Overview of Skin Whitening Agents: Drugs and Cosmetic Products
Abstract
:1. Introduction
2. Different Mechanisms of Action for Achieving a Depigmentation Effect
2.1. Skin Treatments
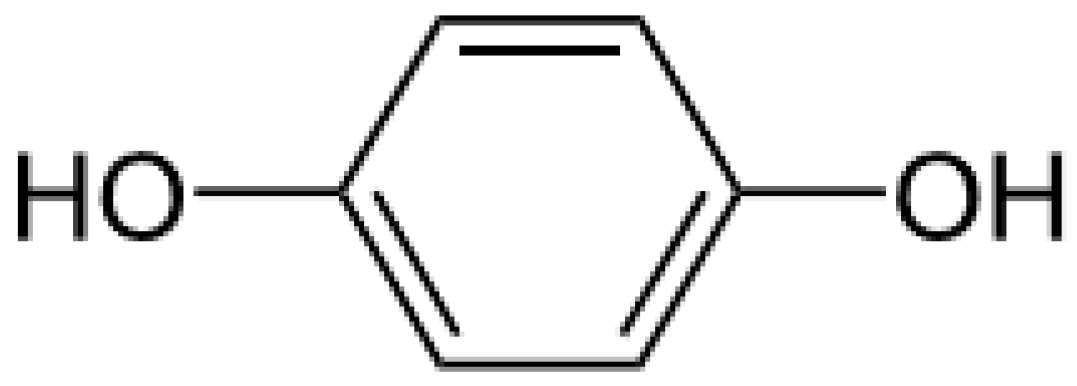
2.1.1. Hydroquinone and Its Derivatives
2.1.2. Retinoic Acid or Tretinoin or Vitamin A Acid
2.2. The Cosmetology Approach
2.2.1. Active Ingredients Having an Indirect Action on Already-Formed Melanins
2.2.2. Active Ingredients Acting on the Melanin Formation Process
Ascorbic Acid and Its Derivatives
Divalent Ion Chelators
Retinol and Retinaldehyde
Tyrosinase Inhibitors
Tyrosinase Inhibitors Offering New Avenues in the Field of Skin Lightening
2.2.3. Value of Combinations
3. Prevention, the Most Effective Means of Combat
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Scarpa, A.; Guerci, A. Depigmenting procedures and drugs employed by melanoderm populations. J. Ethnopharmacol. 1987, 19, 17–66. [Google Scholar] [CrossRef]
- Kamagaju, L.; Bizuru, E.; Minani, V.; Morandini, R.; Stévigny, C.; Ghanem, G.; Duez, P. An ethnobotanical survey of medicinal plants used in Rwanda for voluntary depigmentation. J. Ethnopharmacol. 2013, 150, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Fiori, F.; Andrisano, V. LC–MS method for the simultaneous determination of six glucocorticoids in pharmaceutical formulations and counterfeit cosmetic products. J. Pharm. Biomed. Anal. 2014, 91, 185–192. [Google Scholar]
- Perry, A.; Petit, A.; Bagot, M.; Villa, A.; Bellaiche, M.; Bourrat, E. Intoxication au mercure par procuration chez un enfant: Une nouvelle complication de la dépigmentation volontaire. Arch. Pédiatr. 2014, 21, 22–24. (In French) [Google Scholar] [CrossRef]
- Sène, D.; Huong-Boutin, D.L.T.; Thiollet, M.; Barete, S.; Cacoub, P.; Piette, J.C. Insuffisance surrénalienne haute symptomatique compliquant l’usage de dermocorticoïdes pour dépigmentation volontaire. Rev. Méd. Int. 2008, 29, 1030–1033. (In French) [Google Scholar] [CrossRef] [PubMed]
- Porcheron, A.; Latreille, J.; Jdid, R.; Tschachler, E.; Morizot, F. Influence of skin ageing features on Chinese women’s perception of facial age and attractiveness. Int. J. Cosmet. Sci. 2014, 36, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Pathak, M.; Fitzpatrick, T.B.; Kraus, E.W. Usefulness of retinoic acid in the treatment of melasma. J. Am. Acad. Dermatol. 1986, 15, 894–899. [Google Scholar] [CrossRef]
- Akyol, A.; Can, O.T.; Bayramoglu, M. Treatment of hydroquinone by photochemical oxidation and electrocoagulation combined process. J. Water Process Eng. 2015, 8, 45–54. [Google Scholar] [CrossRef]
- Findlay, G.H. Ochronosis following skin bleaching with hydroquinone. J. Am. Acad. Dermatol. 1982, 6, 1092–1093. [Google Scholar] [CrossRef]
- Papaspyrides, C.D.; Protopapas, S.A. E.s.r. approach on hydroquinone-melanin possible interaction. Int. J. Biol. Macromol. 1988, 10, 62–63. [Google Scholar] [CrossRef]
