1. Introduction
Scalp hair is a defining element of our physical appearance with significant psychological and social impacts in our daily life. Everyone within any society has an abstract, unique and innate idea of beauty. As hair is one of the physical features which is easier to modify in terms of length, color or shape, the pursuit of the desired and idealized hairstyle to achieve beauty drives many consumers and feeds a vast global cosmetic industry.
Each individual is unique regarding hair growth rate, size and shape, but there are general properties of the hair fiber that can be grouped according to the ethnic background. The cosmetic industry considers three primary geo-racial hair types—African, Asian and Caucasian—with distinct hair fiber shape characteristics (diameter, ellipticity, and curvature) that control much of the cosmetic and physical behavior of human hair.
The physicochemical properties and shape of the hair is the direct result of the organization of its various structural elements, proteins being the most significant. Hair shape is defined in the hair follicle: large hair follicles produce “terminal” hairs (scalp), small follicles produce fine “vellus” hairs (body hair), curved follicles produce curly hair in all ethnicities [
1,
2]. Particular hair fiber shapes can be associated with polymorphisms/mutations in certain genes; furthermore, some proteins were shown to be expressed asymmetrically in a curly hair follicle bulb [
2,
3,
4,
5,
6,
7,
8,
9,
10,
11].
While hair styling is an ancient practice, permanent wave or hair straightening treatments only appeared as a commercially available and reliable service for the intentional control of hair shape in the 19th and early 20th centuries, respectively. The available methods for hair straightening/waving rely on the rearrangement of intermolecular bonds, based on cosmetic emulsions of high pH and reducing power. These hair procedures can have very negative consequences for hair, scalp and even consumer’s health. When hair is systematically exposed to permanent chemical treatments, it becomes, sooner or later, damaged. This damage can affect only the hair fiber surface attributes like smoothness, porosity and shine or it can affect the fiber core texture (thickness), and mechanical properties. Because the hair fiber is a non-living structure, its damage caused by cosmetic or environmental factors is irreversible. It is critical to have appropriate hair care procedures to improve function and prevent further damage, as hair fibers cannot be restored to their original structure. If the hair follicles are not affected, the subject has to wait for hair fibers to grow, which can take a long time, depending on their size and growth rate.
The consumer awareness of these problems is the driving force and the source of many potentially major changes occurring in the market, which creates a new niche for alternatives to traditional hair procedures. The future of cosmetic science will be the development of more powerful hair care treatments for damaged hair and of new cosmetics that allow the safe and specific control of hair morphology.
2. General Aspects of Human Hair Biology
Hair is an integrated complex system of several morphological components that act as a unit. The part of the hair seen above the skin is termed the hair fiber and, inside the skin, the hair follicle is the live part of hair from which the hair grows and where the hair fiber is generated [
12,
13].
2.1. Hair Morphogenesis
Hair follicles initially form in the skin of a human embryo as invaginations of the epidermis into the dermis, between the 8th and 12th week of gestation [
14,
15]. Each mammal is born with a fixed number of follicles that typically does not increase further, with exception when wound healing occurs, though this finding was only demonstrated in mouse skin [
16]. The key prerequisite for hair follicle development is the interaction between the epidermis and the underlying mesenchyme [
17], which remains in intimate contact throughout the life of the follicular unit. Reciprocal interactions occur between the epidermal keratinocytes, committed to hair follicle and that engage in specific differentiation, and the mesenchymal cells, that form follicular papilla. These interactions are governed by the series of inductive events or “messages” [
18,
19,
20]. Once the distribution of the follicles has been established, subsequent molecular events in the developing follicle determine the future phenotype of each hair [
21].
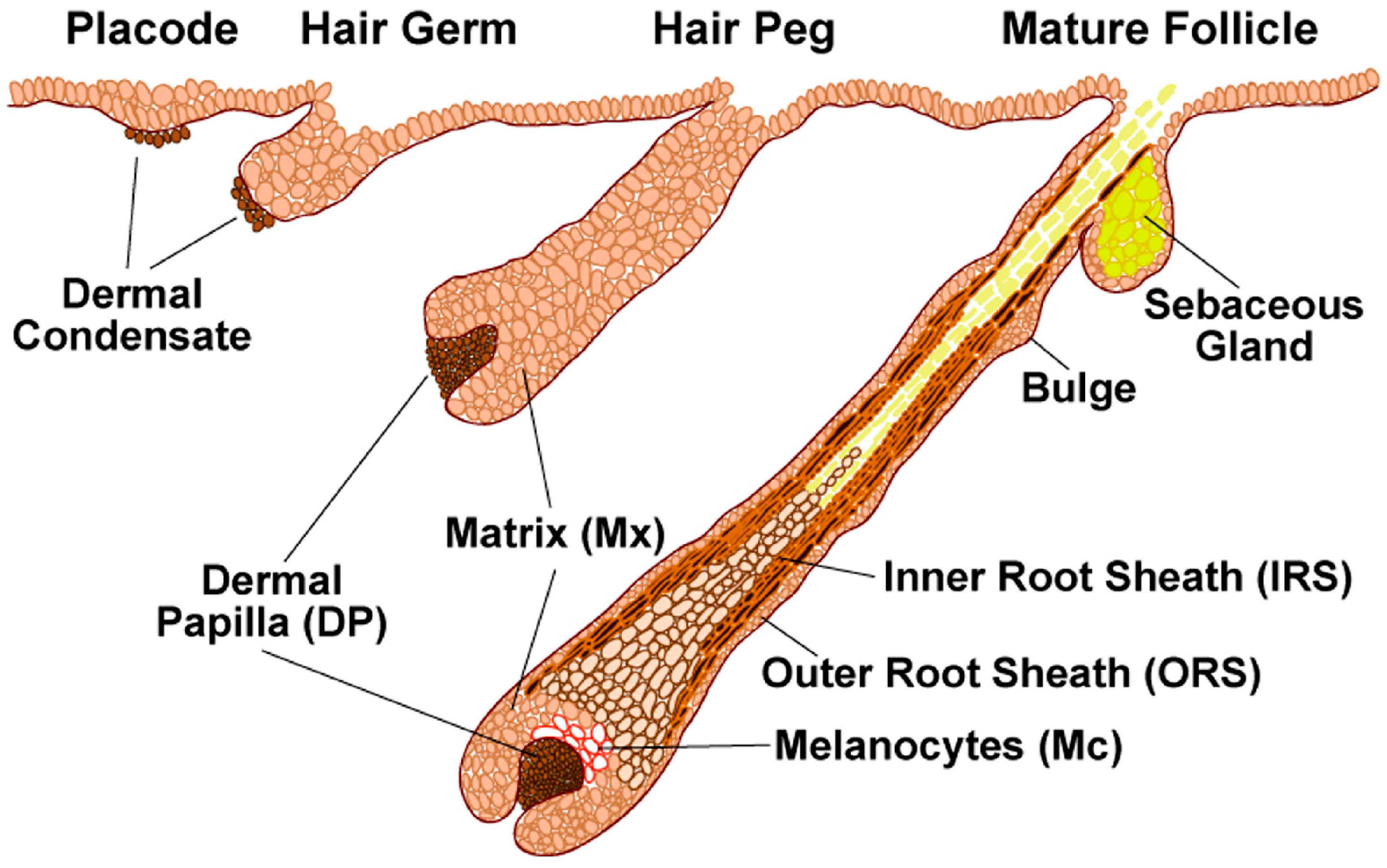
The development and differentiation of hair follicles during embryogenesis is classically divided into three main stages: induction, organogenesis (or progression) and cytodifferentiation (or maturation), which are morphologically characterized as germ, peg and bulbous follicles (
Figure 1) [
20,
22]. During the initial events of hair follicle induction, Wnt mediated signal transduction arises first in mesenchymal cells directing the thickening of overlying epithelial cells to form a placode. This is followed by hair follicle organogenesis and cytodifferentiation, each phase being characterized by specific molecular interactions [
20]. The organogenesis comprises a complex interplay of signals. Epithelial cells direct the underlying dermal cells to proliferate and form a dermal condensate, which in turn signals the epithelial cells to proliferate and grow downwards into the dermis. During cytodifferentiation, the dermal condensate is enveloped with follicular epithelial cells creating a distinct dermal papilla, which instructs the ectoderm to shape the entire hair follicle through the action of morphogens and growth factors [
23,
24].
2.2. Hair Life Cycle
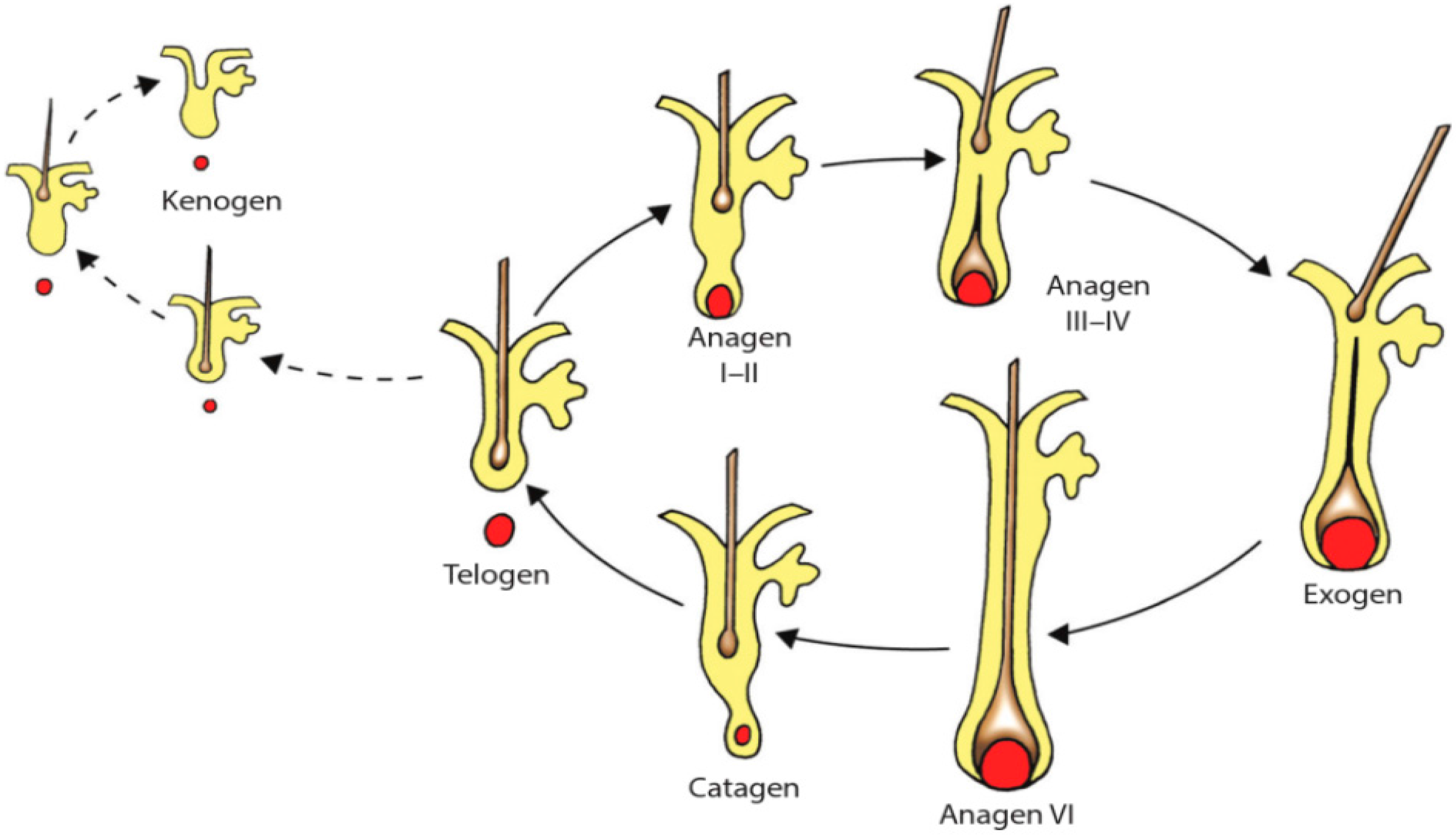
During postnatal life, hair follicles exhibit periodic changes in their activity: periods of steady growth, approximately 1 cm per month and continuously for 3–5 years (anagen phase) (
Figure 2). Growth then stops and is followed by a brief transient stage (catagen) and a 2–4 months resting stage (telogen) during which old hair is shed. Moreover, some authors defend the existence of an additional phase, during which the hair fiber is actively shed, suggesting that the shedding of the hair shaft is an active process (exogen phase). The subsequent interval of the hair cycle, in which the hair follicle remains empty after the telogen hair has been extruded and before a new anagen hair emerges, has been named kenogen [
26,
27,
28]. These cyclic changes comprise rapid remodeling of both epithelial and dermal components through the activation of differentiation of stem cells [
29,
30,
31,
32].
