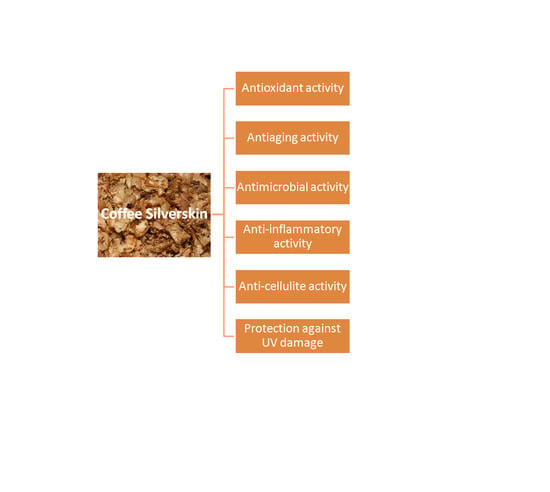
Coffee Silverskin: A Review on Potential Cosmetic Applications
Abstract
:1. Introduction
2. Skin Aging and Related Diseases
2.1. Anti-Aging Activity
2.2. Anti-Inflammatory Activity
2.3. Antimicrobial Activity
2.4. Protection against Skin UV Damage
2.5. Anti-Cellulite Activity
2.6. Anti-Hair Loss Activity
3. Safety and Toxicity
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Alves, R.C.; Rodrigues, F.; Antónia Nunes, M.; Vinha, A.F.; Oliveira, M.B.P.P. State of the art in coffee processing by-products. In Handbook of Coffee Processing by-Products, 1st ed.; Galanakis, C.M., Ed.; Academic Press: London, UK, 2017; pp. 1–26. [Google Scholar]
- Borrelli, R.C.; Esposito, F.; Napolitano, A.; Ritieni, A.; Fogliano, V. Characterization of a new potential functional ingredient: Coffee silverskin. J. Agric. Food Chem. 2004, 52, 1338–1343. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.S.; Alves, R.C.; Vinha, A.F.; Costa, E.; Costa, C.S.; Nunes, M.A.; Almeida, A.A.; Santos-Silva, A.; Oliveira, M.B.P.P. Nutritional, chemical and antioxidant/pro-oxidant profiles of silverskin, a coffee roasting by-product. Food Chem. 2017. [Google Scholar] [CrossRef]
- Costa, A.S.; Alves, R.C.; Vinha, A.F.; Barreira, S.V.; Nunes, M.A.; Cunha, L.M.; Oliveira, M.B.P.P. Optimization of antioxidants extraction from coffee silverskin, a roasting by-product, having in view a sustainable process. Ind. Crops Prod. 2014, 53, 350–357. [Google Scholar] [CrossRef]
- Carneiro, L.; Silva, J.; Mussatto, S.; Roberto, I.; Teixeira, J. Determination of total carbohydrates content in coffee industry residues. In Book of Abstracts of the 8th International Meeting of the Portuguese Carbohydrate Group; GLUPOR: Braga, Portugal, 2009; p. 94. [Google Scholar]
- Napolitano, A.; Fogliano, V.; Tafuri, A.; Ritieni, A. Natural occurrence of ochratoxin A and antioxidant activities of green and roasted coffees and corresponding byproducts. J. Agric. Food Chem. 2007, 55, 10499–10504. [Google Scholar] [CrossRef] [PubMed]
- Toschi, T.G.; Cardenia, V.; Bonaga, G.; Mandrioli, M.; Rodriguez-Estrada, M.T. Coffee silverskin: Characterization, possible uses, and safety aspects. J. Agric. Food Chem. 2014, 62, 10836–10844. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.C.; Costa, A.S.; Jerez, M.A.; Casal, S.; Sineiro, J.; Núñez, M.J.; Oliveira, M.B.P.P. Antiradical activity, phenolics profile, and hydroxymethylfurfural in espresso coffee: Influence of technological factors. J. Agric. Food Chem. 2010, 58, 12221–12229. [Google Scholar] [CrossRef] [PubMed]
- Mesías, M.; Navarro, M.; Martínez-Saez, N.; Ullate, M.; del Castillo, M.; Morales, F. Antiglycative and carbonyl trapping properties of the water soluble fraction of coffee silverskin. Food Res. Int. 2014, 62, 1120–1126. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Haza, A.I.; Ávalos, A.; del Castillo, M.D.; Morales, P. Validation of coffee silverskin extract as a food ingredient by the analysis of cytotoxicity and genotoxicity. Food Res. Int. 2017, 100, 791–797. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Regulation (EC) No. 123/2005 of 26 January 2005 amending Regulation (EC) No. 466/2001 as regards ochratoxin A. Off. J. Eur. Union 2005, L25, 3–5. [Google Scholar]
- Narita, Y.; Inouye, K. Review on utilization and composition of coffee silverskin. Food Res. Int. 2014, 61, 16–22. [Google Scholar] [CrossRef] [Green Version]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Selection of the solvent and extraction conditions for maximum recovery of antioxidant phenolic compounds from coffee silverskin. Food Bioproc. Technol. 2014, 7, 1322–1332. [Google Scholar] [CrossRef] [Green Version]
- Mussatto, S.I.; Machado, E.M.; Martins, S.; Teixeira, J.A. Production, composition, and application of coffee and its industrial residues. Food Bioprocess Technol. 2011, 4, 661–672. [Google Scholar] [CrossRef] [Green Version]