- Devillers, J.; Boule, P.; Vasseur, P.; Prevot, P.; Steiman, R.; Seigle-Murandi, F.; Benoit-Guyod, J.L.; Nendza, M.; Grioni, C.; Dive, D.; et al. Environmental and health risks of hydroquinone. Ecotoxicol. Environ. Saf. 1990, 19, 327–354. [Google Scholar] [CrossRef]
- O’Donaghue, J.L.; David, P.; Richardson, W.; Dyer, M. Hydroquinone and hepatitis. Lancet 1995, 346, 1427–1428. [Google Scholar] [CrossRef]
- Rendon, M.; Berneburg, M.; Arellano, I.; Picardo, M. Treatment of melasma. J. Am. Acad. Dermatol. 2006, 54, S272–S281. [Google Scholar] [CrossRef] [PubMed]
- Tidman, M.J.; Horton, J.J.; MacDonald, D.M. Hydroquinone-induced ochronosis—Light and electronmicroscopic features. Clin. Exp. Dermatol. 1986, 11, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Findlay, G.H.; Morrisson, J.G.L.; Simson, I.W. Exogenous ochronosis and pigmented colloid milium from hydroquinone bleaching creams. Br. J. Dermatol. 1975, 93, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Masumori, S.; Hirata-Koizumi, M.; Ono, A.; Honma, M.; Yokoyama, K.; Hirose, A. Evaluation of in vivo mutagenicity of hydroquinone in Muta™ mice. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2014, 775–776, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.M.; Zhou, Q.; Lei, T.C.; Ding, S.F.; Xu, S.Z. Effects of hydroquinone and its glucoside derivatives on melanogenesis and antioxidation: Biosafety as skin whitening agents. J. Dermatol. Sci. 2009, 55, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Kligman, A.M.; Willis, I. A new formula for depigmenting human skin. Arch. Dermatol. 1975, 111, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Katsambas, A.D.; Stratigos, A.J. Depigmenting and bleaching agents: Coping with hyperpigmentation. Clin. Dermatol. 2001, 19, 483–488. [Google Scholar] [CrossRef]
- Callender, V.D. An open-label study of the use of adapalene cream and a triple-combination therapy for the treatment of mild-to-moderate acne and post-inflammatory hyperpigmentation. J. Am. Acad. Dermatol. 2004, 50. [Google Scholar] [CrossRef]
- Callender, V.D. Maintaining remission of melasma with triple-combination cream therapy. J. Am. Acad. Dermatol. 2005, 52. [Google Scholar] [CrossRef]
- Taylor, S. Open-label case study on triple-combination cream in patients with pseudofolliculitis barbae. J. Am. Acad. Dermatol. 2005, 52. [Google Scholar] [CrossRef]
- Grimes, P.E. Management of Hyperpigmentation in Darker Racial Ethnic Groups. Semin. Cutan. Med. Surg. 2009, 28, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Goldman, M.; Lori, A.; Luz, E.; Gold, M.; Gottschalk, R. Results from a split-face study comparing sequential treatment with triple combination cream and intense pulsed light versus a control cream and intense pulsed light in patients with moderate to severe melasma. J. Am. Acad. Dermatol. 2009, 60, AB161. [Google Scholar]
- Hexsel, D.; Sidou, F.; Kerrouche, N.; Cestari, T. Combination of a triple combination cream and tretinoin cream in subjects with mottled hyperpigmentation associated with photodamage. J. Am. Acad. Dermatol. 2010, 62. [Google Scholar] [CrossRef]