All body hairs undergo a similar life cycle, although its extent, duration of its phases and the length of individual fibers vary between different body areas and between persons, depending on genetic programming, gender, age and health status [
34]. Furthermore, the extent of the cycle phases determines the length of the hair and its replacement rate [
1]. The hair length is defined by the duration of anagen. At any time, around 85% to 90% of all scalp hairs are at the anagen stage [
18]. In man, the cycle regulates the characteristics of hair across diverse body sites and also helps to explain what occurs during hair loss and hirsutism [
35].
The hair cyclic transformations are controlled by finely tuned changes in the local signaling milieu. This signaling is based on altered expression of several growth factors, cytokines, hormones, neurotransmitters and their receptors as well as transcription factors and enzymes, which act through endocrine, paracrine or autocrine paths. The hair follicle cycling as such is an autonomous phenomenon that is capable of continuing even in isolated hair follicles in organ culture [
20,
36]. In fact, hair cycling parallels morphogenesis even in multiple signaling events incorporating developmental pathways during the different hair cycle stages [
37].
2.3. Hair Follicle Anatomy
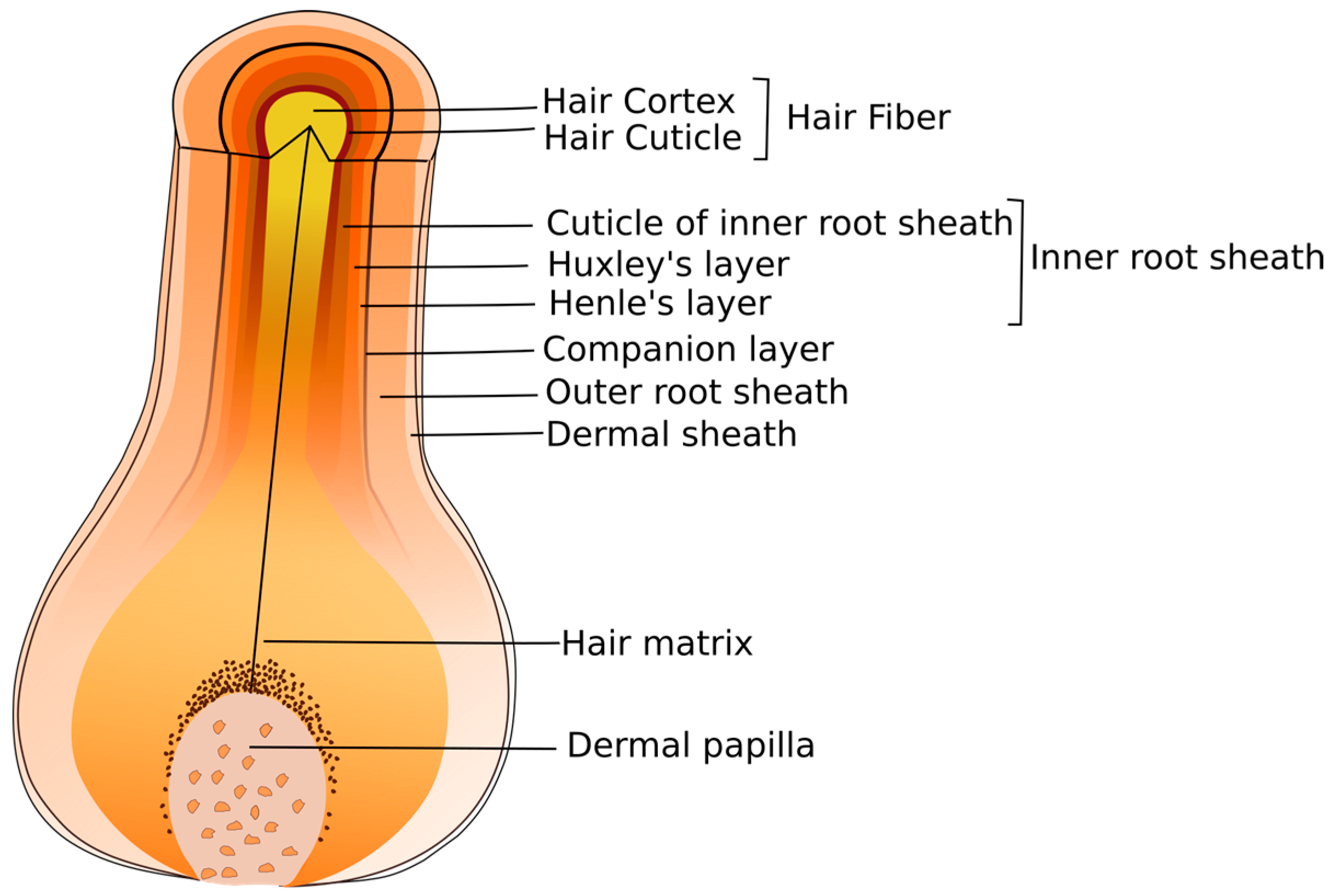
The hair follicle is a complex epithelial structure and is enclosed by an outer root sheath (ORS), which helps to support hair growth, and an inner root sheath (IRS), and follows the hair fiber up to the opening of the sebaceous gland [
38] (
Figure 3). The ORS and IRS are separated by the companion layer. The IRS can be subdivided into three distinct cell layers: Henle’s layer, Huxley’s layer and the cuticle of IRS. Besides these two layers, ORS and IRS, the hair follicles are composed of four other different epidermal layers: hair matrix, medulla, cortex and cuticle, as well as two dermal tissues: dermal papilla and dermal sheath [
39,
40]. Among these layers, only the medulla is not always present, given that some hairs have no medulla and others have a medulla relatively large. Each layer itself can comprise numerous individualized cell layers characterized by specific programs of differentiation [
32].
Within the skin, the terminal region of the hair follicle is called hair bulb, which is the structure formed by actively growing cells that produce the long, fine and cylindrically shaped hair fibers. The keratinocytes of the hair bulb have the highest proliferation rate among cells in the human body. The hair bulb comprises the hair matrix that will differentiate into the different precursors of the hair fiber, dermal papilla and surrounding dermal sheath. Additionally, the hair bulb also contains very specialized cells, the melanocytes, which produce the pigment melanin that gives color to the hair fiber [
30,
31,
41].
In combination with its associated structures (sebaceous and apocrine gland,
arrector pili muscle), the hair follicle forms the pilosebaceous unit. The hair follicle primarily acts as a factory for pigmented, multifunctional and exceptionally durable proteinaceous fibers—hair [
22].
2.4. Hair Fiber Structure
The hair fiber, about 50–100 µm in diameter, has both protective and cosmetic functions [
42]. Hair protects the scalp from sunburn and mechanical abrasion, provides thermoregulation and social communication [
42]. The human hair scalp, eyebrows, and lashes are long, thick and pigmented terminal hair fibers. However, the body is covered with hairs of 2–4 cm in length, under 40 µm in diameter, and often unpigmented, named vellus hairs [
42,
43,
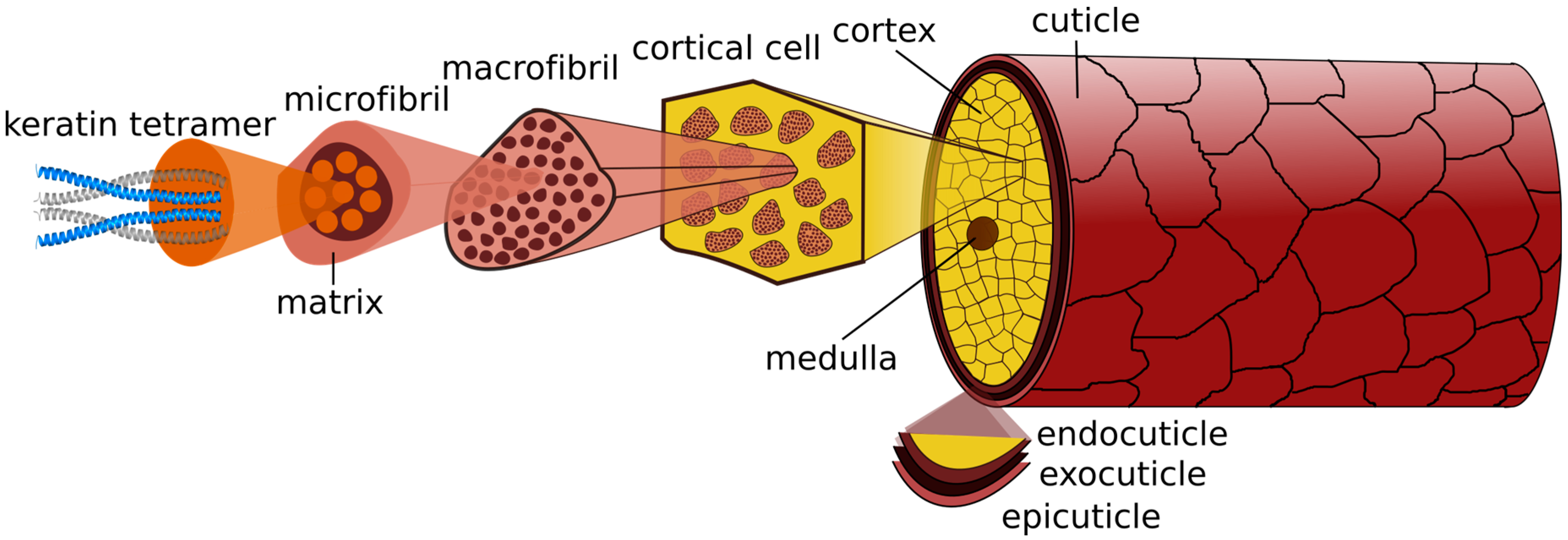
44]. Human hair fibers are divided into three main morphological constituents, also components of the hair follicle: cuticle, cortex and, in some cases, medulla (
Figure 4).
