Castanea sativa Bur: An Undervalued By-Product but a Promising Cosmetic Ingredient
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Materials
2.3. Preparation of C. sativa Bur Hydro-Alcoholic Extracts
2.4. Preparation of Hydrogels Containing C. sativa Bur Hydro-Alcoholic Extracts
2.5. Technological Characterization of Hydrogels
2.5.1. Colour Evaluation
2.5.2. Determination of pH
2.5.3. Determination of Moisture
2.5.4. Texture Analysis
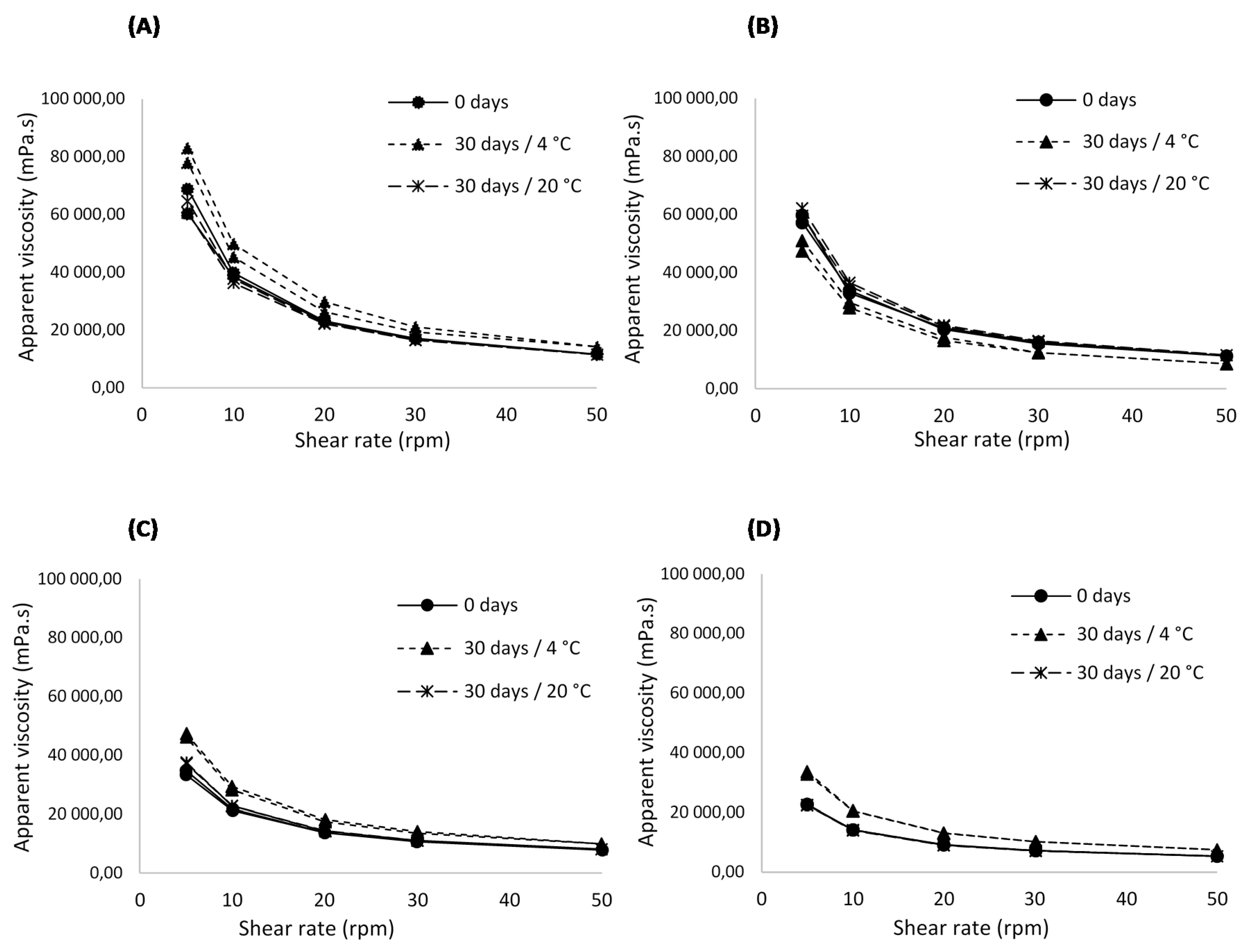
2.5.5. Rheological Behaviour Analysis
2.6. Determination of Hydrogels Bioactive Compounds
2.6.1. Total Phenolic Content
2.6.2. Total Flavonoid Content
2.7. In Vitro Antioxidant Activity of Hydrogels
2.7.1. DPPH Free Radical Scavenging Assay
2.7.2. Ferric Reducing Antioxidant Power Assay
2.8. Microbiological Properties
2.9. Stability Study
2.10. Statistical Analysis
3. Results and Discussion
3.1. Technological Characterization of Hydrogels
3.1.1. Colour Evaluation
3.1.2. Determination of pH
3.1.3. Determination of Moisture
3.1.4. Texture Analysis
3.1.5. Rheological Behaviour Analysis
3.2. Bioactive Compounds in Hydrogels
3.2.1. Total Phenolic Content
3.2.2. Total Flavonoid Content
3.3. In Vitro Antioxidant Activity of Hydrogels
3.3.1. DPPH Free Radical Scavenging Assay
3.3.2. Ferric Reducing Antioxidant Power Assay
3.4. Correlation between Total Phenolic Content and Antioxidant Activity
3.5. Correlation between Total Flavonoid Content and Antioxidant Activity
3.6. Correlation between Total Phenolic and Total Flavonoid Contents
3.7. Microbiological Properties
3.8. Stability Study
4. Conclusions and Future Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rodrigues, F.; Santos, J.; Pimentel, F.B.; Braga, N.; Palmeira-de-Oliveira, A.; Oliveira, M.B.P.P. Promising new applications of Castanea sativa shell: Nutritional composition, antioxidant activity, amino acids and vitamin E profile. Food Funct. 2015, 6, 2854–2860. [Google Scholar] [CrossRef] [PubMed]
- Braga, N.; Rodrigues, F.; Oliveira, M.B.P.P. Castanea sativa by-products: A review on added value and sustainable application. Nat. Prod. Res. 2015, 29, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Pinto, D.; Rodrigues, F.; Braga, N.; Santos, J.; Pimentel, F.B.; Palmeira-de-Oliveira, A.; Oliveira, M.B.P.P. The Castanea sativa bur as a new potential ingredient for nutraceutical and cosmetic outcomes: Preliminary studies. Food Funct. 2017, 8, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Vázquez, G.; Fernández-Agulló, A.; Gómez-Castro, C.; Freire, M.S.; Antorrena, G.; González-Álvarez, J. Response surface optimization of antioxidants extraction from chestnut (Castanea sativa) bur. Ind. Crops Prod. 2012, 35, 126–134. [Google Scholar] [CrossRef]