- Scissors, B.; Gathers, R.C. A case of exogenous ochronosis caused by a triple combination bleaching cream. J. Am. Acad. Dermatol. 2010, 62. [Google Scholar] [CrossRef]
- Hsieh, P.W.; Al-Suwayeh, S.A.; Fang, C.L.; Lin, H.F.; Chen, C.C.; Fang, J.Y. The co-drug of conjugated hydroquinone and azelaic acid to enhance topical skin targeting and decrease penetration through the skin. Eur. J. Pharm. Biopharm. 2012, 81, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, A.B., Jr.; Schwartzel, E.H.; Colby, S.I.; Altman, D.J. The combination of 2% 4-hydroxyanisole (Mequinol) and 0.01% tretinoin is effective in improving the appearance of solar lentigines and related hyperpigmented lesions in two double-blind multicenter clinical studies. J. Am. Acad. Dermatol. 2000, 42, 459–467. [Google Scholar] [CrossRef]
- Ortonne, J.P.; Pandya, A.G.; Lui, H.; Hexsel, D. Treatment of solar lentigines. J. Am. Acad. Dermatol. 2006, 54, S262–S271. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Gover, M.D.; Nouri, K.; Taylor, S. The treatment of melasma: A review of clinical trials. J. Am. Acad. Dermatol. 2006, 55, 1048–1065. [Google Scholar] [CrossRef] [PubMed]
- Tzimas, G.; Collins, M.D.; Nau, H. Identification of 14-hydroxy-4,14-retro-retinol as an in vivo metabolite of vitamin A. Biochim. Biophys. Acta (BBA) Lipids Lipid Metab. 1996, 1301, 1–6. [Google Scholar] [CrossRef]
- Clark, E.; Scerri, L. Superficial and medium-depth chemical peels. Clin. Dermatol. 2008, 26, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Inan, S.; Oztukcan, S.; Vatansever, S.; Ermertcan, A.T.; Zeybek, D.; Oksal, A.; Giray, G.; Muftuoglu, S. Histopathological and ultrastructural effects of glycolic acid on rat skin. Acta Histochem. 2006, 108, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Okuda, M.; Donahue, D.A.; Kaufman, L.E.; Avalos, J.; Simion, F.A.; Story, D.C.; Sakaguchi, H.; Fautz, R.; Fuchs, A. Negligible penetration of incidental amounts of alpha-hydroxy acid from rinse-off personal care products in human skin using an in vitro static diffusion cell model. Toxicol. Vitr. 2011, 25, 2041–2047. [Google Scholar] [CrossRef] [PubMed]
- Denda, S.; Denda, M.; Inoue, K.; Hibino, T. Glycolic acid induces keratinocyte proliferation in a skin equivalent model via TRPV1 activation. J. Dermatol. Sci. 2010, 57, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Kempiak, S.J.; Uebelhoer, N. Superficial Chemical Peels and Microdermabrasion for Acne Vulgaris. Semin. Cutan. Med. Surg. 2008, 27, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Kornhauser, A.; Wie, R.R.; Yamaguchi, Y.; Coelho, S.G.; Kaidbey, K.; Barton, C.; Takahashi, K.; Beer, J.Z.; Miller, S.A.; Hearing, V.J. The effects of topically applied glycolic acid and salicylic acid on ultraviolet radiation-induced erythema, DNA damage and sunburn cell formation in human skin. J. Dermatol. Sci. 2009, 55, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Madan, R.K.; Jacob, L. A review of toxicity from topical salicylic acid preparations. J. Am. Acad. Dermatol. 