2.4.1. Cuticle
The hair fiber is enclosed in the cuticle, a barrier protecting the underlying cortex from external environmental damage. It contains 6–10 layers of overlapping scales, in a way that only approximately one-sixth of each surface is exposed. The cuticle’s proximal end is firmly attached to the cortex and the distal open end of the overlapping tiles points towards the tip of the fiber [
45,
46]. Adjacent hairs grow and move outwards in relation to each other, facilitating the elevation of dirt and scales and assisting easy removal [
45]. The shape and orientation of the cuticle cells are responsible for limiting friction between hair fibers.
The outermost layer of cuticle cells is the epicuticle, a lipid layer that includes 18-methyl eicosanoic acid (18-MEA) and free lipids, providing lubricity to the hair and consequently constituting the first line of defense against environmental assaults. Immediately below is the A-layer, with approximately 30% cystine content, highly cross-linked, which confers structural strength and rigidity to the cuticle. The following layers gradually have less cystine content and consequently less rigidity. The B-layer, or the exocuticle, is immediately below with approximately 15% cysteine content. The last layer corresponds to the endocuticle, which is mainly composed by remaining cell organelles, and consists of proteins with low cystine content (3% cysteine content). Hence, this layer swells more in water than the layers richer in cystine, and it is mechanically softer. Finally, the cellular membrane complex (CMC) is the intercellular cement that holds the cuticle cells together, primarily composed of non-keratinous protein with low cystine content (2%) [
46,
47]. The CMC comprises the δ-layer enclosed on both sides by 2-lipid endowed β-layers [
45].
2.4.2. Cortex
The cuticle encircles the cortex, the major part of the hair mass. The cortex is composed of cortical cells and the CMC [
47]. The elongated cortical cells enclose melanosomes containing eumelanin (brown/black pigment) and/or pheomelanin (red pigment), responsible for the hair color. These cells are tightly packed and contain macrofibrils which are parallel and longitudinal oriented to the hair fiber axis [
47]. Each macrofibril is arranged in a spiral formation and comprises intermediate filaments proteins (IFPs), also called microfibrils, and keratin associated proteins (KAPs), also known as matrix proteins. The matrix is formed by crystalline proteins of high cystine content (approximately 21%). The intermediate filaments, low in cysteine (~6%), contain subfilamentous units, protofilaments, incorporating short sections of α-helical polypeptide chains in coiled coil formation. The cortex is responsible for the great hair tensile strength.
Three types of cortical cells have been observed in the hair fiber with different ratio of intermediate filaments and matrix arrangements: orthocortical, paracortical and mesocortical cells. Orthocortical cells contain less matrix among the intermediate filaments composed of keratin and a low cystine content (~3%); paracortical cells have higher matrix content and more regular intermediate filaments, have smooth and rounded edges, are smaller in diameter and have a higher cystine content high (~5%); and mesocortical cells contain an intermediate level of cysteine [
9,
42,
48,
49]. The bilateral asymmetric structure of these fibers is one possible factor contributing to the shape of the hair. However, recent studies describe the orientation of the keratins in human hair and divide them into different cell types. They propose a different nomenclature not based on wool-cell types ortho, meso and paracortical, since human hair macrofibril-cell type relationships are less clear. In these studies, the classifications of cortical cells are type A (small discrete high-intensity double-twist macrofibrils), type B (close-packed macrofibrils with a mixture of intensities) and type C (large distorted fused macrofibrils) [
48,
49].
2.4.3. Medulla
Fine hair fibers are composed only by cuticle and cortex. With an increase in the hair fiber diameter, a third region, the medulla, may be found in the core of the hair fiber. Cells from medulla are spherical hollow vacuoles, which are loosely packed along the fiber, being bound together by a CMC-type framework. These cells only constitute a small percentage of the mass of keratin fibers. Medulla may be continuous, discontinuous or even entirely absent in the hair fiber [
42,
50]. Medulla is believed to contribute negligibly to the mechanical properties of hair fibers [
47].
3. Hair Fiber Chemical Composition
Human hair fibers are composed of different morphologic components and several different chemical species, acting together [
45,
50]. The main component is protein, corresponding to 65%–95% of the hair weight. Other constituents are water, lipids, pigment, and trace elements [
50].
3.1. Hair Proteins
The main components of human hair are keratin proteins. Keratins are complex natural composites with a heterogeneous morphological structure that belong to the family of fibrous structural proteins. They are the building block of fibers such as hair and wool and are part of the structural material of the human skin and nails [
51].
During hair formation, keratin existing inside the cells becomes more crystalline as the cells differentiate, giving rise to the hair fiber. These keratinized cells comprise an extremely organized material intended to provide significant resistance to countless environmental constraints and attacks, such as friction, tension, flexion, chemical assault and UV radiation [
52].
As keratin is the main content of hair fiber and its isoelectric point is acidic, under most pH conditions the surface of hair carries a negative charge.
Keratins have a molecular weight ranging from 40 to 70 kDa. Alpha-keratins are found in tissues such as hair, nails, claws of mammals, including humans and are mainly in α-helix conformation. Beta-keratins are found in reptiles and birds in tissues such as claws, shells, feathers and beaks, and are mainly in β-sheets conformations. However, both secondary protein conformations can be found in both α- and β-keratins. Alpha-keratins can be divided in type I and type II. Type I keratins have, in general, smaller size (44 to 46 kDa molecular weight) with acidic isoelectric points (pI) (pI range: 4.5–5.5) as compared to type II keratins, which are larger (50 to 60 kDa) and neutral to slightly basic (pI range: 6.5–7.5) [
42,
53]. Conway and Parry [
54] proposed to further divide keratins into “a” (type Ia and type IIa) for “hard” keratins, such as in hair and in nails, and “b” (type Ib and IIb) for epidermal and other “soft” keratins, such as in epidermis. The intermediate filament proteins in keratin fibers are formed by type Ia and type IIa “hard” keratin chains, arranged parallel to one another and in the axial register, to form a dimer [
42].
The amino acid composition of human hair keratins is typically different from the remaining keratins. The most significant difference corresponds to the cysteine residue content (7.6% in human hair keratin and 2.9% for
stratum corneum keratin) and glycine content (5.6% in human hair keratin and 11.6% for
stratum corneum keratin) [
53]. The higher amount of cystines in human hair keratins translates into a higher amount of disulfide bonds, producing a tougher and more durable structure with good mechanical, thermal and chemical properties. Notwithstanding, all keratin types have a high content of aspartic and glutamic acid residues, accounting for the relatively acidic character of these proteins [
53]. Several factors can induce changes in amino acid hair content, such as gender, genetic variation, weathering, diet, cosmetic treatment, as well as the extraction and analytical methods used. For example, in general, male hair contains more cystine than female hair and the tip of scalp hair contains, owing to weathering, significantly less cystine and cysteine than the root end; the converse applies for cysteic acid [
50].
KAPs have been less characterized than keratins, as they do not include well-defined spatial organization in the hair. They include high sulfur proteins, that contain in average 20% cystine residues and having a very high molecular weight (50–75 kDa); ultra-high sulfur proteins, with a higher content of cystine (30%–40%) and a lower molecular weight (15–50 kDa); high glycine tyrosine proteins, containing large amounts of these two amino acids and a low molecular weight (10 kDa) [
52].
3.2. Water Content
Water is an essential factor for the stabilization of proteins structure [
52]. Therefore, water content of hair is an important parameter regarding its physical and cosmetic properties. The water moisture content of keratin fibers depends on the conditions of dryness of the fiber as well as on the air relative humidity (RH) [
42]. Hair is hygroscopic, capable of absorbing large amounts of water [
52]. Hair readily absorbs water, as 75% of the maximum amount of water is absorbed within four minutes [
50]. Hair impregnated with water has an increase in weight by 12%–18% [
50]. Hair swelling is anisotropic, since hair fiber length increases approximately 2%, while hair fiber diameter increases more than 15%, from 0% to 100% RH [
52]. This occurs because water is assumed to adsorb to the hydrophilic matrix of the cortical cells, in the boundary with the microfibrils. Hence, water is able to slightly distort the structure of microfibrils: they oppose the longitudinal swelling of the matrix and so hair fiber volume is increased by diametric swelling [
52]. There are numerous water binding sites in keratin including peptide bonds and acidic and basic side chains. Although water permeates the hair readily, there is some binding selectivity within the molecular structure of the hair cortex [
50,
52]. Water absorption is also related to the quantity of lipids in hair, as well as to the pH level [
52].
3.3. Hair Lipids
Hair lipids are distributed across the hair fiber, its average content is of the order of 4% of the fiber weight. They can be external or internal lipids, and the latter can be free or part of the structure of the CMC [
42]. Internal lipids are thought to be located in the intercellular spaces, as a part of the β-layers [
52]. The majority of lipids are cholesterol, cholesterol esters, cholesterol sulfate, free fatty acids, triglycerides, paraffins, squalene and ceramides [
52]. A major component of exogenous lipids is 18-MEA, covalently attached to the cuticle surface. This lipid works as a lubricant decreasing friction between hair fibers and its absence influences the sensory perception of hair such as dry hair or difficulty in combing [
55]. Lipid content may vary depending on several factors, such as ethnicity, gender and age [
50,
56,
57].
3.4. Trace Elements
Besides the elements already referred, hair also contains a variable amount of inorganic elements, usually lower than 1% of the content [
52]. The most frequent are alkaline elements (K and Na), alkaline earth metals (Mg, Ca, and Sr), other metals (Ca, Zn, Fe, Mn; Hg, Cd, Pb, As, and Se) and metalloids (Si and P) [
52].
The detection of trace elements can be used for diagnostic medicine, as the accumulation of several elements may be a symptom of systemic disease and correlate to the amount of those trace elements in internal organs [
42]. Also, hair analysis can be used for detection of drugs, such as cocaine, opium, amphetamines, and environmental toxics as collection is noninvasive and relatively easy to perform. Hair may provide a long-term information of drug intake and toxin exposure, extended from months or even years if the scalp hair is long [
58]. However, the limits of such methods are still controversial [
59,
60] and may be affected by hair care practices. Hair can also be used to sensor air pollution, as pollutants can be absorbed by hair fibers. Likewise, hair cosmetics may provide additional trace elements to the hair fiber [
50].
The incorporation of drugs into hair can be due to three different processes, including exogenous or endogenous ones. When the incorporation occurs from internal origins, the compound can pass through passive diffusion from the blood into the hair matrix, being incorporated in the hair fiber during keratinization, where they are bound to proteins, lipids or pigments. Another process, external, corresponds to the transfer from the sebum and sweat. The drug or toxics can also be taken up by the hair in contact with the environment, such as atmospheric dust, water ions, and elements from cosmetics [
52].
3.5. Chemical Interactions within Hair Fibers
The stability of macromolecular structure of keratins derives from a variety of interactions between and within the protein chains, holding them together. These interactions range from covalent bonds, such as disulfide bonds and isopeptide crosslinks, to weaker interactions such as hydrogen bonds, Coulombic interactions, van der Waals forces and hydrophobic interactions. These interactions depend on the presence of reactive groups in the fiber, but also on their availability due to the fiber morphology and molecular structure [
42,
45].