- Almeida, I.F.; Valentão, P.; Andrade, P.B.; Seabra, R.M.; Pereira, T.M.; Amaral, M.H.; Costa, P.C.; Bahia, M.F. In vivo skin irritation potential of a Castanea sativa (Chestnut) leaf extract, a putative natural antioxidant for topical application. Basic Clin. Pharmacol. Toxicol. 2008, 103, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Barreira, J.C.M.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P.; Pereira, J.A. Antioxidant activities of the extracts from chestnut flower, leaf, skins and fruit. Food Chem. 2008, 107, 1106–1113. [Google Scholar] [CrossRef]
- Barreira, J.C.M.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P.; Pereira, J.A. Antioxidant Potential of Chestnut (Castanea sativa L.) and Almond (Prunus dulcis L.) By-products. Food Sci. Technol. Int. 2010, 16, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Almeida, I.F.; Maleckova, J.; Saffi, R.; Monteiro, H.; Góios, F.; Amaral, M.H.; Costa, P.C.; Garrido, J.; Silva, P.; Pestana, N.; et al. Characterization of an antioxidant surfactant-free topical formulation containing Castanea sativa leaf extract. Drug Dev. Ind. Pharm. 2015, 41, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, M.C.B.M.; Bennett, R.N.; Quideau, S.; Jacquet, R.; Rosa, E.A.S.; Ferreira-Cardoso, J.V. Evaluating the potential of chestnut (Castanea sativa Mill.) fruit pericarp and integument as a source of tocopherols, pigments and polyphenols. Ind. Crops Prod. 2010, 31, 301–311. [Google Scholar] [CrossRef]
- Federici, F.; Fava, F.; Kalogerakis, N.; Mantzavinos, D. Valorisation of agro-industrial by-products, effluents and waste: Concept, opportunities and the case of olive mill wastewaters. J. Chem. Technol. Biotechnol. 2009, 84, 895–900. [Google Scholar] [CrossRef]
- Vázquez, G.; González-Alvarez, J.; Freire, M.S.; Fernández-Agulló, A.; Santos, J.; Antorrena, G. Chestnut Burs as a Source of Natural Antioxidants. In Chemical Engineering Transactions; United States Environmental Protection Agency (EPA): Washington, DC, USA, 2009; pp. 855–860. [Google Scholar]
- Mujić, A.; Grdović, N.; Mujić, I.; Mihailović, M.; Živković, J.; Poznanović, G.; Vidaković, M. Antioxidative effects of phenolic extracts from chestnut leaves, catkins and spiny burs in streptozotocin-treated rat pancreatic β-cells. Food Chem. 2011, 125, 841–849. [Google Scholar] [CrossRef]
- Basile, A.; Sorbo, S.; Giordano, S.; Ricciardi, L.; Ferrara, S.; Montesano, D.; Castaldo, R.C.; Vuotto, M.L.; Ferrara, L. Antibacterial and allelopathic activity of extract from Castanea sativa leaves. Fitoterapia 2000, 71 (Suppl. 1), S110–S116. [Google Scholar] [CrossRef]
