Oxidative Stress in Reproduction: A Mitochondrial Perspective
Abstract
:1. Introduction
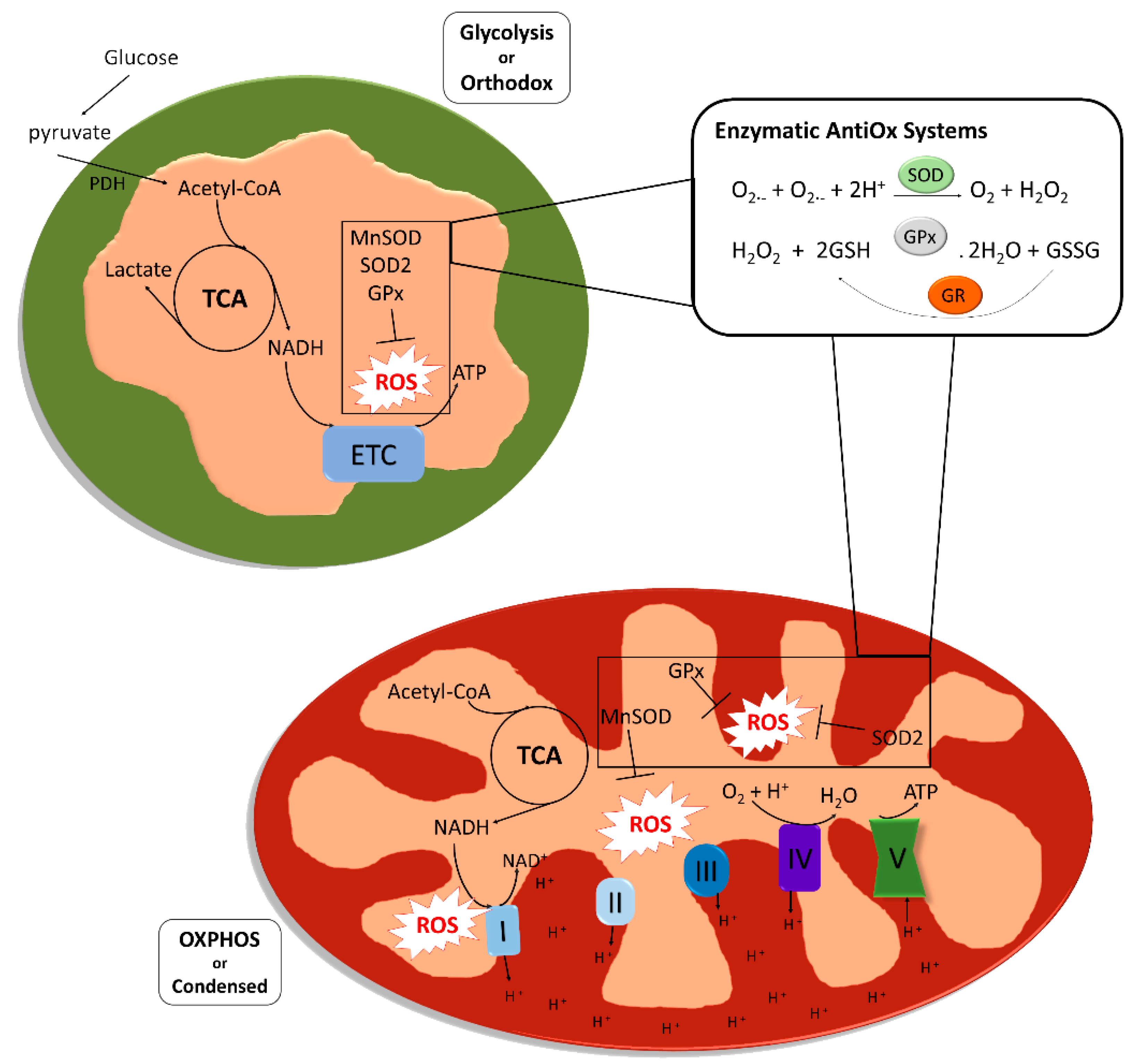
2. Oxidative Stress and Mitochondrial Activity in Gamete Development
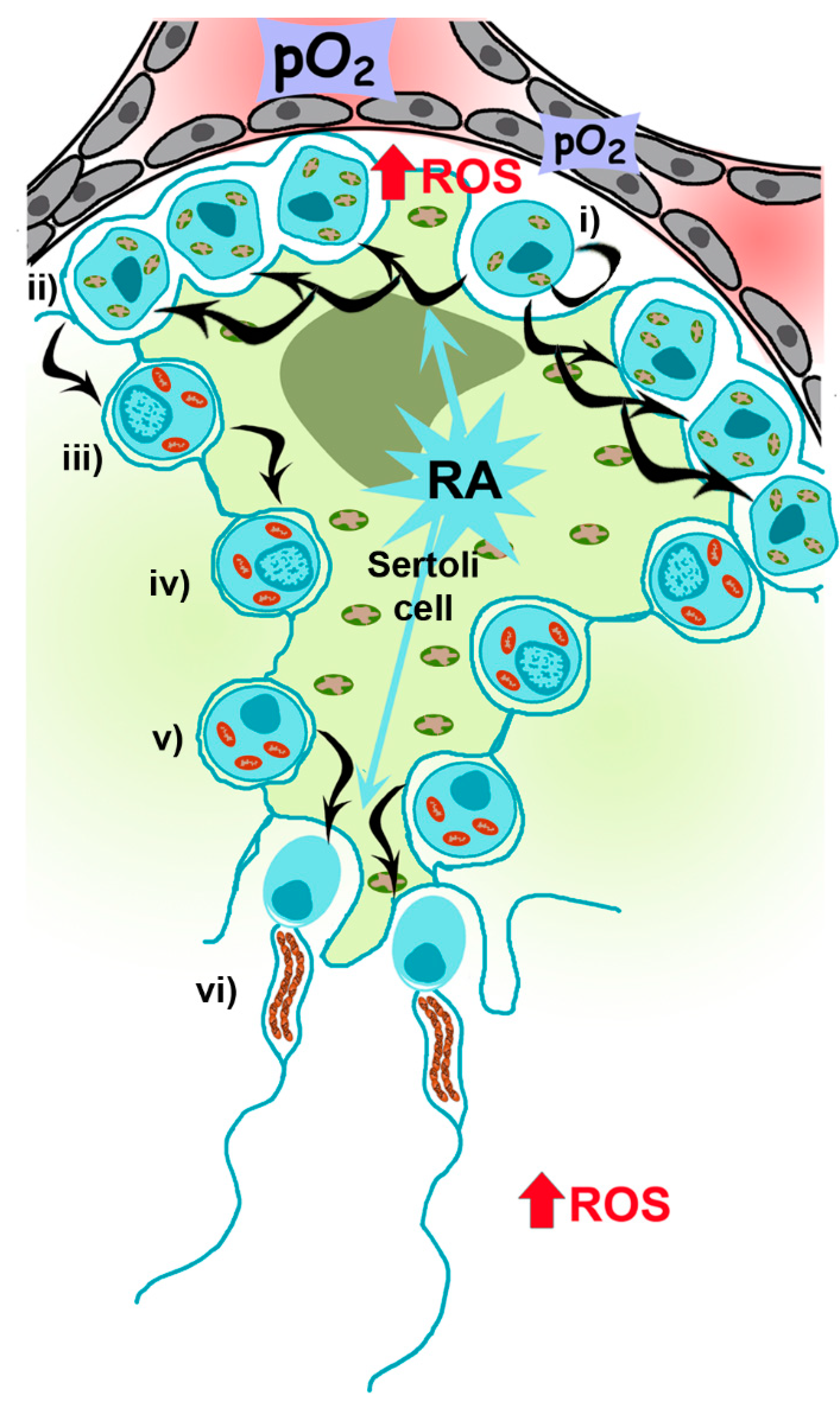
2.1. Mitochondrial Metabolism during Spermatogenesis
2.2. Role of Mitochondria and ROS during Spermatogenesis
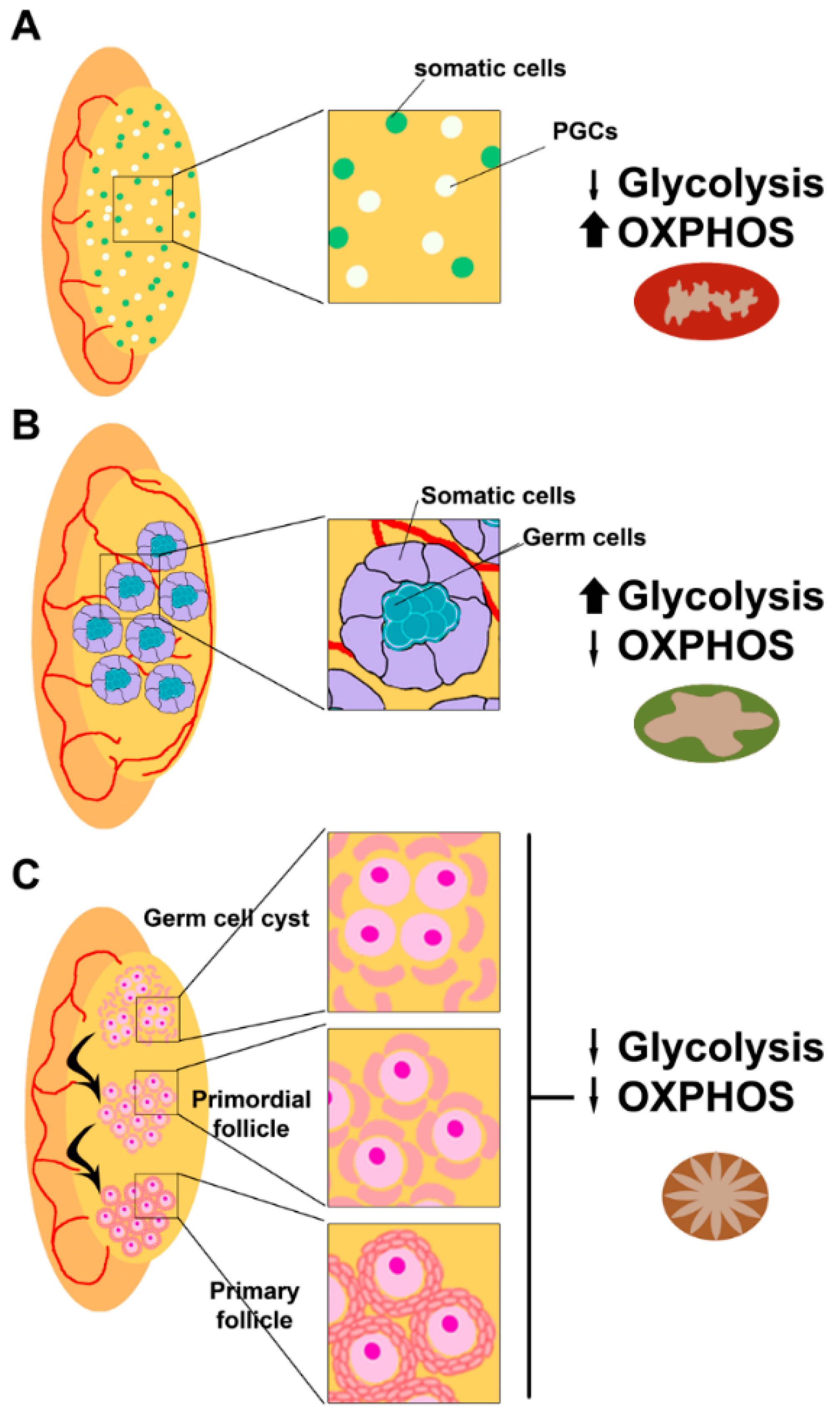
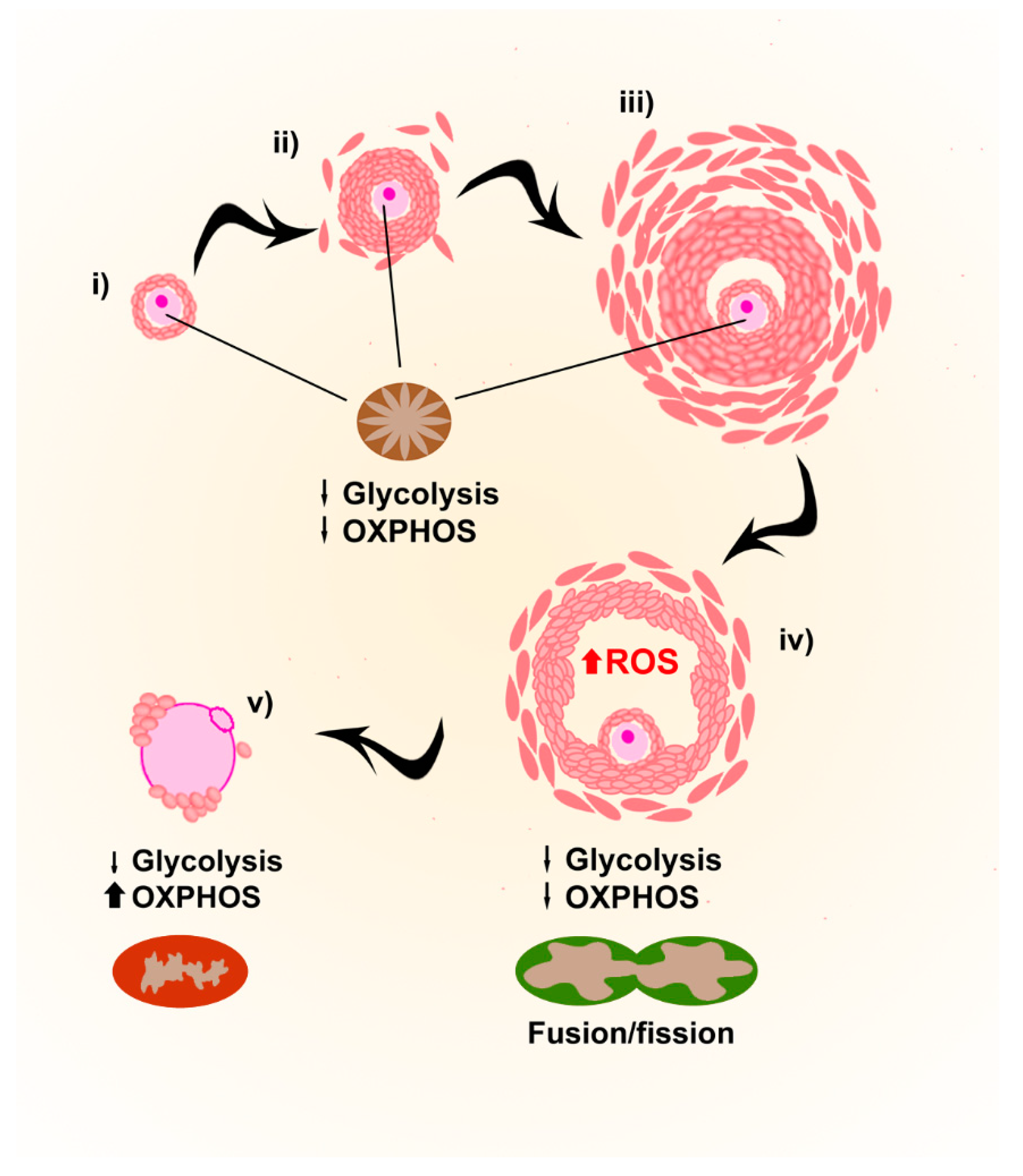
2.3. Mitochondria and ROS in Folliculogenesis and Oogenesis
2.4. ROS (in)Balance in Follicles and Oocytes
3. Mitochondria and ROS in Embryos
4. Mitochondrial DNA and OS
5. Mitochondria, Oxidative Stress and Aging in Reproduction
6. Mitochondrial OS during ART Procedures
7. Mitochondrial OS Testing in ART Outcomes
8. ART Outcome Improvement through OS Management
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hackenbrock, C.R. Energy-Linked Condensed-Orthodox Ultrastructural Transformations in Mitochondria. Chemotherapy 1981, 27, 21–26. [Google Scholar] [CrossRef]