Hydrogen bonds, although weak and easily broken by water, are the most frequent bonds in the hair and the interchain hydrogen bonding along the polypeptide chain is essential for the α-keratin structure stability. Coulombic interactions, due to the high content of acidic and basic side chains, are relatively stable in an aqueous environment but are easily broken by acids or alkalis. Hydrophobic interactions occur between non-polar groups along the keratin [
45].
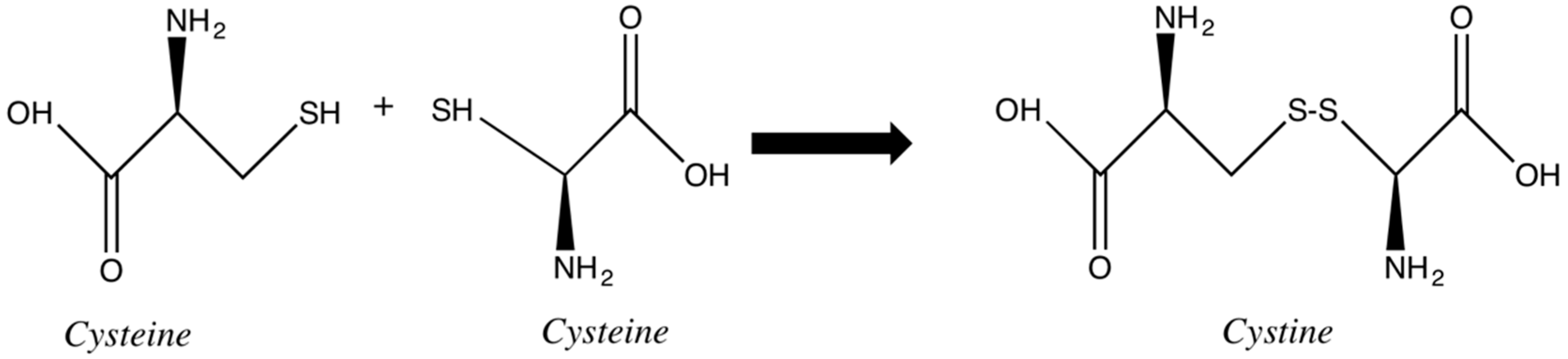
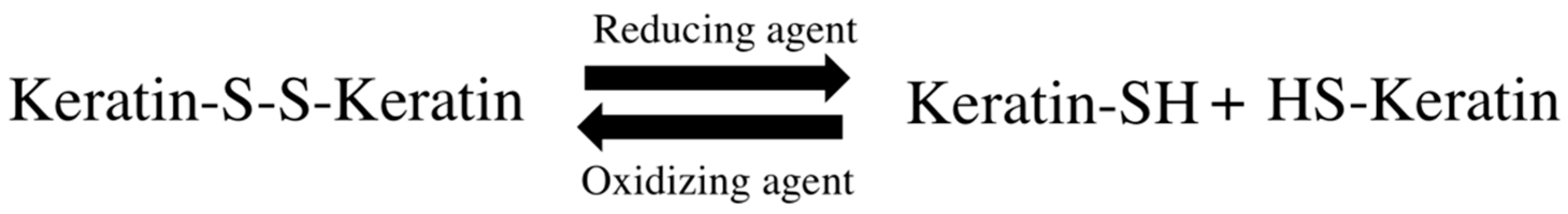
Disulfide bonds are key to the stability of the keratin. Two adjacent cysteines are linked together, generating cystine, forming a bridge between two chains or two portions of the same chain (
Figure 5). The susceptibility of these bonds to reduction and oxidation are the basis of most chemical modifications of hair and consequent change in its physicochemical properties. Some of these changes are the goal of the cosmetic procedures, such as hair waving or straightening. Some other cosmetic processes, such as oxidative hair coloring, bleaching and hair weathering, involve changes in the disulfide bonds as an undesired side effect. In both cases, intentionally or not, during the procedures involving redox chemistry, the physicochemical properties of hair are affected [
42,
45].
4. Classification of Hair Fiber Shape and the Underlying Structural Differences
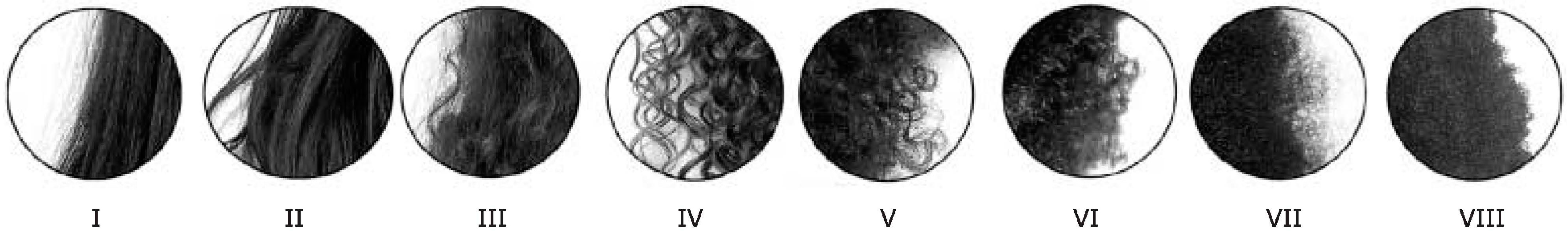
The classification of hair shape is not free of controversy. Hair can be classified generically into straight, waved, curly or kinky hair, regarding hair ethnicity into Asian, Caucasian and African hair. However, further studies analyzed hair among various world populations to classify the hair geometry based on more rigorous criteria [
24,
61]. De La Mettrie et al. [
61,
62] divided the human hair types into eight classes, based on the parameter hair curve diameter, curl index and number of waves (
Figure 6).
For the purpose of this review, the typical hair from different ethnicity will be further analyzed, considering it corresponds to very distinct partitions of the hair shape classification: Asian hair as indubitably straight hair, African hair as kinky hair and, the intermediate between those two, Caucasian hair as waved hair.
Some authors have proposed that the origin of human hair shape is based on morphological features [
2,
12,
18,
63]. In the hair follicle bulb, fiber shape has been thought to be determined by the hardening of the IRS layers inside the follicle [
64]. In line with this, it is logical to suggest that the shape of the follicle in the zone of keratinization determines the shape of the hair fiber and not the angle or emergence from the skin surface. It is a single characteristic enabled by communication among stem cell populations and is programmed within the hair bulb [
2]. Thus, if the follicle where the fiber is formed is curved in the area of keratinization, the emerging hair fiber will be highly wavy, but if the follicle is quite straight, the emerging hair will be straight [
56,
65].
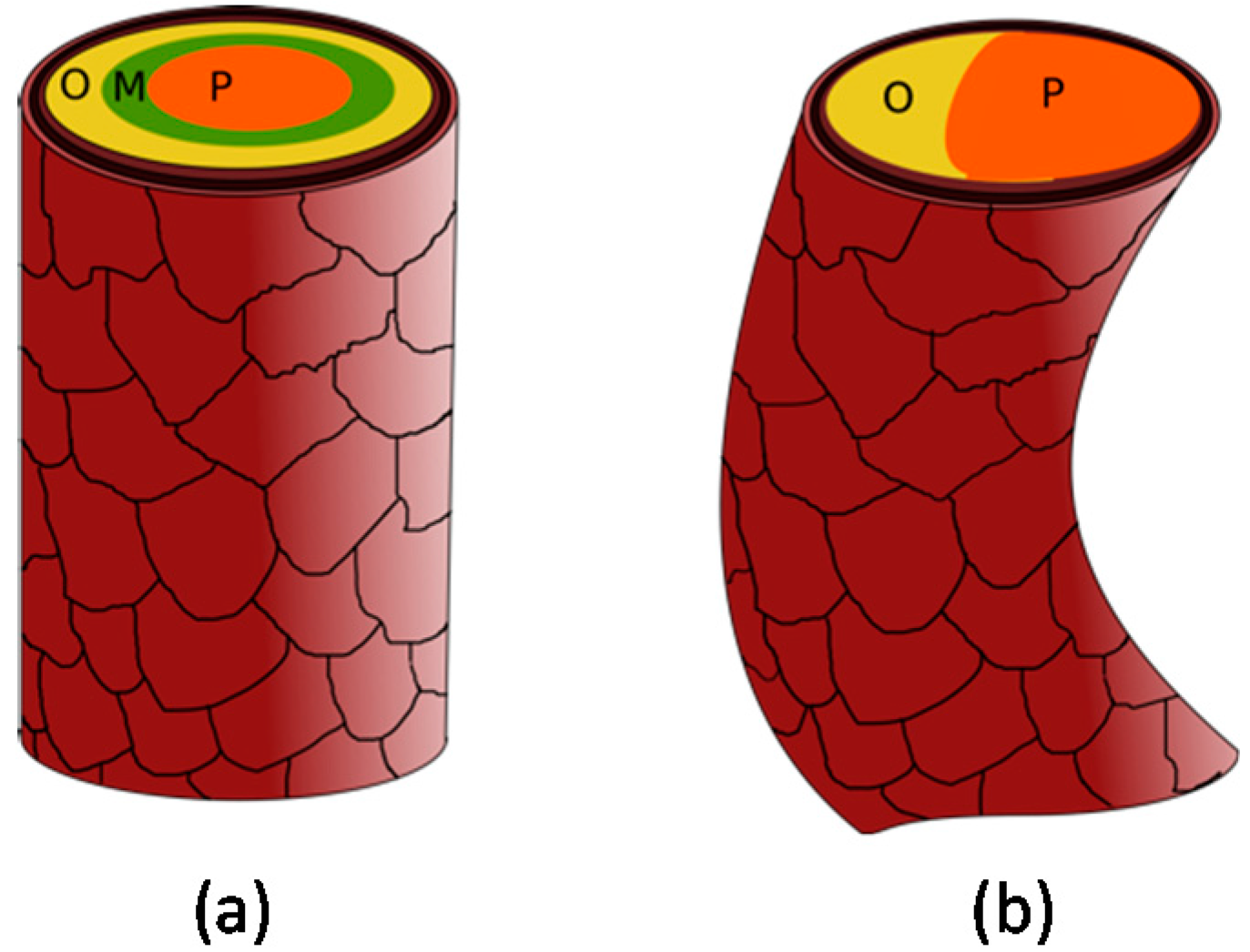
Possibly due to these different shapes of the hair follicle, there is a heterogeneous distribution of cortical cells in different hair fiber shapes (
Figure 7). Straight hair has a homogeneous and annular distribution of these cells, with the orthocortical cells delimitating the fiber externally, surrounding a putative part of mesocortical cells, which are in turn around the core of paracortical cells [
9,
42,
66,
67]. Curliness of Merino wool originates primarily from heterogeneity of the intermediate filaments, with paracortical cells on the concave side of the capillary curve, orthocortical cells on the convex side, in higher proportion, and absence of mesocortical cells [
9,
42,
67]. The smaller the interior angle of the hair fiber curvature, the more paracortical cells are restricted to the concave side of the curvature [
9]. The different halves of the hair fiber grow at different rates, generating the curvature driving force and, consequently, a curled fiber [
42]. Nevertheless, the exact processes underlying curly pattern of the hair are still undefined.