- Almeida, I.F.; Costa, P.C.; Bahia, M.F. Evaluation of Functional Stability and Batch-to-Batch Reproducibility of a Castanea sativa Leaf Extract with Antioxidant Activity. AAPS PharmSciTech 2010, 11, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, G.; Calvo, M.; Freire, M.S.; González-Álvarez, J.; Antorrena, G. Chestnut shell as heavy metal adsorbent: Optimization study of lead, copper and zinc cations removal. J. Hazard. Mater. 2009, 172, 1402–1414. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, G.; González-Alvarez, J.; Santos, J.; Freire, M.S.; Antorrena, G. Evaluation of potential applications for chestnut (Castanea sativa) shell and eucalyptus (Eucalyptus globulus) bark extracts. Ind. Crops Prod. 2009, 29, 364–370. [Google Scholar] [CrossRef]
- Okayama, Y. Oxidative stress in allergic and inflammatory skin diseases. Curr. Drug Targets Inflamm. Allergy 2005, 4, 517–519. [Google Scholar] [CrossRef] [PubMed]
- Sander, C.S.; Chang, H.; Hamm, F.; Elsner, P.; Thiele, J.J. Role of oxidative stress and the antioxidant network in cutaneous carcinogenesis. Int. J. Dermatol. 2004, 43, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Nishigori, C.; Hattori, Y.; Toyokuni, S. Role of reactive oxygen species in skin carcinogenesis. Antioxid. Redox Signal. 2004, 6, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Ou-Yang, H.; Stamatas, G.; Saliou, C.; Kollias, N. A chemiluminescence study of UVA-induced oxidative stress in human skin in vivo. J. Investig. Dermatol. 2004, 122, 1020–1029. [Google Scholar] [CrossRef] [PubMed]
- Rechner, A.R.; Kuhnle, G.; Bremner, P.; Hubbard, G.P.; Moore, K.P.; Rice-Evans, C.A. The metabolic fate of dietary polyphenols in humans. Free Radic. Biol. Med. 2002, 33, 220–235. [Google Scholar] [CrossRef]
- Rodrigues, F.; Palmeira-de-Oliveira, A.; Neves, J.; Sarmento, B.; Amaral, M.H.; Oliveira, M.B.P.P. Coffee silverskin: A possible valuable cosmetic ingredient. Pharm. Biol. 2015, 53, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.M.; Lin, P.H.; Yao, Q.; Chen, C. Chemical and molecular mechanisms of antioxidants: Experimental approaches and model systems. J. Cell Mol. Med. 2010, 14, 840–860. [Google Scholar] [CrossRef] [PubMed]
- Craft, B.D.; Kerrihard, A.L.; Amarowicz, R.; Pegg, R.B. Phenol-Based Antioxidants and the in vitro Methods Used for Their Assessment. Compr. Rev. Food Sci. Food Saf. 2012, 11, 148–173. [Google Scholar] [CrossRef]