2014, 70, 788–792. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.J.; Dreher, F.; Chew, A.L.; Zhai, H.; Levin, C.; Stern, R.; Maibach, H.I. Cutaneous bioassay of salicylic acid as a keratolytic. Int. J. Pharm. 2005, 292, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Landau, M. Chemical peels. Clin. Dermatol. 2008, 26, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Kodali, S.; Guevara, I.L.; Carrigan, C.R.; Daulat, S.; Blanco, G.; Boker, A.; Hynan, L.S.; Pandya, A.G. A prospective, randomized, split-face, controlled trial of salicylic acid peels in the treatment of melasma in Latin American women. J. Am. Acad. Dermatol. 2010, 63, 1030–1035. [Google Scholar] [CrossRef] [PubMed]
- Baden, H.P.; Alper, J.C. A Keratolytic Gel Containing Salicylic Acid in Propylene Glycol. J. Investig. Dermatol. 1973, 61, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Gallarate, M.; Carlotti, M.E.; Trotta, M.; Bovo, S. On the stability of ascorbic acid in emulsified systems for topical and cosmetic use. Int. J. Pharm. 1999, 188, 233–241. [Google Scholar] [CrossRef]
- Jutley, G.S.; Rajaratnam, R.; Halpern, J.; Salim, A.; Emmett, C. Systematic review of randomized controlled trials on interventions for melasma: An abridged Cochrane review. J. Am. Acad. Dermatol. 2014, 70, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Bowe, W.P.; Shalita, A.R. Effective over-the-counter acne treatments. Semin. Cutan. Med. Surg. 2008, 27, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Słoczyńska, K.; Gunia-Krzyżak, A.; Żelaszczyk, D.; Waszkielewicz, A.M.; Marona, H. Skin metabolism established with the use of MetaSite for selected retinoids employed in topical and systemic treatment of various skin disorders and found in cosmeceuticals. Acta Biochim. Pol. 2015, 62, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Yourick, J.; Jung, C.; Bronaugh, R. In vitro and in vivo percutaneous absorption of retinol from cosmetic formulations: Significance of the skin reservoir and prediction of systemic absorption. Toxicol. Appl. Pharmacol. 2008, 231, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Nohynek, G.J.; Meuling, W.J.; Vaes, W.H.; Lawrence, R.S.; Shapiro, S.; Schulte, S.; Steiling, W.; Bausch, J.; Gerber, E.; Sasa, H.; et al. Repeated topical treatment, in contrast to single oral doses, with Vitamin A-containing preparations does not affect plasma concentrations of retinol, retinyl esters or retinoic acids in female subjects of child-bearing age. Toxicol. Lett. 2006, 163, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Bae-Hwan, K.; Yong-Soon, L.; Kyung-Sun, K. The mechanism of retinol-induced irritation and its application to anti-irritant development. Toxicol. Lett. 2003, 146, 65–73. [Google Scholar]