5. Differences on Ethnic Hair Fiber Characteristics and Properties
Each individual is unique regarding hair production rate, size and shape, but there are general properties of the hair fiber that differ in a typical way according to ethnic background [
68,
69].
Regarding hair geometry, Asian hair is almost invariably straight while African hair is invariably curly [
70]. The geometrical form of Caucasian hair varies from straight to wavy, with approximately 45% being straight hair, 40% wavy hair, and 15% curly hair [
4,
71]. The curly nature of African hair is attributed to the curved shape of the follicle.
Asian hair fiber has the greatest diameter with circular geometry [
71,
72]. African hair has elliptical cross section diameters changing along the hair fiber length; it is a flattening twisted oval rod-shaped fiber with random reversals in direction [
71]. Caucasian hair has an intermediate diameter and section shape. The hair cuticle is usually 6–10 scales thick in Asian hair, slightly less in Caucasian and even less in African hair [
42]. The density of African hair follicles is also, on average, slightly lower (90,000) than in Caucasians typical hair type (120,000) [
73,
74,
75].
The different ethnic hair types have considerably different mechanical properties associated. Asian hair has better mechanical properties than any of the other ethnic hair groups. African hair has the lowest tensile strength and is more brittle than Caucasian hair [
46]. At a similar relative humidity, African hair exhibits lower water uptake than Caucasian and Asian hair fibers; these last two types similarly absorb water. This is reflected in hair diameter swelling [
52,
76].
In spite of all the differences in hair physical properties, when analyzing their protein composition it is remarkable how uniform the amino acid makeup of protein components is across ethnic hair groups [
45,
66,
76,
77,
78].
The hair lipid content across different ethnic groups is still a subject under study. People with curly and wavy hairs are more likely to have oily scalp but less oil on the hair fiber surface. Sebaceous glands of African descendants are frequently less active when compared with the Caucasian ones [
79]. A study [
57] revealed that, in the composition of African hair fiber, the lipid content in terms of cholesterol-ester, free-fatty-acids and cholesterol-sulfate is higher than in the other two ethnic hair types. Furthermore, in the same study, authors proposed that the existence of a higher concentration of lipids in African hair fiber composition may interfere with the typical arrangement of hair keratins structure, directly interfering with hair physical properties and shape.
Factors such as genetic variation, weathering, diet or cosmetic treatments affect the constitution of the hair and underlie the variations in hair characteristics across hair ethnic groups, such as diameter, ellipticity and curliness.
6. Hair Cosmetic Treatments: Hair Cleansing and Hair Shape Modulation
Hair is one of the physical features easier to modify. Haircare industry has developed plenty products to provide beauty and modify some hair characteristics. In this review, we focus on hair cleansing products and cosmetic treatments that impact on hair shape (straightening and waving permanent procedures) and their influence on hair fiber health. Hair cleansing products were included in this review because, firstly, they are extensively used and they affect mainly the features of hair fiber surface (smoothness, shine, combability and hydrophobicity) and, secondly, they are also used in the finishing of hair shape chemical modifications to recover hair properties (pI, hydrophobicity besides the fiber surface features).
6.1. Hair Cleansing
6.1.1. Shampoo
The arrangement of the hair cuticles allows self-cleaning properties of the hair fibers, repelling by itself some dirt and greasy residues. However, with time accumulation of grease and dirt occurs and the hair needs to be cleaned. Shampoos’ primary goal is to clean the hair and scalp of these residues. Nowadays, it is expected that shampoos have secondary benefits such as to prevent hair fiber damage, keep the hair aesthetically presentable, preserve its softness, combability and shine [
42]. These secondary functions are usually the reason to purchase a particular shampoo. Therefore, the most significant interactions for shampoos are the ones happening near the fiber surface and first few cuticle layers. Nonetheless, if the hair surface is damaged and the cortex exposed, shampoos interact also with the exposed cortex [
42].
A shampoo usually has an average of 80% water content and pH from 5 to 7 [
80]. They are typically composed of 10 to 30 ingredients; that can be grouped into cleansing agents, conditioning agents, special care ingredients, additives, preservatives and aesthetic agents (
Table 1) [
47,
80,
81,
82].
Surfactants, as cleaning agents, are among the most important ingredients on shampoos, providing foaming and detergent properties. Typically, dirt is excess of sebum, produced by scalp and dirt adsorbed to this sebum. Surfactants weaken the physicochemical adhesive force that binds lipid residues to the hair, transfer it into the aqueous rinse, and disperse them, avoiding redeposition on the hair fiber. Surfactants have, generally, a tail of fatty hydrocarbons and a polar head. In contact with water, they attain the structure formation of a micelle, with hydrophilic exterior and hydrophobic interior, where the residues are trapped and kept in a dispersed form within the aqueous rinse [
42,
50,
81].
The surfactants can be classified into four groups according to the electric charge of the polar extremity: anionic, cationic, amphoteric and nonionic [
79,
80]. Modern shampoos contain a mixture of surfactants to provide different cleaning levels according to hair type [
80]. Usually, a primary surfactant for cleaning and foaming and a secondary surfactant for foam and/or viscosity enhancement [
42].
The anionics are the primary cleansing agents, while the cationics, amphoterics and nonionics are additives that give certain product attributes (see below). The main anionic detergent used is soap, which corresponds to a salt of a fatty acid obtained from alkali treatment of vegetable or animal fats. Soap tends to hydrolyze in water, releasing an alkali residue, harmful to the skin and hair. This residue precipitates as calcium salts and attaches to the hair fiber, leading to a tangled and opaque appearance [
81]. Therefore, anionic detergents are excellent cleansers but may leave the hair feeling dry and coarse, as well as cause an increase in negative electrical charges on the hair surface and consequently increase frizz and friction. Oily-hair shampoos generally employ anionic detergents for an improved removal of sebum [
83]. Other anionic surfactants, derived from sulfonating fatty alcohols and polyoxyethylene analogs, impart less damage to the hair and skin, but have less efficient cleansing and foaming properties [
79,
81,
83].
Cationic surfactants have a high affinity for hair fiber. They provide softness to the hair and make combing the hair easier. Yet, they are poor cleansing surfactants. Therefore, they are usually combined with nonionic surfactants in specific shampoos specially designed for damaged hair; they also reduce the static electricity effects cause by anionic surfactants, decreasing the frizz effect [
79,
81].
Amphoterics surfactants, such as betaines and amphoacetates, reduce the anionics tendency to adsorb onto proteins. They are mild cleansers, leave the hair manageable but do not irritate the eyes. Hence, they are usually combined with other surfactants of mild shampoos to modulate cleansing efficiency. Baby shampoos employ amphoteric surfactants to minimize irritation of the eyes [
81,
83].
Nonionics, such as ethoxylated fatty alcohols, tweens and alkyl polyglucosides, are the mildest surfactants. They are not so effective at removing dirt and sebum but leave the hair more manageable. They have good dispersing, emulsifying and detergent properties but poor foaming capacity. Consequently, they are usually auxiliary cleansing agents [
81,
83].
The primary functions of conditioning agents are to provide softness and gloss, resistance to static electricity, and to facilitate combing and manageability. They are particularly beneficial for the care of dry and damaged hair [
81,
83].
Special care ingredients are addressed to specific problems relating to the superficial condition of the scalp or hair; for example, they can reduce unaesthetic consequences of dandruff and excess greasiness, for example [
81].
Additives and preservatives agents, such as foam stabilizers, viscosity builders (gum, salt, and amide), chelating agents, pearlescents or opacifiers for visual effects, other colorants also for visual effects, fragrances, and UV absorbers, contribute to the stability and aesthetic sensibility of the shampoo [
42,
81].
6.1.2. Conditioners
Conditioners reduce friction, detangle the hair, minimize frizz and improve combability, restore hydrophobicity, enhance shine, smoothness and manageability [
42].
The mechanism by which conditioners work to provide hair manageability relies on decreasing static electricity and reducing friction among hair fibers. Static electricity is reduced through the deposition of positively charged ions/molecules on the hair fiber surface which possesses a natural negative dipole moment; besides negative charges are induced by combing, brushing and are more exposed on damaged hair. Friction is reduced by some components that flatten cuticles along the longitudinal axis of the fiber. Smooth cuticles reflect more light which improves hair shine and color and provides softness to the hair. Conditioners may also seal the gaps that expose the cortex to environmental damage. The substances that compose the conditioner may reach the cuticle surface or the inner part of the cortex, depending mainly on their molecular weight [
13,
42,
47]. Therefore, bleached and chemical treated hair have a higher affinity to conditioning ingredients due to their lower isoelectric point (higher concentration of negative sites) and higher porosity when compared to virgin hair [
42,
79].
Conditioners are usually emulsions of oil or wax in water, with a cationic charge. Conditioners usually contain polymers, oils or waxes, cationic agents, additives, preservatives and aesthetic agents (
Table 2). Some cationic molecules are also combined with bridging agents to enhance the adsorption of hydrophobic ingredients to the hair [
42]. Conditioners may include UV filters for color protection [
80]. The most frequently used hair conditioners are based primarily on cationic surfactants with additives like silicones or cationic polymers.
The most used conditioner agent is silicone [
84,
85]. Silicones may be of different types, having different deposition, adherence and wash out capacities. They spread over the hair surface and form a uniform, thin, hydrophobic layer that increases luster and gloss and reduce the combing force. Cationic polymers are highly substantive to hair due to the hair's low isoelectric point (pH ~3.67), therefore, negative charge. Cationic polymers differ from cationic surfactants as the cationic ends are part of a macromolecular structure and are not attached to a fatty hydrocarbon chain [
81]. Alkaline cosmetics intensify the net negative charge of hair surface which attracts the cationic charged molecules of conditioners [
42,
50]. Typically, there is no need for preservatives in the final composition of a conditioner since its pH is usually between 3 and 5, and that is a harsh environment for microorganisms [
80]. Besides ionic and electrostatic interactions, other forces like van der Waals forces and entropy are important to bind the conditioner molecules to the fiber [
42].
6.1.3. Health and Hair Hazards of Hair Cleansing Procedures
Shampoos and hair conditioners are usually perceived as products that do not damage hair. However, there is evidence indicating these products may contribute to hair damage through abrasive or erosive actions. They may result in the degradation of the non-keratinous components of the endocuticle and CMC [
42]. Regarding scalp, shampoos are not a frequent cause of irritant or allergic contact dermatitis due to their short contact time with the skin. Shampoo’s components, such as anionic surfactants can still contribute to xerosis and eczematous dermatoses due to the ability to remove sebum [
83]. Common allergens in shampoos are: cocamidopropyl betaine, formaldehyde-releasing preservatives, methylchloroisothiazolinone, propylene glycol, vitamin E (tocopherol), parabens and benzophenones [
79].
Even below the maximum amount legally allowed, shampoos may also contain preservatives, such as quaternium-15, imidiazolidinyl urea, and diazolidinyl urea, with antimicrobial effect that function through the release of trace amounts of formaldehyde, a potential sensitizer as referred previously and an inhalation carcinogenic [
86].