- Roleira, F.M.; Siquet, C.; Orru, E.; Garrido, E.M.; Garrido, J.; Milhazes, N.; Podda, G.; Paiva-Martins, F.; Reis, S.; Carvalho, R.A.; et al. Lipophilic phenolic antioxidants: Correlation between antioxidant profile, partition coefficients and redox properties. Biorgan. Med. Chem. 2010, 18, 5816–5825. [Google Scholar] [CrossRef] [PubMed]
- Prochazkova, D.; Bousova, I.; Wilhelmova, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, M.C.B.M.; Bennett, R.N.; Rosa, E.A.S.; Ferreira-Cardoso, J.V. Primary and Secondary Metabolite Composition of Kernels from Three Cultivars of Portuguese Chestnut (Castanea sativa Mill.) at Different Stages of Industrial Transformation. J. Agric. Food Chem. 2007, 55, 3508–3516. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.C.; Costa, A.S.G.; Jerez, M.; Casal, S.; Sineiro, J.; Nunez, M.J.; Oliveira, M.B.P.P. Antiradical activity, phenolics profile, and hydroxymethylfurfural in espresso coffee: Influence of technological factors. J. Agric. Food Chem. 2010, 58, 12221–12229. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.S.G.; Alves, R.C.; Vinha, A.F.; Barreira, S.V.P.; Nunes, M.A.; Cunha, L.M.; Oliveira, M.B.P.P. Optimization of antioxidants extraction from coffee silverskin, a roasting by-product, having in view a sustainable process. Ind. Crops Prod. 2014, 53, 350–357. [Google Scholar] [CrossRef]
- Barros, L.; Baptista, P.; Ferreira, I.C.F.R. Effect of Lactarius piperatus fruiting body maturity stage on antioxidant activity measured by several biochemical assays. Food Chem. Toxicol. 2007, 45, 1731–1737. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Shoaib Khan, H.M.; Iqbal, A.; Khan, B.A.; Bashir, S. Glycyrrhiza glabra extract cream: Effects on skin pigment “melanin”. In Proceedings of the International Conference on Bioscience, Biochemistry and Bioinformatics, Singapore, 26–28 February 2011; Volume 5, pp. 434–439. [Google Scholar]
- Jones, D.S.; Muldoon, B.C.; Woolfson, A.D.; Sanderson, F.D. An examination of the rheological and mucoadhesive properties of poly(acrylic acid) organogels designed as platforms for local drug delivery to the oral cavity. J. Pharm. Sci. 2007, 96, 2632–2646. [Google Scholar] [CrossRef] [PubMed]
- Rozman, B.; Gasperlin, M. Stability of vitamins C and E in topical microemulsions for combined antioxidant therapy. Drug Deliv. 2007, 14, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.P.; Leong, L.P.; William Koh, J.H. Antioxidant activities of aqueous extracts of selected plants. Food Chem. 2006, 99, 775–783. [Google Scholar] [CrossRef]
- ISO Standard 11930:2012. Cosmetics—Microbiology—Evaluation of the Antimicrobial Protection of a Cosmetic Product; International Organization for Standardization (ISO): Geneva, Switzerland, 2012.