- Mélot, M.; Pudney, P.D.A.; Williamson, A.M.; Caspers, P.J.; Van Der Pol, A.; Puppels, G.J. Studying the effectiveness of penetration enhancers to deliver retinol through the stratum cornum by in vivo confocal Raman spectroscopy. J. Controll. Release 2009, 138, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Miller, D. Skin benefits of a retinol-containing daily moisturizer with photostable SPF-30. J. Am. Acad. Dermatol. 2008, 58, AB23. [Google Scholar]
- Séhédic, D.; Hardy-Boismartel, A.; Couteau, C.; Coiffard, L.J. Are cosmetic products which include an SPF appropriate for daily use? Arch. Dermatol. Res. 2009, 301, 603–608. [Google Scholar] [CrossRef] [PubMed]
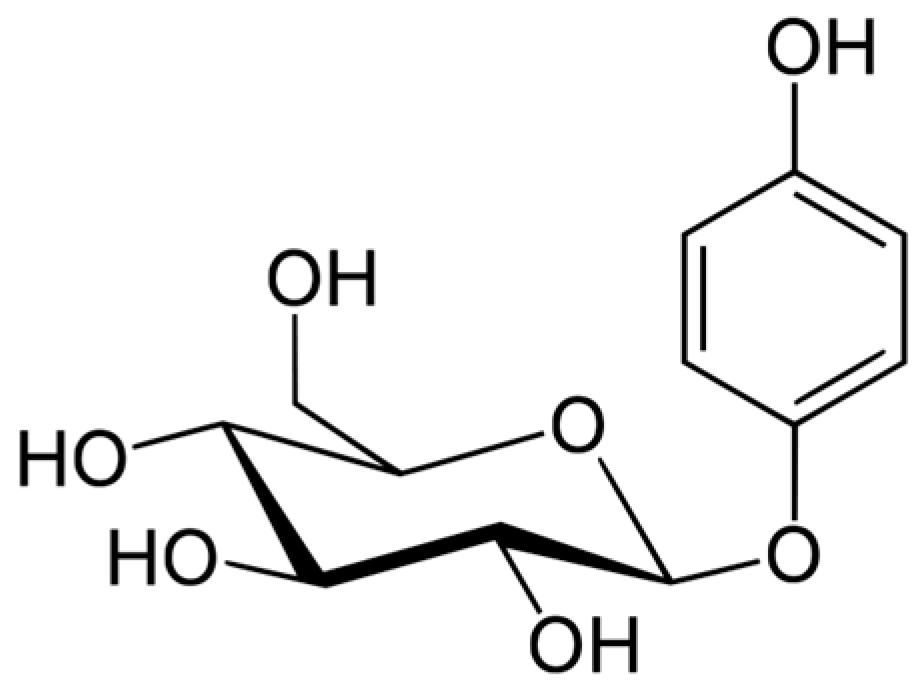
- Lukas, B.; Schmiderer, C.; Mitteregger, U.; Novak, J. Arbutin in marjoram and oregano. Food Chem. 2010, 121, 185–190. [Google Scholar] [CrossRef]
- Migas, P.; Krauze-Baranowska, M. The significance of arbutin and its derivatives in therapy and cosmetics. Phytochem. Lett. 2015, 13, 35–40. [Google Scholar] [CrossRef]
- Suau, R.; Cuevas, A.; Valpuesta, V.; Reid, M.S. Arbutin and sucrose in the leaves of the resurrection plant Myrothamnus flabellifolia. Phytochemistry 1991, 30, 2555–2556. [Google Scholar] [CrossRef]
- Nordlund, J.J.; Grimes, P.E.; Ortonnes, J.P. The safety of hydroquinone. J. Eur. Acad. Dermatol. Venereol. 2006, 20, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, K.; Nomura, K.; Nishimura, T.; Kiso, T.; Sugimoto, K.; Kuriki, T. Syntheses of α-arbutin-α-glycosides and their inhibitory effects on human tyrosinase. J. Biosci. Bioeng. 2005, 99, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Couteau, C.; Coiffard, L.J.M. Photostability determination of arbutin, a vegetable whitening agent. II Farmaco 2000, 55, 410–413. [Google Scholar] [CrossRef]
- Degen, G.H. Opinion of the Scientific Committee on Consumer Safety (SCCS)—Opinion on the safety of the use of α-arbutin in cosmetic products. Regul. Toxicol. Pharmacol. 2016, 74, 75–76. [Google Scholar]
- Degen, G.H. Opinion of the Scientific Committee on Consumer Safety (SCCS)—Opinion on the safety of the use of β-arbutin in cosmetic products. Regul. Toxicol. Pharmacol. 2015, 73, 866–867. [Google Scholar] [PubMed]
- Shin, J.W.; Yoon, S.W.; Jeong, J.B.; Park, K.C. Different responses of the melanin index to ultraviolet irradiation in relation to skin color and body site. Photodermatol. Photoimmunol. Photomed. 2014, 30, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Hamed, S.H.; Sriwiriyanont, P.; deLong, M.A.; Visscher, M.O.; Wickett, R.R.; Boissy, R.E. Comparative efficacy and safety of deoxyarbutin, a new tyrosinase-inhibiting agent. J. Cosmet. Sci. 2006, 57, 291–308. [Google Scholar] [PubMed]