6.2. Chemical Hair Cosmetics Procedures that Modulate Hair Shape
Hair waving and straightening are procedures that change hair physical appearance, regarding shape, in a durable way. There is an essential difference between these procedures and hair setting. Temporary straightening or waving requires physicochemical techniques such as dryer, flat iron, hot comb and lasts only until the next wash. Hair has to be wetted to break the hydrogen bonds of keratin, allowing a temporary opening of its original structure. Fast hair drying and mechanical aid maintain the flat or waved strand of the hair. Consequently, the new shape is moisture sensitive [
12,
45].
Permanent hair straightening or waving requires changes in hair disulfide bonds. These techniques involve manipulation of the physicochemical interactions that stabilize the keratin structure, through keratin softening, molding to the intended shape and providing a new geometry. This occurs through breakage of disulfide bonds between keratin filaments and their posterior rearrangement into the desired shape, affecting the structural integrity and hair cystine content [
45] (
Figure 8).
6.2.1. Hair Straightening
It is common that people with characteristically tightly curled African hair seek methods to straighten the hair in a durable way. This procedure uses an alkaline agent, an oil phase and a water phase of a high-viscosity emulsion to relax and reforms disulfide bonds [
87].
There are two classes of nucleophilic agents that are applied to the hair to straighten it: reductive agents, such as mercaptans, sulfites, and agents containing hydroxide. Mercaptans and sulfites cleave the disulfide bonds selectively so that they can be recombined at the end of the process, instead of disrupting the entire protein [
45]. For example, in the procedure with the mercaptan ammonium thioglycolate, the disulfide bonds are converted to sulfhydryl groups to allow the mechanical relaxation of the keratins. After relaxation, free sulfhydryl groups are reoxidized to reestablish the disulfide bonds with the desired conformation. A hot iron may be used to induce enough additional stress to permanently straight the hair [
12,
88]. By using mercaptans or sulfites, 90% of the cysteine content is retained with 10% additional cystine transformed to cysteic acid. This process causes less protein loss than the use of alkaline agents [
79].
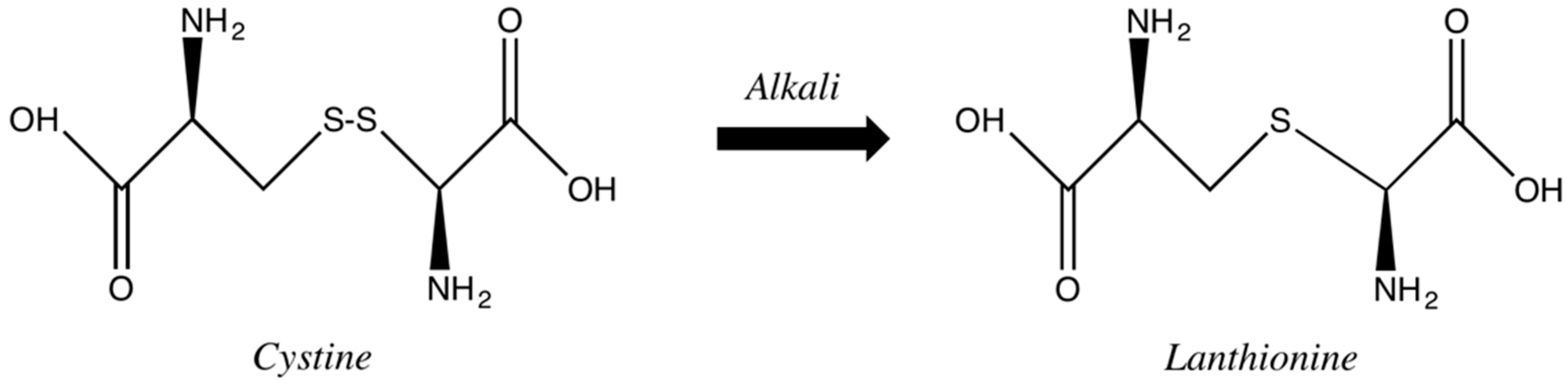
Alkaline agents containing hydroxides, usually higher than pH 9.0, cleave disulfide bonds less selectively with a permanent fission. The high pH induces hair swelling, cuticle scales opening and, consequently, induces a deep penetration of the alkaline agent up to the hair cortex. There, the hydroxyl ions disrupt the disulfide bonds of the hair keratins so that a rearrangement of disulfide bridges can occur. In some cases, in such drastic conditions, there is a conversion of the disulfide bonds into monosulfide cross-links. The late reaction, lanthionization, consists in substituting part of the amino acid cystine for lanthionine (
Figure 9). This process results in a more effective straightening, although with an impairment of the fiber integrity, as approximately one-third of the disulfide bonds are permanently converted into lanthionine bonds. This results in a decrease of elasticity and tensile strength along with cuticular damage [
13,
45]. Salt bridges and other ionic bonds are also easily broken at such high pH [
42,
80,
89]. With this procedure, due to supercontraction, there is no need of heat. This phenomenon provides enough stress to straighten the fiber permanently [
42]. At the molecular level, fiber supercontraction is the result of changes in the secondary structure, due to the transition of α to β phase [
90]. It is believed that the irreversible molecular conformational changes due to supercontraction leads to permanent hair straightening [
90].
When the alkali agents contain sodium or potassium hydroxide (1%–10% content) are designated lye relaxers (
Table 3). If they contain other substances, such as lithium hydroxide, guanidine, ammonium thioglycolate, or calcium hydroxide, they are designated no-lye relaxers. Each category may be further sub-divided. The no-lye relaxers can be divided in two sub-categories: mix relaxers (base containing calcium hydroxide and an activator of the relaxation reaction containing guanidine carbonate) and no-mix relaxers (lithium hydroxide). Within the lye relaxers, there are the sub-categories no-base relaxers (high-oil-content emulsions, and less damaging for hair scalp) and base relaxers (low oil content and relatively high percentage of lye) [
80].
Besides the nucleophilic agent to straighten the hair, these products also contain two more main components: an oil phase and a water phase in an emulsion form. The oil phase contains lipophilic components, such as oils and waxes, and also, surfactants to provide shine, hair combability and a barrier-type protection to the scalp. The water phase is the vehicle for the alkaline component. The stability of the formulation, the potential to irritate the scalp and the ability of straightening highly depends on the type and the proportion of the emulsifying agent used [
89].
Following the hair relaxer, the hair is mechanically straightened with a comb to realign the position of disulfide bonds between the keratins fibers. The new bonds are consolidated with an oxidizing agent (
Table 4) [
13]. Finally, a neutralizing shampoo should be used to bring the pH back to normal, usually acidic shampoos (pH 4.5–6.0) or a neutralizer to aid closing the cuticle scales. A conditioner is usually also applied [
13,
80].
The straightening procedure has to be repeated after 12 weeks, on average, due to hair growth. The treatment should be focused in this new part of the hair, as repeated treatments lead to hair breakage. Therefore, usually, hair breakage occurs at the junction between the previously treated hair and the new part of the hair fiber [
79].
6.2.2. Hair Waving
Similar to hair straightening, hair waving includes a reducing agent to break the disulfide bonds, and a posterior oxidizing agent to create new disulfide bonds in the positions mechanically set to fix the hair curl (
Table 4) [
92].
The chemical principle involved is similar in all perming solutions. The hair is initially washed and styled in hair curlers according to the degree of curl desired. Afterward, the permanent waving solution, usually ammonium salts of reducing agents such as thioglycolic acid or sulfite, is applied on the hair [
13]. The permanent waving solution also includes ammonium salts or ammonia to induce as alkaline agent to provide appropriate swelling to the hair, chelating agents as stabilizers, non-ionic surfactants to aid permeation and emsulsification and cationic surfactants and oils to raise utility [
92]. This solution passes through the hair cuticle into the hair cortex cleaving some of the disulfide bonds, in an equilibrium process. Once the bonds are broken, there is a molecular rearrangement and the new bonds are created according to the new shape of the hair set. The perm solution is then rinsed off, and the bonds are solidified by re-oxidation using a neutralizing solution, containing an oxidizing agent (
Table 4). The neutralizing solution besides the oxidizing agent includes pH buffer for the reaction occur under a constant pH and surfactants and oils to raise utility [
13].
6.2.3. Health and Hair Hazards of Hair Shape Modification
One side effect of hair straightening or waving is the damage to the hair fiber. The chemical procedures affect the structure of the hair fiber and weakens it, as the rearrangement of the disulfide bonds may incur in hair fiber structural distortion and damage, mainly in the distal part of the hair fiber [
50]. Both processes leave the hair with reduced tensile strength, and drier, as they induce the removal of the monomolecular layer of fatty acids covalently bound to the cuticle, including 18-MEA. Elimination of this lipid decreases the luster of the hair and makes it more hydrophilic, more susceptible to static electricity and frizzing induced by humidity, as that hydrophobic layer reduces water penetration in the hair fiber [
12,
50].
Nevertheless, the hair damage caused can be minimized or even avoided with a correct usage of the products and few repetitions of the procedure [
79]. Incorrect or over usage of hair straightening and waving products may lead to problems including hair thinning and weakening, discoloration, scalp irritation, itching, skin burn, scalp damage, lack of hair growth and hair loss, apart from the allergic reactions to chemicals [
93,
94,
95].
In treatments with lye-based straighteners, petrolatum needs to be applied to the scalp and hairline prior to the procedure to avoid skin burn. The strong alkaline pH is caustic to the skin, scalp, and eyes [
79,
96]. Therefore, chemical relaxers should not be applied directly to the scalp to avoid skin burn. Petrolatum should be applied to the skin along the hair line and to the ears before the application of the relaxers [
79].
Typically, alkaline straighteners, due to their high pH can make the hair susceptible to friction and reduce its resistance and strength, as they can crack the endocuticle and the CMC [
79].
Besides the above-mentioned chemical straighteners, there is another one that is, unfortunately, very popular and dangerous: formaldehyde (and, more recently, glutaraldehyde). They are cheaper and provide a quicker process. Formaldehyde is able to crosslink the keratin filament. Formaldehyde and glutaraldehyde may cause skin and mucosal irritation and severe damage to the tissues of the upper respiratory tract for the user and the hair care professional. They also have carcinogenic and teratogenic potential [
12,
97,
98].
7. Final Remarks
Hair is a very distinctive personal feature playing a major role in self-perception. It is one of the physical features that can be easily changed in terms of length, color or shape. However, common chemical styling processes are also known to induce changes in hair cuticle and cortex, damaging the fiber and in some cases the health of the person or the hair care professional.
The cosmetics industry has traditionally focused on the development of products or procedures for hair cleansing and to modulate the shape of hair fibers after their exit from the skin surface, as referred in this review. Due to the potential damage to the hair fiber caused by many traditional methods, mainly of hair shape modulation, there is increasing interest in understanding the genetic basis associated with hair shape, exploring whether hair appearance can be modified as the fiber is generated in the hair follicle [
13,
99,
100]. Studies on hair follicle biology and on the hair fiber, together with the development of improved formulation components, will be essential to develop new and safer hair cosmetics.
Acknowledgments
We thank the Portuguese Foundation for Science and Technology (FCT) for providing Célia F. Cruz the grant for PhD studies (scholarship SFRH/BD/100927/2014) and Teresa Matamá the grant for post-doctoral research (SFRH/BPD/102153/2014). This work was also supported by FCT under the scope of the strategic funding of UID/BIO/04469/2013 and UID/BIA/04050/2013 units, COMPETE 2020 (POCI-01-0145-FEDER-006684 and POCI-01-0145-FEDER-007569) and under the Project RECI/BBB-EBI/0179/2012 (FCOMP-01-0124-FEDER-027462).