- Anchisi, C.; Maccioni, A.M.; Sinico, C.; Valenti, D. Stability studies of new cosmetic formulations with vegetable extracts as functional agents. Farmaco 2001, 56, 427–431. [Google Scholar] [CrossRef]
- Bouftira, I.; Abdelly, C.; Sfar, S. Characterization of cosmetic cream with Mesembryanthemum crystallinum plant extract: Influence of formulation composition on physical stability and anti-oxidant activity. Int. J. Cosmet. Sci. 2008, 30, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Guaratini, T.; Gianeti, M.D.; Campos, P.M. Stability of cosmetic formulations containing esters of vitamins E and A: Chemical and physical aspects. Int. J. Pharm. 2006, 327, 12–16. [Google Scholar] [CrossRef] [PubMed]
| Composition of Hydrogels | ||||
|---|---|---|---|---|
| Compounds | Total Weight (%) | |||
| F1 | F2 | F3 | F4 | |
| Glycerine | 10 | 10 | 10 | 10 |
| Carbopol® 940 | 0.5 | 0.5 | 0.5 | 0.5 |
| Preservative | 0.5 | 0.5 | 0.5 | 0.5 |
| C. sativa bur hydro-alcoholic extract | - | 25 | 50 | 89 |
| Triethanolamine | q.s. | q.s. | q.s | q.s. |
| Deionized water | 89 | 64 | 39 | - |
| Formulations | Technological Properties | |||||||
|---|---|---|---|---|---|---|---|---|
| L* (Lightness) | a* (Redness) | b* (Yellowness) | pH | Moisture (%) | Adhesiveness (N.mm) | Firmness (N) | ||
| F1 | T0 | 10.69 ± 0.01 a | −0.64 ± 0.04 a | 3.37 ± 0.02 c | 4.46 ± 0.04 a | 30.69 ± 1.64 a | −1.480 ± 0.237 a | 0.554 ± 0.013 b |
| T30/4 °C | 10.01 ± 0.05 c | −0.72 ± 0.06 a | 4.01 ± 0.04 a | 4.48 ± 0.01 a | 30.28 ± 0.70 a | −1.943 ± 0.232 a | 0.749 ± 0.023 a | |
| T30/20 °C | 10.11 ± 0.01 b | −0.73 ± 0.04 a | 3.57 ± 0.02 b | 4.52 ± 0.01 a | 29.35 ± 0.55 a | −1.413 ± 0.220 a | 0.531 ± 0.031 b | |
| F2 | T0 | 10.74 ± 0.02 c | 2.70 ± 0.08 a | 6.39 ± 0.06 b | 5.27 ± 0.03 a | 20.27 ± 0.39 a | −1.158 ± 0.148 a | 0.461 ± 0.020 a |
| T30/4 °C | 10.93 ± 0.01 a | 2.65 ± 0.04 a | 6.71 ± 0.06 a | 5.00 ± 0.02 b | 20.01 ± 0.66 a | −1.193 ± 0.013 a | 0.471 ± 0.019 a | |
| T30/20 °C | 10.83 ± 0.02 b | 2.74 ± 0.08 a | 6.51 ± 0.06 b | 4.99 ± 0.01 b | 20.12 ± 0.49 a | −1.029 ± 0.077 a | 0.429 ± 0.004 b | |
| F3 | T0 | 10.31 ± 0.02 b | 0.43 ± 0.03 a | 3.03 ± 0.01 a | 5.67 ± 0.01 b | 15.43 ± 3.13 a | −0.932 ± 0.122 a | 0.367 ± 0.012 b |
| T30/4 °C | 10.96 ± 0.02 a | 0.42 ± 0.02 a | 2.89 ± 0.02 c | 5.65 ± 0.01 b | 14.15 ± 0.53 a | −1.125 ± 0.033 b | 0.439 ± 0.011 a | |
| T30/20 °C | 10.27 ± 0.02 b | 0.49 ± 0.03 a | 2.98 ± 0.01 b | 5.72 ± 0.01 a | 13.89 ± 0.73 a | −0.857 ± 0.035 a | 0.381 ± 0.005 b | |
| F4 | T0 | 11.25 ± 0.02 c | −0.28 ± 0.09 a | 1.69 ± 0.01 c | 8.28 ± 0.01 b | 14.04 ± 0.63 a | −0.707 ± 0.082 a | 0.288 ± 0.008 a |