- Lynch, B.; Simon, R.; Roberts, A. In vitro and in vivo assessment of the genotoxic activity of aloesin. Regul. Toxicol. Pharmacol. 2011, 61, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Draelos, Z.D. The cosmeceutical realm. Clin. Dermatol. 2008, 26, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Gao, J. The Use of Botanical Extracts as Topical Skin-Lightening Agents for the Improvement of Skin Pigmentation Disorders. J. Investig. Dermatol. Symp. Proc. 2008, 13, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Yimam, M.; Brownell, L.; Jia, Q. In vivo safety evaluation of UP780, a standardized composition of aloe chromone aloesin formulated with an Aloe vera inner leaf fillet. Regul. Toxicol. Pharmacol. 2014, 69, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Park, K.C.; Huh, S.Y.; Choi, H.R.; Kim, D.S. Biology of melanogenesis and the search for hypopigmenting agents. Dermatol. Sin. 2010, 28, 53–57. [Google Scholar] [CrossRef]
- Somjen, D.; Katzburg, S.; Vaya, J.; Kaye, A.M.; Hendel, D.; Posner, G.H.; Tamir, S. Estrogenic activity of glabridin and glabrene from licorice roots on human osteoblasts and prepubertal rat skeletal tissues. J. Steroid Biochem. Mol. Biol. 2004, 91, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.; Li, H.; Wang, X.; Lee, F.S.; Cui, S. Isolation and identification of flavonoids in licorice and a study of their inhibitory effects on tyrosinase. J. Agric. Food Chem. 2005, 53, 7408–7414. [Google Scholar] [CrossRef] [PubMed]
- Amer, M.; Metwalli, M. Topical liquiritin improves melasma. Int. J. Dermatol. 2000, 39, 299–301. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, Y.; Wang, Y.; Zhang, Y. Bioassay-guided screening and isolation of α-glucosidase and tyrosinase inhibitors from leaves of Morus alba. Food Chem. 2012, 131, 617–622. [Google Scholar] [CrossRef]
- Wang, K.H.; Lin, R.D.; Hsu, F.L.; Huang, Y.H.; Chang, H.C.; Huang, C.Y.; Lee, M.H. Cosmetic applications of selected traditional Chinese herbal medicines. J. Ethnopharmacol. 2006, 106, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Nohynek, G.J.; Kirkland, D.; Marzin, D.; Toutain, H.; Leclerc-Ribaud, C.; Jinnai, H. An assessment of the genotoxicity and human health risk of topical use of kojic acid. Food Chem. Toxicol. 2004, 42, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.N.; Kwak, S.Y.; Seo, H.S.; Seo, J.H.; Kim, B.G.; Lee, Y.S. Kojic acid-amino acid conjugates as tyrosinase inhibitors. Bioorg. Med. Chem. Lett. 2009, 19, 5586–5589. [Google Scholar] [CrossRef] [PubMed]
- Mitani, H.; Koshiishi, I.; Sumita, T.; Imanari, T. Prevention of the photodamage in the hairless mouse dorsal skin by kojic acid as an iron chelator. Eur. J. Pharmacol. 2001, 411, 169–174. [Google Scholar] [CrossRef]
- Burnett, C.L.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Final report of the safety assessment of Kojic acid as used in cosmetics. Int. J. Toxicol. 2010, 29, 244S–273S. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.W.; Park, K.C. Current clinical use of depigmenting agents. Dermatol Sin. 2014, 32, 205–210. [Google Scholar] [CrossRef]
- Nazzaro-Porro, M. Azelaic acid. J. Am. Acad. Dermatol. 1987, 17, 1033–1041. [Google Scholar] [CrossRef]