- Pourfarzad, A.; Mahdavian-Mehr, H.; Sedaghat, N. Coffee silverskin as a source of dietary fiber in bread-making: Optimization of chemical treatment using response surface methodology. LWT-Food Sci. Technol. 2013, 50, 599–606. [Google Scholar] [CrossRef]
- Martinez-Saez, N.; Ullate, M.; Martin-Cabrejas, M.A.; Martorell, P.; Genovés, S.; Ramon, D.; del Castillo, M.D. A novel antioxidant beverage for body weight control based on coffee silverskin. Food Chem. 2014, 150, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.; Pereira, C.; Pimentel, F.; Alves, R.; Ferreira, M.; Sarmento, B.; Amaral, M.H.; Oliveira, M.B.P.P. Are coffee silverskin extracts safe for topical use? An in vitro and in vivo approach. Ind. Crops Prod. 2015, 63, 167–174. [Google Scholar] [CrossRef]
- Rodrigues, F.; Matias, R.; Ferreira, M.; Amaral, M.H.; Oliveira, M.B.P.P. In vitro and in vivo comparative study of cosmetic ingredients coffee silverskin and hyaluronic acid. Exp. Dermatol. 2016, 25, 572–574. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.; Antónia Nunes, M.; Alves, R.; Oliveira, M. Applications of recovered bioactive compounds in cosmetics and other products. In Handbook of Coffee Processing by-Products, 1st ed.; Galanakis, C.M., Ed.; Academic Press: London, UK, 2017; pp. 195–220. [Google Scholar]
- Furusawa, M.; Narita, Y.; Iwai, K.; Fukunaga, T.; Nakagiri, O. Inhibitory effect of a hot water extract of coffee “silverskin” on hyaluronidase. Biosci. Biotechnol. Biochem. 2011, 75, 1205–1207. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.; Palmeira-de-Oliveira, A.; das Neves, J.; Sarmento, B.; Amaral, M.H.; Oliveira, M.B.P.P. Coffee silverskin: A possible valuable cosmetic ingredient. Pharm. Biol. 2015, 53, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.S.; Nunes, F.M.; Domingues, M.R.; Coimbra, M.A. Coffee melanoidins: Structures, mechanisms of formation and potential health impacts. Food Funct. 2012, 3, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Lephart, E.D. Skin aging and oxidative stress: Equol’s anti-aging effects via biochemical and molecular mechanisms. Ageing Res. Rev. 2016, 31, 36–54. [Google Scholar] [CrossRef] [PubMed]
- Berthon, J.-Y.; Nachat-Kappes, R.; Bey, M.; Cadoret, J.-P.; Renimel, I.; Filaire, E. Marine algae as attractive source to skin care. Free Rad. Res. 2017, 51, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Bessada, S.M.; Barreira, J.C.; Oliveira, M.B.P.P. Asteraceae species with most prominent bioactivity and their potential applications: A review. Ind. Crops Prod. 2015, 76, 604–615. [Google Scholar] [CrossRef]
- Del Castillo, M.; Fernandez-Gomez, B.; Martinez Saez, N.; Iriondo De Hond, A.; Martirosyan, D.; Mesa, M. Coffee silverskin extract for aging and chronic diseases. In Functional Foods for Chronic Diseases, 1st ed.; Martirosyan, D.M., Ed.; CreateSpace Independent Publishing Platform: Colorado, TX, USA, 2016; pp. 386–409. [Google Scholar]
- Tobin, D.J. Introduction to skin aging. J. Tissue Viability 2017, 26, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Paur, I.; Balstad, T.R.; Blomhoff, R. Degree of roasting is the main determinant of the effects of coffee on NF-κb and epre. Free Radic. Biol. Med. 2010, 48, 1218–1227. [Google Scholar] [CrossRef] [PubMed]
- Sahasrabudhe, A.; Deodhar, M. Anti-hyaluronidase, anti-elastase activity of Garcinia indica. Int. J. Bot. 2010, 6, 1–10. [Google Scholar] [CrossRef]
- Rodrigues, F.; Gaspar, C.; Palmeira-de-Oliveira, A.; Sarmento, B.; Helena Amaral, M.; Oliveira, M.B.P.P. Application of coffee silverskin in cosmetic formulations: Physical/antioxidant stability studies and cytotoxicity effects. Drug Dev. Ind. Pharm. 2016, 42, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.; Sarmento, B.; Amaral, M.H.; Oliveira, M.B.P.P. Exploring the antioxidant potentiality of two food by-products into a topical cream: Stability, in vitro and in vivo evaluation. Drug Dev. Ind. Pharm. 2016, 42, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Iriondo-DeHond, A.; Martorell, P.; Genovés, S.; Ramón, D.; Stamatakis, K.; Fresno, M.; Molina, A.; del Castillo, M.D. Coffee silverskin extract protects against accelerated aging caused by oxidative agents. Molecules 2016, 21, 721. [Google Scholar] [CrossRef] [PubMed]
