able to ask questions or play games with the child. | To allow for the child to be distracted, games and quizzes can be used to focus on the robot and not on the procedure. | Should-have |
| Singing | The robot must be able to use singing in a sing-along style. | To allow for children aged 4–9 to be distracted, singing can be used to focus on the robot and not on the procedure. | Should-have |
| Jokes | The robot must be able to tell jokes to children aged 6–12. | To allow for children aged 6–12 to be distracted, humor can be used to focus on the robot and not on the procedure. | Should-have |
| Original songs | The robot must play songs with the original sound. | To allow for the child to be distracted, music can be used to focus on the robot and not on the procedure. | Should-have |
| Hand movements | The robot must be able to use hand movements. | To mimic human behavior, the robot should be able to wave goodbye to enforce a humanlike relationship. | Should-have |
| Colors | The robot must make use of colors, preferably, primary colors (red, yellow, and blue). | To allow for the child to be distracted, colors can be used to focus on the robot and not on the procedure. | Should-have |
| Name | Description | Rationale | Rationale for Rejection |
|---|---|---|---|
| Duration | The robot must provide distraction regardless of the duration of the blood draw. | To ensure the child will be distracted through the entire blood draw in case this takes longer than anticipated. | Because of a lack of programming skills and time, the project group was not able to program a script with a varying duration into the design of the social robot. |
| Different scripts for regular basis | The robot must have different behaviors for children who undergo blood draw regularly. | To ensure that a child who undergoes blood draw regularly will not undergo the blood draw with the same script as before. | Because of a lack of programming skills and time, the project group was not able to program multiple scripts for the same age group into the design of the social robot. |
| Operable by speech | The robot must be operable by speech. | Blood draw employees are busy with preparing the child for the procedure and the blood draw itself, thus, they are not able to press any buttons. | Because of the used platform, the robot could not use its camera and microphone. The platform makes use of the internet, and all these data will also be transported over the internet. This is not allowed due to privacy concerns. |
| Operability | The robot must be operable by using code words. | To avoid children with a mental illness getting confronted with their mental handicap, code words should be used. | Because of the used platform, the robot could not use its camera and microphone. The platform makes use of the internet, and all these data will also be transported over the internet. This is not allowed due to privacy concerns. |
| Placement | The robot must be placed on the eye level of the child. | To ensure the child has a clear view of the robot. | The attribute to get the robot on the eye level of the child would be a hindrance to the phlebotomist. Therefore, the robot was placed on the floor, about one meter distance from the child. |
| - | N | Missing Data | Mean | SD | p |
|---|---|---|---|---|---|
| All | 126 | 6 | 1.35 | 1.71 | 0.095 |
| Experimental | 69 | 3 | 1.12 | 1.65 | |
| Control | 63 | 3 | 1.60 | 1.76 |
| Questions | N | M | SD | Min | Max |
|---|---|---|---|---|---|
| How much knowledge do you have of robotics? | 69 | 2.31 | 1.19 | 1 | 5 |
| How much knowledge does your child have of robotics? | 69 | 2.12 | 1.23 | 1 | 5 |
| How tense was your child before coming to the clinic? | 128 | 3.64 | 1.65 | 1 | 6 |
| Do you think your child would like to be distracted by the robot? | 69 | 4.97 | 0.98 | 2 | 6 |
| What was the effect of the robot on the stress and anxiety of the child? | 69 | 5.08 | 1.04 | 3 | 6 |
| Do you think your child would like to use the robot again during blood draw? | 69 | 5.44 | 0.97 | 2 | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smakman, M.H.J.; Smit, K.; Buser, L.; Monshouwer, T.; van Putten, N.; Trip, T.; Schoof, C.; Preciado, D.F.; Konijn, E.A.; van der Roest, E.M.; et al. Mitigating Children’s Pain and Anxiety during Blood Draw Using Social Robots. Electronics 2021, 10, 1211. https://doi.org/10.3390/electronics10101211
Smakman MHJ, Smit K, Buser L, Monshouwer T, van Putten N, Trip T, Schoof C, Preciado DF, Konijn EA, van der Roest EM, et al. Mitigating Children’s Pain and Anxiety during Blood Draw Using Social Robots. Electronics. 2021; 10(10):1211. https://doi.org/10.3390/electronics10101211
Chicago/Turabian StyleSmakman, Matthijs H. J., Koen Smit, Lotte Buser, Tom Monshouwer, Nigel van Putten, Thymen Trip, Coen Schoof, Daniel F. Preciado, Elly A. Konijn, Esther M. van der Roest, and et al. 2021. "Mitigating Children’s Pain and Anxiety during Blood Draw Using Social Robots" Electronics 10, no. 10: 1211. https://doi.org/10.3390/electronics10101211