- Lowe, N.J.; Rizk, D.; Grimes, P.; Billips, M.; Pincus, S. Azelaic acid 20% cream in the treatment of facial hyperpigmentation in darker-skinned patients. Clin. Ther. 1998, 20, 945–959. [Google Scholar] [CrossRef]
- Webster, G. Combination azelaic acid therapy for acne vulgaris. J. Am. Acad. Dermatol. 2000, 43, S47–S50. [Google Scholar] [CrossRef] [PubMed]
- Draelos, Z. Multiple mechanisms of action of azelaic acid: New findings. J. Am. Acad. Dermatol. 2009, 60, AB86. [Google Scholar]
- Mingrone, G.; Greco, A.V.; Ciardiello, A.; Passo, S.; Nazzaro-Porro, M. Distribution of radiolabelled azelaic acid in eye membranes and fluids of rabbits. Exp. Pathol. 1984, 25, 85–88. [Google Scholar] [CrossRef]
- Schäfer, T.; Merkl, J.; Klemm, E.; Wichmann, H.E.; Ring, J.; KORA Study Group. The Epidemiology of Nevi and Signs of Skin Aging in the Adult General Population: Results of the KORA-Survey 2000. J. Investig. Dermatol. 2006, 126, 1490–1496. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.X.; Nguyen, N.T.; Nguyen, M.H.; Le, T.H.; Van Do, T.N.; Hung, T.M.; Nguyen, M.T. Tyrosinase inhibitory activity of flavonoids from Artocarpus heterophyllous. Chem. Cent. J. 2016, 10. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.J.; Lin, C.C.; Lu, T.M.; Li, J.H.; Chen, I.S.; Kuo, Y.H.; Ko, H.H. Chemical constituents derived from Artocarpus xanthocarpus as inhibitors of melanin biosynthesis. Phytochemistry 2015, 117, 424–435. [Google Scholar] [CrossRef] [PubMed]
- Jagtap, U.B.; Bapat, V.A. Artocarpus: A review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2010, 129, 142–166. [Google Scholar] [CrossRef] [PubMed]
- Germanò, M.P.; Cacciola, F.; Donato, P.; Dugo, P.; Certo, G.; D’Angelo, V.; Mondello, L.; Rapisarda, A. Betula pendula leaves: Polyphenolic characterization and potential innovative use in skin whitening products. Fitoterapia 2012, 83, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Maack, A.; Pegard, A. Populus nigra (Salicaceae) absolute rich in phenolic acids, phenylpropanoïds and flavonoids as a new potent tyrosinase inhibitor. Fitoterapia 2016, 111, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Himani, S.; Madhuri, K.L.; Koushalya, D. Evaluation and comparison of polyphenols and bioactivities of wild edible fruits of North-West Himalaya, India. Asian Pac. J. Trop. Dis. 2015, 5, 888–893. [Google Scholar]
- Batubara, I.; Julita, I.; Latifah, K.; Darusman, A.M.M.; Mitsunaga, T. Flower Bracts of Temulawak (Curcuma Xanthorrhiza) for Skin Care: Anti-acne and Whitening Agents. Procedia Chem. 2015, 14, 216–224. [Google Scholar] [CrossRef]