| T30/4 °C | 12.44 ± 0.03 a | −0.15 ± 0.09 a | 1.96 ± 0.05 a | 8.69 ± 0.01 a | 14.03 ± 0.13 a | −0.659 ± 0.029 a | 0.259 ± 0.007 b | |
| T30/20 °C | 11.32 ± 0.02 b | −0.35 ± 0.09 c | 1.78 ± 0.01 b | 8.21 ± 0.02 c | 13.99 ± 0.23 a | −0.679 ± 0.060 a | 0.261 ± 0.007 b | |
| Formulations | Bioactive Compounds | ||||
|---|---|---|---|---|---|
| TPC (mg GAE/g gel) | TFC (mg CAE/g gel) | FRAP (µmol FSE/g gel) | DPPH (µg TE/g gel) | ||
| F1 | T0 | 0.79 ± 0.01 a | 0.05 ± 0.01 a | 98.41 ± 7.65 a | 431.96 ± 7.71 a |
| T30/4 °C | 0.82 ± 0.01 a | 0.06 ± 0.01 a | 85.05 ± 5.01 a | 395.01 ± 3.86 b | |
| T30/20 °C | 0.69 ± 0.01 b | 0.06 ± 0.01 a | 76.71 ± 5.78 b | 280.46 ± 8.12 c | |
| F2 | T0 | 2.48 ± 0.02 b | 0.31 ± 0.01 a | 509.17 ± 16.10 a | 658.37 ± 5.26 a |
| T30/4 °C | 2.81 ± 0.01 a | 0.27 ± 0.01 b | 510.84 ± 13.25 a | 656.07 ± 10.11 a | |
| T30/20 °C | 1.96 ± 0.01 c | 0.23 ± 0.01 c | 412.32 ± 16.10 b | 611.24 ± 10.21 b | |
| F3 | T0 | 5.30 ± 0.01 a | 0.73 ± 0.01 a | 774.66 ± 10.43 a | 803.15 ± 12.71 a |
| T30/4 °C | 4.41 ± 0.02 b | 0.69 ± 0.01 b | 727.91 ± 15.30 b | 795.03 ± 7.58 a | |
| T30/20 °C | 3.48 ± 0.01 c | 0.63 ± 0.01 c | 714.55 ± 10.43 b | 745.91 ± 13.36 b | |
| F4 | T0 | 9.65 ± 0.04 a | 1.23 ± 0.02 a | 1013.43 ± 12.61 a | 990.84 ± 14.06 a |
| T30/4 °C | 8.00 ± 0.01 b | 1.16 ± 0.01 b | 1030.13 ± 18.97 a | 933.98 ± 11.58 b | |
| T30/20 °C | 7.55 ± 0.04 c | 1.08 ± 0.02 c | 888.20 ± 12.61 b | 867.11 ± 11.57 c | |
| Formulations | Microbiological Properties | |||
|---|---|---|---|---|
| Total Aerobic Count (UFC/mL gel) | Total Mesophilic Aerobic Bacteria Count (UFC/mL gel) | Total Yeast and Mold Count (UFC/mL gel) | ||
| F1 | T0 | ≤10 | ≤10 | ≤10 |
| T30/4 °C | ≤10 | ≤10 | ≤10 | |
| T30/20 °C | ≤10 | ≤10 | ≤10 | |
| F2 | T0 | ≤10 | ≤10 | ≤10 |
| T30/4 °C | ≤10 | ≤10 | ≤10 | |
| T30/20 °C | ≤10 | ≤10 | ≤10 | |
| F3 | T0 | ≤10 | ≤10 | 195 |
| T30/4 °C | ≤10 | ≤10 | ≤10 | |
| T30/20 °C | ≤10 | ≤10 | ≤10 | |
| F4 | T0 | ≤10 | 15 | 15 |
| T30/4 °C | ≤10 | 25 | 30 | |
| T30/20 °C | ≤10 | ≤10 | ≤10 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto, D.; Braga, N.; Rodrigues, F.; Oliveira, M.B.P.P. Castanea sativa Bur: An Undervalued By-Product but a Promising Cosmetic Ingredient. Cosmetics 2017, 4, 50. https://doi.org/10.3390/cosmetics4040050
Pinto D, Braga N, Rodrigues F, Oliveira MBPP. Castanea sativa Bur: An Undervalued By-Product but a Promising Cosmetic Ingredient. Cosmetics. 2017; 4(4):50. https://doi.org/10.3390/cosmetics4040050
Chicago/Turabian StylePinto, Diana, Nair Braga, Francisca Rodrigues, and M. Beatriz P. P. Oliveira. 2017. "Castanea sativa Bur: An Undervalued By-Product but a Promising Cosmetic Ingredient" Cosmetics 4, no. 4: 50. https://doi.org/10.3390/cosmetics4040050