- Martinez, R.M.; Pinho-Ribeiro, F.A.; Steffen, V.S.; Silva, T.C.; Caviglione, C.V.; Bottura, C.; Fonseca, M.J.; Vicentini, F.T.; Vignoli, J.A.; Baracat, M.M. Topical formulation containing naringenin: Efficacy against ultraviolet b irradiation-induced skin inflammation and oxidative stress in mice. PLoS ONE 2016, 11, e0146296. [Google Scholar] [CrossRef] [PubMed]
- Menezes, A.C.; Campos, P.M.; Euletério, C.; Simões, S.; Praça, F.S.G.; Bentley, M.V.L.B.; Ascenso, A. Development and characterization of novel 1-(1-naphthyl) piperazine-loaded lipid vesicles for prevention of uv-induced skin inflammation. Eur. J. Pharm. Biopharm. 2016, 104, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.S.; Satsu, H.; Bae, M.-J.; Zhao, Z.; Ogiwara, H.; Totsuka, M.; Shimizu, M. Anti-inflammatory effect of chlorogenic acid on the IL-8 production in Caco-2 cells and the dextran sulphate sodium-induced colitis symptoms in c57bl/6 mice. Food Chem. 2015, 168, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-H.; Koh, E.-J.; Lee, Y.-J.; Chio, J.; Song, J.-H.; Seo, Y.-J.; Lee, B.-Y. Anti-inflammatory effect of caffeine by regulating NF-κb activation in murine macrophage. FASEB J. 2016, 30, lb256. Available online: http://www.fasebj.org/content/30/1_Supplement/lb256.short (accessed on 3 January 2018).
- Vitaglione, P.; Morisco, F.; Mazzone, G.; Amoruso, D.C.; Ribecco, M.T.; Romano, A.; Fogliano, V.; Caporaso, N.; D’argenio, G. Coffee reduces liver damage in a rat model of steatohepatitis: The underlying mechanisms and the role of polyphenols and melanoidins. Hepatology 2010, 52, 1652–1661. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Zamora, A.; Pastoriza, S.; Rufián-Henares, J.A. Revalorization of coffee by-products. Prebiotic, antimicrobial and antioxidant properties. LWT-Food Sci. Technol. 2015, 61, 12–18. [Google Scholar] [CrossRef]
- Rufian-Henares, J.A.; de la Cueva, S.P. Antimicrobial activity of coffee melanoidins: A study of their metal-chelating properties. J. Agric. Food Chem. 2009, 57, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Antonio, A.G.; Moraes, R.S.; Perrone, D.; Maia, L.C.; Santos, K.R.N.; Iório, N.L.; Farah, A. Species, roasting degree and decaffeination influence the antibacterial activity of coffee against streptococcus mutans. Food Chem. 2010, 118, 782–788. [Google Scholar] [CrossRef]
- Cardenia, V.; Rodriguez-Estrada, M.T.; Boselli, E.; Lercker, G. Cholesterol photosensitized oxidation in food and biological systems. Biochimie. 2013, 95, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, S.; Yoshii, K.; Morita, S.-Y.; Teraoka, R. Efficient topical delivery of chlorogenic acid by an oil-in-water microemulsion to protect skin against UV-induced damage. Chem. Pharm. Bull. 2011, 59, 793–796. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.; Qin, Z.; Xia, W.; Shao, Y.; Voorhees, J.J.; Fisher, G.J. Matrix-degrading metalloproteinases in photoaging. J. Investig. Dermatol. Symp. Proc. 2009, 14, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-H.; Bahuguna, A.; Kim, H.-H.; Kim, D.-I.; Kim, H.-J.; Yu, J.-M.; Jung, H.-G.; Jang, J.-Y.; Kwak, J.-H.; Park, G.-H. Potential effect of compounds isolated from Coffea arabica against UV-B induced skin damage by protecting fibroblast cells. J. Photochem. Photobiol. B Biol. 2017, 174, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-P.; Lou, Y.-R.; Xie, J.-G.; Peng, Q.-Y.; Liao, J.; Yang, C.S.; Huang, M.-T.; Conney, A.H. Topical applications of caffeine or (−)-epigallocatechin gallate (EGCG) inhibit carcinogenesis and selectively increase apoptosis in UVB-induced skin tumors in mice. Proc. Natl. Acad. Sci. USA 2002, 99, 12455–12460. [Google Scholar] [CrossRef] [PubMed]