- Sung, H.C.; Liang, C.J.; Lee, C.W.; Yen, F.L.; Hsiao, C.Y.; Wang, S.H.; Jiang-Shieh, Y.F.; Tsai, J.S.; Chen, Y.L. The protective effect of eupafolin against TNF-α-induced lung inflammation via the reduction of intercellular cell adhesion molecule-1 expression. J. Ethnopharmacol. 2015, 170, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.H.; Chiang, Y.C.; Tsai, M.H.; Liang, C.J.; Hsu, L.F.; Li, S.Y.; Wang, M.C.; Yen, F.L.; Lee, C.W. Eupafolin, a skin whitening flavonoid isolated from Phyla nodiflora, downregulated melanogenesis: Role of MAPK and Akt pathways. J. Ethnopharmacol. 2014, 151, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Seité, S.; Oresajo, C. A double-blind, placebo controlled clinical trial to evaluate the efficacy and safety of a new skin whitening combination formula in patients with solar lentigines. J. Am. Acad. Dermatol. 2016, 74, AB10. [Google Scholar]
- Holman, D.M.; Berkowitz, Z.; Gery, G.P., Jr.; Hawkins, N.A.; Saraiya, M.; Watson, M. Patterns of sunscreen use on the face and other exposed skin among US adults. J. Am. Acad. Dermatol. 2015, 73, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Couteau, C.; Chauvet, C.; Paparis, E.; Coiffard, L.J. Influence of certain ingredients on the SPF determined in vivo. Arch. Dermatol. Res. 2012, 304, 817–821. [Google Scholar] [CrossRef] [PubMed]

| Mechanism of Action | Molecules |
|---|---|
| Inhibition of tyrosinase transcription | Tretinoin, glucosamine, retinol, retinaldehyde, N-acetyl glucosamine |
| Tyrosinase inhibition | Hydroquinone, mequinol, arbutin, azelaic acid, kojic acid, ellagic acid, resveratrol, oxyresvaretral |
| Epidermal turnover accelerant | Vitamin C, vitamin E, thioctic acid, retinoids, lactic acid, glycolic acid, salicylic acid, liquiritin |
| Inhibition of melanosome transfer | Linoleic acid |
| Anti-inflammatory | Niacinamide, soy milk |
| Free radical trapping agents | Topical steroids, glycyrrhetinic acid |
| Peel Level | Active Ingredient |
|---|---|
| Superficial or very superficial | Fruit acids, trichloroacetic acid (10%–20%), salicylic acid, tretinoin |
| Medium | Trichloroacetic acid (35%) |
| Deep | Phenol |
| Trade name | Actives |
|---|---|
| Depiwhite (ACM) | Kojic acid |
| Revitalift Laser x 3 lotion (L’Oréal) | Glycolic acid |
| White objective (Bioderma) | Niacinamide, Glycryrrhiza glabra root extract |
| D-Pigment (Avène) | Retinaldehyde |
| Effaclar AI (La Roche Posay) | Niacinamide |
| Aqua lotion (Amarte) | Arbutin |
| Whitelan (Dermica) | Morus alba root extract, Kojic acid, Glycyrrhiza glabra root extract |
| Hydra system (Institut Estherderm) | Salicylic acid, Morus alba leaf extract, Niacinamide |
| Depiderm (Uriage) | Ascorbic acid |
| Vinoperfect (Caudalie) | Tocopheryl acetate |
| Prescription anti-taches intensif (Liérac) | Glycolic acid |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Couteau, C.; Coiffard, L. Overview of Skin Whitening Agents: Drugs and Cosmetic Products. Cosmetics 2016, 3, 27. https://doi.org/10.3390/cosmetics3030027
Couteau C, Coiffard L. Overview of Skin Whitening Agents: Drugs and Cosmetic Products. Cosmetics. 2016; 3(3):27. https://doi.org/10.3390/cosmetics3030027
Chicago/Turabian StyleCouteau, Céline, and Laurence Coiffard. 2016. "Overview of Skin Whitening Agents: Drugs and Cosmetic Products" Cosmetics 3, no. 3: 27. https://doi.org/10.3390/cosmetics3030027