Author Contributions
Célia F. Cruz, Cristiana Costa and Teresa Matamá wrote different parts of the review paper (the order of authors’ names reflects the weight of their contribution); Célia F. Cruz joined the different text parts into the final full text and made all the artwork for the review (except when stated otherwise). Andreia C. Gomes, Teresa Matamá and Artur Cavaco-Paulo supervised and made critical suggestions and revisions.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 18-MEA | 18-methyleicosanoic acid |
| CMC | cellular membrane complex |
| IRS | Inner root sheath |
| KAP | keratin associated protein |
| ORS | outer root sheath |
| pI | isoelectric point |
| RH | relative humidity |
References
- Westgate, G.E.; Botchkareva, N.V.; Tobin, D.J. The Biology of Hair Diversity. Int. J. Cosmet. Sci. 2013, 35, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Thibaut, S.; Gaillard, O.; Bouhanna, P.; Cannell, D.W.; Bernard, B.A. Human hair shape is programmed from the bulb. Br. J. Dermatol. 2005, 152, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, A.; Kimura, R.; Ohashi, J.; Omi, K.; Yuliwulandari, R.; Batubara, L.; Mustofa, M.S.; Samakkarn, U.; Settheetham-Ishida, W.; Ishida, T.; et al. A scan for genetic determinants of human hair morphology: EDAR is associated with Asian hair thickness. Hum. Mol. Genet. 2008, 17, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Medland, S.E.; Nyholt, D.R.; Painter, J.N.; McEvoy, B.P.; McRae, A.F.; Zhu, G.; Gordon, S.D.; Ferreira, M.A.R.; Wright, M.J.; Henders, A.K.; et al. Common Variants in the Trichohyalin Gene Are Associated with Straight Hair in Europeans. Am. J. Hum. Genet. 2009, 85, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, Y.; Wajid, M.; Petukhova, L.; Kurban, M.; Christiano, A.M. Autosomal-Dominant Woolly Hair Resulting from Disruption of Keratin 74 (KRT74), a Potential Determinant of Human Hair Texture. Am. J. Hum. Genet. 2010, 86, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, Y.; Wajid, M.; Ishii, Y.; Shapiro, L.; Petukhova, L.; Gordon, D.; Christiano, A.M. Disruption of P2RY5, an orphan G protein-coupled receptor, underlies autosomal recessive woolly hair. Nat. Genet. 2008, 40, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, Y.; Wajid, M.; Petukhova, L.; Shapiro, L.; Christiano, A.M. Mutations in the lipase H gene underlie autosomal recessive woolly hair/hypotrichosis. J. Invest. Dermatol. 2009, 129, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, G.; Zhang, J. Mutations with Hair Shape Phenotypes Abnormalities—The Morphogenetic Waves and Related Diseases. J. Cosmet. Dermatol. Sci. Appl. 2013, 3, 26–34. [Google Scholar] [CrossRef]
- Thibaut, S.; Barbarat, P.; Leroy, F.; Bernard, B.A. Human hair keratin network and curvature. Int. J. Dermatol. 2007, 46, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Sriwiriyanont, P.; Hachiya, A.; Pickens, W.L.; Moriwaki, S.; Kitahara, T.; Visscher, M.O.; Kitzmiller, W.J.; Bello, A.; Takema, Y.; Kobinger, G.P. Effects of IGF-binding protein 5 in dysregulating the shape of human hair. J. Invest. Dermatol. 2011, 131, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Mou, C.; Thomason, H.A.; Willan, P.M.; Clowes, C.; Harris, W.E.; Drew, C.F.; Dixon, J.; Dixon, M.J.; Headon, D.J. Enhanced ectodysplasin—A receptor (EDAR) signaling alters multiple fiber characteristics to produce the East Asian hair form. Hum. Mutat. 2008, 29, 1405–1411. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Vilela, A.L.; Botelho, A.J.; Muehlmann, L.A. An overview of chemical straightening of human hair: Technical aspects, potential risks to hair fibre and health and legal issues. Int. J. Cosmet. Sci. 2014, 36, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Bolduc, C.; Shapiro, J. Hair care products: Waving, straightening, conditioning, and coloring. Clin. Dermatol. 2001, 19, 431–436. [Google Scholar] [CrossRef]
- Uyttendaele, H.; Panteleyev, A.A.; de Berker, D.; Tobin, D.T.; Christiano, A.M. Activation of Notch1 in the hair follicle leads to cell-fate switch and Mohawk alopecia. Differentiation 2004, 72, 396–409. [Google Scholar] [CrossRef] [PubMed]
- McElwee, K.J.; Sinclair, R. Hair physiology and its disorders. Drug Discov. Today Dis. Mech. 2008, 5, e163–e171. [Google Scholar] [CrossRef]
- Chuong, C.-M.; Cotsarelis, G.; Stenn, K. Defining hair follicles in the age of stem cell bioengineering. J. Invest. Dermatol. 2007, 127, 2098–2100. [Google Scholar] [CrossRef] [PubMed]
- Headon, D.J. Ectodysplasin signaling in cutaneous appendage development: Dose, duration, and diversity. J. Invest. Dermatol. 2009, 129, 817–819. [Google Scholar] [CrossRef] [PubMed]
- Bernard, B.A. Hair shape of curly hair. J. Am. Acad. Dermatol. 2003, 48, S120–S126. [Google Scholar] [CrossRef] [PubMed]
- Botchkarev, V.A.; Paus, R. Molecular biology of hair morphogenesis: Development and cycling. J. Exp. Zool. Part B Mol. Dev. Evol. 2003, 298, 164–180. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.R.; Schmidt-Ullrich, R.; Paus, R. The hair follicle as a dynamic miniorgan. Curr. Biol. 2009, 19, R132–R142. [Google Scholar] [CrossRef] [PubMed]
- Paus, R.; Cotsarelis, G. The biology of hair follicles. N. Engl. J. Med. 1999, 341, 491–497. [Google Scholar] [PubMed]
- Schmidt-Ullrich, R.; Paus, R. Molecular principles of hair follicle induction and morphogenesis. Bioessays 2005, 27, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Rishikaysh, P.; Dev, K.; Diaz, D.; Qureshi, W.M.S.; Filip, S.; Mokry, J. Signaling involved in hair follicle morphogenesis and development. Int. J. Mol. Sci. 2014, 15, 1647–1670. [Google Scholar] [CrossRef] [PubMed]
- Nissimov, J.N.; Das Chaudhuri, A.B. Hair curvature: A natural dialectic and review. Biol. Rev. 2014, 89, 723–766. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, E. Skin stem cells: Rising to the surface. J. Cell Biol. 2008, 180, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Rebora, A.; Guarrera, M. Kenogen. A new phase of the hair cycle? Dermatology 2002, 205, 108–110. [Google Scholar] [CrossRef] [PubMed]
- Guarrera, M.; Rebora, A. Kenogen in female androgenetic alopecia. A longitudinal study. Dermatology 2005, 210, 18–20. [Google Scholar] [CrossRef] [PubMed]
- Bernard, B.A. The human hair follicle, a bistable organ? Exp. Dermatol. 2012, 21, 401–403. [Google Scholar] [CrossRef] [PubMed]
- Claudinot, S.; Nicolas, M.; Oshima, H.; Rochat, A.; Barrandon, Y. Long-term renewal of hair follicles from clonogenic multipotent stem cells. Proc. Natl. Acad. Sci. USA 2005, 102, 14677–14682. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.J.; Liu, Y.; Marles, L.; Yang, Z.; Trempus, C.; Li, S.; Lin, J.S.; Sawicki, J.A.; Cotsarelis, G. Capturing and profiling adult hair follicle stem cells. Nat. Biotechnol. 2004, 22, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, M. Hair follicle bulge: A fascinating reservoir of epithelial stem cells. J. Dermatol. Sci. 2007, 46, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Oshima, H.; Rochat, A.; Kedzia, C.; Kobayashi, K.; Barrandon, Y. Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell 2001, 104, 233–245. [Google Scholar] [CrossRef]
- Vogt, A.; McElwee, K.J.; Blume-Peytavi, U. Biology of the hair follicle. In Hair Growth and Disorders; Blume-Peytavi, U., Tosti, A., Whiting, D.A., Trueb, R., Eds.; Springer-Verlag: Heidelberg, Germany, 2008; pp. 1–22. [Google Scholar]
- Araújo, R.; Fernandes, M.; Cavaco-Paulo, A.; Gomes, A. Biology of Human Hair: Know Your Hair to Control It; Springer-Verlag: Heidelberg, Germany, 2010; pp. 1–23. [Google Scholar]
- Randall, V.A. Androgens and hair growth. Dermatol. Ther. 2008, 21, 314–328. [Google Scholar] [CrossRef] [PubMed]
- Paus, R.; Foitzik, K. In search of the “hair cycle clock”: A guided tour. Differentiation 2004, 72, 489–511. [Google Scholar] [CrossRef] [PubMed]
- Blume-Peytavi, U.; Tosti, A.; Whiting, D.A.; Trüeb, R.M. Hair Growth and Disorders, 1st ed.; Springer-Verlag: Heidelberg, Germany, 2008. [Google Scholar]
- Lai-Cheong, J.E.; McGrath, J.A. Structure and function of skin, hair and nails. Medicine (Baltimore) 2013, 41, 317–320. [Google Scholar] [CrossRef]
- Legué, E.; Sequeira, I.; Nicolas, J.-F. Hair Follicle Stem Cells; Hayat, M.A., Ed.; Springer-Verlag: Heidelberg, Germany, 2012. [Google Scholar]
- Yu, Z.; Gordon, S.W.; Nixon, A.J.; Bawden, C.S.; Rogers, M.A.; Wildermoth, J.E.; Maqbool, N.J.; Pearson, A.J. Expression patterns of keratin intermediate filament and keratin associated protein genes in wool follicles. Differentiation 2009, 77, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Blanpain, C.; Lowry, W.E.; Geoghegan, A.; Polak, L.; Fuchs, E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell 2004, 118, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Robbins, C.R. Chemical and Physical Behavior of Human Hair, 4th ed.; Springer-Verlag: Heidelberg, Germany, 2012. [Google Scholar]
- Krause, K.; Foitzik, K. Biology of the hair follicle: The basics. Semin. Cutan. Med. Surg. 2006, 25, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Headington, J.T. Transverse Microscopic Anatomy of the Human Scalp. Arch. Dermatol. 1984, 120, 449. [Google Scholar] [CrossRef] [PubMed]
- Wolfram, L.J. Human hair: A unique physicochemical composite. J. Am. Acad. Dermatol. 2003, 48, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, I.P.; Bhushan, B. Effect of ethnicity and treatments on in situ tensile response and morphological changes of human hair characterized by atomic force microscopy. Acta Mater. 2008, 56, 3585–3597. [Google Scholar] [CrossRef]
- Bhushan, B. Nanoscale characterization of human hair and hair conditioners. Prog. Mater. Sci. 2008, 53, 585–710. [Google Scholar] [CrossRef]