- Koo, S.W.; Hirakawa, S.; Fujii, S.; Kawasumi, M.; Nghiem, P. Protection from photodamage by topical application of caffeine after ultraviolet irradiation. Br. J. Dermatol. 2007, 156, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.-S.; Park, E.D.; Park, Y.; Han, S.H.; Hong, K.B.; Suh, H.J. Topical application of spent coffee ground extracts protects skin from ultraviolet B-induced photoaging in hairless mice. Photochem. Photobiol. Sci. 2016, 15, 779–790. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, Z.; Halabchi, F.; Mazaheri, R.; Abolhasani, M.; Tabesh, M. Review of the mechanisms and effects of noninvasive body contouring devices on cellulite and subcutaneous fat. Int. J. Endocrinol. Metab. 2016, 14, e36727. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, A. Cellulite and its treatment. Int. J. Cosmet. Sci. 2006, 28, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.C.; Oliveira, M.B.P.P.; Casal, S. Coffee authenticty. In Current Topics on Food Authetication, 1st ed.; Oliveira, M.B.P.P., Mafra, I., Amaral, J.S., Eds.; Transworld Research Network: Kerala, India, 2017; pp. 57–72. [Google Scholar]
- Herman, A.; Herman, A. Caffeine’s mechanisms of action and its cosmetic use. Skin Pharmacol. Physiol. 2013, 26, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.; Alves, A.C.; Nunes, C.; Sarmento, B.; Amaral, M.H.; Reis, S.; Oliveira, M.B.P.P. Permeation of topically applied caffeine from a food by-product in cosmetic formulations: Is nanoscale in vitro approach an option? Int. J. Pharm. 2016, 513, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Fischer, T.; Hipler, U.; Elsner, P. Effect of caffeine and testosterone on the proliferation of human hair follicles in vitro. Int. J. Dermatol. 2007, 46, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Teichmann, A.; Richter, H.; Knorr, F.; Antoniou, C.; Sterry, W.; Lademann, J. Investigation of the penetration and storage of a shampoo formulation containing caffeine into the hair follicles by in vivo laser scanning microscopy. Laser Phys. Lett. 2007, 4, 464–468. [Google Scholar] [CrossRef]
- Lademann, J.; Richter, H.; Schanzer, S.; Klenk, A.; Sterry, W.; Patzelt, A. Analysis of the penetration of a caffeine containing shampoo into the hair follicles by in vivo laser scanning microscopy. Laser Phys. 2010, 20, 551–556. [Google Scholar] [CrossRef]
- Otberg, N.; Patzelt, A.; Rasulev, U.; Hagemeister, T.; Linscheid, M.; Sinkgraven, R.; Sterry, W.; Lademann, J. The role of hair follicles in the percutaneous absorption of caffeine. Br. J. Clin. Pharm. 2008, 65, 488–492. [Google Scholar] [CrossRef] [PubMed]
| Compound | Biological Activities | References |
|---|---|---|
| Caffeine | Antioxidant and anti-aging activity; Thermogenic and anti-cellulite activity; Protection against UV damage; Increase of blood circulation in the skin; Inhibition of 5α-reductase and hyaluronidase activities. | [7,19,20] |
| Caffeoylquinic acids/Feruloylquinic acids/p-coumaroylquinic acids | Anti-aging activity; Protection against UV damage; Antimicrobial and anti-inflammatory activity. | [17,21] |
| Melanoidins | Antioxidant, anti-aging, antimicrobial and anti-inflammatory activities. | [22] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bessada, S.M.F.; C. Alves, R.; P. P. Oliveira, M.B. Coffee Silverskin: A Review on Potential Cosmetic Applications. Cosmetics 2018, 5, 5. https://doi.org/10.3390/cosmetics5010005
Bessada SMF, C. Alves R, P. P. Oliveira MB. Coffee Silverskin: A Review on Potential Cosmetic Applications. Cosmetics. 2018; 5(1):5. https://doi.org/10.3390/cosmetics5010005
Chicago/Turabian StyleBessada, Sílvia M. F., Rita C. Alves, and M. Beatriz P. P. Oliveira. 2018. "Coffee Silverskin: A Review on Potential Cosmetic Applications" Cosmetics 5, no. 1: 5. https://doi.org/10.3390/cosmetics5010005