- Bryson, W.G.; Harland, D.P.; Caldwell, J.P.; Vernon, J.A.; Walls, R.J.; Woods, J.L.; Nagase, S.; Itou, T.; Koike, K. Cortical cell types and intermediate filament arrangements correlate with fiber curvature in Japanese human hair. J. Struct. Biol. 2009, 166, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Harland, D.P.; Walls, R.J.; Vernon, J.A.; Dyer, J.M.; Woods, J.L.; Bell, F. Three-dimensional architecture of macrofibrils in the human scalp hair cortex. J. Struct. Biol. 2014, 185, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Dawber, R. Hair: Its Structure and Response to Cosmetic Preparations. Clin. Dermatol. 1996, 14, 105–112. [Google Scholar] [CrossRef]
- Plowman, J.E. The proteomics of keratin proteins. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 849, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, C.; Wilkinson, J. The Science of Hair Care, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Yu, J.; Yu, D.-W.; Checkla, D.D.M.; Freedberg, I.M.; Bertolino, A.P. Human Hair Keratins. J. Investig. Dermatol. 1993, 101, 56S–59S. [Google Scholar] [CrossRef] [PubMed]
- Conway, J.F.; Parry, D.A.D. Intermediate filament structure: 3. Analysis of sequence homologies. Int. J. Biol. Macromol. 1988, 10, 53–54. [Google Scholar] [CrossRef]
- Tanamachi, H.; Tokunaga, S.; Tanji, N.; Oguri, M.; Inoue, S. 18-MEA and hair appearance. J. Cosmet. Sci. 2010, 61, 147–160. [Google Scholar] [PubMed]
- Breakspear, S.; Smith, J.R.; Luengo, G. Effect of the covalently linked fatty acid 18-MEA on the nanotribology of hair’s outermost surface. J. Struct. Biol. 2005, 149, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Cruz, C.F.; Fernandes, M.M.; Gomes, A.C.; Coderch, L.; Martí, M.; Méndez, S.; Gales, L.; Azoia, N.G.; Shimanovich, U.; Cavaco-Paulo, A. Keratins and lipids in ethnic hair. Int. J. Cosmet. Sci. 2013, 35, 244–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piraccini, B.M.; Pazzaglia, M.; Tosti, A. Hair in forensic medicine. In Hair Growth and Disorders; Blume-Peytavi, U., Whiting, D.A., Tosti, A., Trueb, R.M., Eds.; Springer-Verlag: Heidelberg, Germany, 2008; pp. 539–542. [Google Scholar]
- Xiang, P.; Shen, M.; Drummer, O.H. Review: Drug concentrations in hair and their relevance in drug facilitated crimes. J. Forensic Leg. Med. 2015, 36, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Kidwell, D.A.; Smith, F.P.; Shepherd, A.R. Ethnic hair care products may increase false positives in hair drug testing. Forensic Sci. Int. 2015, 257, 160–164. [Google Scholar] [CrossRef] [PubMed]
- De La Mettrie, R.; Saint-Leger, D.; Loussouarn, G.; Garcel, A. Shape Variability and Classification of Human Hair: A Worldwide Approach. Hum. Biol. 2007, 79, 265–281. [Google Scholar] [CrossRef] [PubMed]
- Loussouarn, G.; Garcel, A.-L.; Lozano, I.; Collaudin, C.; Porter, C.; Panhard, S.; Saint-Léger, D.; de La Mettrie, R. Worldwide diversity of hair curliness: A new method of assessment. Int. J. Dermatol. 2007, 46, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Taguchi, H.; Hachiya, A.; Kitahara, T.; Boissy, R.E.; Visscher, M.O. The natural trait of the curvature of human hair is correlated with bending of the hair follicle and hair bulb by a structural disparity in the root sheath. J. Dermatol. Sci. 2014, 75, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.S. The Inner Root Sheath and the Men Associated with it Eponymically. Int. J. Trichol. 2011, 3, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Thibaut, S.; Bernard, B.A. The biology of hair shape. Int. J. Dermatol. 2005, 44, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Kajiura, Y.; Watanabe, S.; Itou, T.; Nakamura, K.; Iida, A.; Inoue, K.; Yagi, N.; Shinohara, Y.; Amemiya, Y. Structural analysis of human hair single fibres by scanning microbeam SAXS. J. Struct. Biol. 2006, 155, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Leon, H. Structural aspects of keratin fibres. J. Soc. Cosmet. Chem. 1972, 23, 427–445. [Google Scholar]
- Hearle, J.W.S. A critical review of the structural mechanics of wool and hair fibres. Int. J. Biol. Macromol. 2000, 27, 123–138. [Google Scholar] [CrossRef]
- Matsunaga, R.; Abe, R.; Ishii, D.; Watanabe, S.; Kiyoshi, M.; Nocker, B.; Tsuchiya, M.; Tsumoto, K. Bidirectional binding property of high glycine-tyrosine keratin-associated protein contributes to the mechanical strength and shape of hair. J. Struct. Biol. 2013, 183, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Berardesca, E.; Lévêque, J.-L.; Maibach, H.I. Ethnic Skin and Hair, 1st ed.; Informa Healthcare: San Francisco, NC, USA, 2007. [Google Scholar]
- Rodney, I.J.; Onwudiwe, O.C.; Callender, V.D.; Halder, R.M. Hair and scalp disorders in ethnic populations. J. Drugs Dermatol. 2013, 12, 420–427. [Google Scholar] [PubMed]
- Lee, H.J.; Ha, S.J.; Lee, J.H.; Kim, J.W.; Kim, H.O.; Whiting, D.A. Hair counts from scalp biopsy specimens in Asians. J. Am. Acad. Dermatol. 2002, 46, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Sperling, L.C. Hair density in African Americans. Arch. Dermatol. 1999, 135, 656–658. [Google Scholar] [CrossRef] [PubMed]
- Khumalo, N.P.; Doe, P.T.; Dawber, R.P.; Ferguson, D.J. What is normal black African hair? A light and scanning electron-microscopic study. J. Am. Acad. Dermatol. 2000, 43, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Loussouarn, G. African hair growth parameters. Br. J. Dermatol. 2001, 145, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Franbourg, A.; Hallegot, P.; Baltenneck, F.; Toutain, C.; Leroy, F. Current research on ethnic hair. J. Am. Acad. Dermatol. 2003, 48, S115–S119. [Google Scholar] [CrossRef] [PubMed]
- Nappe, C.; Kermici, M. Electrophoretic analysis of alkylated proteins of human hair from various ethnic groups. J. Soc. Cosmet. Chem. 1988, 40, 91–99. [Google Scholar]
- Saint Olive Baque, C.; Zhou, J.; Gu, W.; Collaudin, C.; Kravtchenko, S.; Kempf, J.Y.; Saint-Léger, D. Relationships between hair growth rate and morphological parameters of human straight hair: A same law above ethnical origins? Int. J. Cosmet. Sci. 2012, 34, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Gavazzoni Dias, M.F. Hair cosmetics: An overview. Int. J. Trichology 2015, 7, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Gray, J. Hair Care and Hair Care Products. Clin. Dermatol. 2001, 19, 227–236. [Google Scholar] [CrossRef]
- Trüeb, R.M. The value of hair cosmetics and pharmaceuticals. Dermatology 2001, 202, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Draelos, Z.D. Essentials of Hair Care often Neglected: Hair Cleansing. Int. J. Trichol. 2010, 2, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Draelos, Z.K. Cosmetics: An overview. Curr. Probl. Dermatol. 1995, 7, 45–64. [Google Scholar] [CrossRef]
- LaTorre, C.; Bhushan, B.; Yang, J.-Z.; Torgerson, P.M. Nanotribological effects of silicone type, silicone deposition level, and surfactnat type on human hair using atomic force microscopy. J. Costmet. Sci. 2006, 56, 37–56. [Google Scholar]
- Nazir, H.; Lv, P.; Wang, L.; Lian, G.; Zhu, S.; Ma, G. Uniform-sized silicone oil microemulsions: Preparation, investigation of stability and deposition on hair surface. J. Colloid Interface Sci. 2011, 364, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Wexler, P. Encyclopedia of Toxicology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Bhushan, B. Introduction—Human hair, skin and hair care products. In Biophysics of Human Hair; Springer: Berlin, Germany, 2010; pp. 1–19. [Google Scholar]
- Feughelman, M.A.X. A note on the permanent setting of human hair. J. Soc. Cosmet. Chem. 1990, 212, 209–212. [Google Scholar]
- De Sá Dias, T.C.; Baby, A.R.; Kaneko, T.M.; Robles Velasco, M.V. Relaxing/straightening of Afro-ethnic hair: Historical overview. J. Cosmet. Dermatol. 2007, 6, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.; Wis-Surel, G.; Epps, J. Mechanism of hair straightening. J. Cosmet. Chem. 1994, 45, 347–352. [Google Scholar]
- Manuszak, M.A.; Borish, E.T.; Wickett, R.R.; Borush, E.T.; Wickett, R.R. Reduction of human hair by cysteamine and ammonium thioglycolate: A correlation of amino acid analysis and single-fiber tensile kinetic data. J. Soc. Cosmet. Chem. 1996, 47, 213–227. [Google Scholar]
- Mitsui, T. New Cosmetic Science; Elsevier: Amsterdam, The Netherlands, 1997. [Google Scholar]
- Olsasode, O.A. Chemical hair relaxation and adverse outcomes among Negroid women in South West Nigeria. J. Pak. Assoc. Dermatol. 2009, 19, 203–207. [Google Scholar]
- McMichael, A.J. Hair breakage in normal and weathered hair: Focus on the Black patient. J. Investig. Dermatol. Symp. Proc. 2007, 12, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Kaur, B.J.; Singh, H.; Lin-Greenberg, A. Irritant contact dermatitis complicated by deep-seated staphylococcal infection caused by a hair relaxer. J. Natl. Med. Assoc. 2002, 94, 121–123. [Google Scholar] [PubMed]
- Smith, R.S.; Shear, G. Corneal Alkali Burns Arising From Accidental Instillation of a Hair Straightener. Am. J. Ophthalmol. 1975, 79, 602–605. [Google Scholar] [CrossRef]
- Maneli, M.H.; Smith, P.; Khumalo, N.P. Elevated formaldehyde concentration in Brazilian keratin type hair-straightening products: A cross-sectional study. J. Am. Acad. Dermatol. 2014, 70, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Galli, C.L.; Bettin, F.; Metra, P.; Fidente, P.; de Dominicis, E.; Marinovich, M. Novel analytical method to measure formaldehyde release from heated hair straightening cosmetic products: Impact on risk assessment. Regul. Toxicol. Pharmacol. 2015, 72, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, K.; Fontanil, T.; Cal, S.; Mendoza-Revilla, J.; Fuentes-Guajardo, M.; Chacón-Duque, J.-C.; Al-Saadi, F.; Johansson, J.A.; Quinto-Sanchez, M.; Acuña-Alonzo, V.; et al. A genome-wide association scan in admixed Latin Americans identifies loci influencing facial and scalp hair features. Nat. Commun. 2016, 7, 10815. [Google Scholar] [CrossRef] [PubMed]
- Matamá, T.; Gomes, A.C.; Cavaco-Paulo, A. Hair Coloration by Gene Regulation: Fact or Fiction? Trends Biotechnol. 2015, 33, 707–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).