- Zhao, R.; Jiang, S.; Zhang, L.; Yu, Z. Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Dan Dunn, J.; Alvarez, L.; Zhang, X.; Soldati, T. Reactive oxygen species and mitochondria: A nexus of cellular homeostasis. Redox Biol. 2015, 6, 472–485. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-Mediated Cellular Signaling. Oxidative Med. Cell. Longev. 2016, 2016, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sena, L.A.; Chandel, N.S. Physiological Roles of Mitochondrial Reactive Oxygen Species. Mol. Cell 2012, 48, 158–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Meo, S.; Reed, T.; Venditti, P.; Víctor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxidative Med. Cell. Longev. 2016, 2016, 1–44. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briviba, K.; Sies, H. Nonenzymatic Antioxidant Defense Systems. In Natural Antioxidants in Human Health and Disease; Elsevier: Amsterdam, The Netherlands, 1994; pp. 107–128. [Google Scholar]
- Leese, H.J.; Sturmey, R.G.; Baumann, C.G.; McEvoy, T.G. Embryo viability and metabolism: Obeying the quiet rules. Hum. Reprod. 2007, 22, 3047–3050. [Google Scholar] [CrossRef]
- Agarwal, A.; Sharma, R.; Gupta, S.; Harlev, A.; Ahmad, G.; Du Plessis, S.S.; Esteves, S.C.; Wang, S.M.; Durairajanayagam, D. Oxidative Stress in Human Reproduction; Springer Science and Business Media LLC.: Berlin, Germany, 2017. [Google Scholar]
- Kobayashi, T.; Surani, M.A. On the origin of the human germline. Development 2018, 145, dev150433. [Google Scholar] [CrossRef] [Green Version]
- Nicholls, P.K.; Schorle, H.; Naqvi, S.; Hu, Y.-C.; Fan, Y.; Carmell, M.A.; Dobrinski, I.; Watson, A.L.; Carlson, D.F.; Fahrenkrug, S.C.; et al. Mammalian germ cells are determined after PGC colonization of the nascent gonad. Proc. Natl. Acad. Sci. USA 2019, 116, 25677–25687. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Otsuka, K.; Ebina, M.; Igarashi, K.; Takehara, A.; Matsumoto, M.; Kanai, A.; Igarashi, K.; Soga, T.; Matsui, Y. Distinct requirements for energy metabolism in mouse primordial germ cells and their reprogramming to embryonic germ cells. Proc. Natl. Acad. Sci. USA 2017, 114, 8289–8294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griswold, M.D. Spermatogenesis: The Commitment to Meiosis. Physiol. Rev. 2016, 96, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vertika, S.; Singh, K.K.; Rajender, S. Mitochondria, spermatogenesis, and male infertility—An update. Mitochondrion 2020, 54, 26–40. [Google Scholar] [CrossRef]
- Gewiss, R.; Topping, T.; Griswold, M.D. Cycles, waves, and pulses: Retinoic acid and the organization of spermatogenesis. Androlology 2019, 8, 892–897. [Google Scholar] [CrossRef] [Green Version]
- Bishop, P.D.; Griswold, M.D. Uptake and metabolism of retinol in cultured Sertoli cells: Evidence for a kinetic model. Biochemistry 1987, 26, 7511–7518. [Google Scholar] [CrossRef]
- Lufkin, T.; Lohnes, D.; Mark, M.; Dierich, A.; Gorry, P.; Gaub, M.P.; LeMeur, M.; Chambon, P. High postnatal lethality and testis degeneration in retinoic acid receptor alpha mutant mice. Proc. Natl. Acad. Sci. USA 1993, 90, 7225–7229. [Google Scholar] [CrossRef] [Green Version]
- Perrotta, I.; Perri, M.; Santoro, M.; Panza, S.; Caroleo, M.C.; Guido, C.; Mete, A.; Cione, E.; Aquila, S. Expression and Subcellular Localization of Retinoic Acid Receptor-α (RARα) in Healthy and Varicocele Human Spermatozoa. Appl. Immunohistochem. Mol. Morphol. 2015, 23, 374–381. [Google Scholar] [CrossRef]
- Tourniaire, F.; Mušinović, H.; Gouranton, E.; Astier, J.; Marcotorchino, J.; Arreguin, A.; Bernot, D.; Palou, A.; Bonet, M.L.; Ribot, J.; et al. All-transretinoic acid induces oxidative phosphorylation and mitochondria biogenesis in adipocytes. J. Lipid Res. 2015, 56, 1100–1109. [Google Scholar] [CrossRef] [Green Version]
- Lunt, S.Y.; Heiden, M.G.V. Aerobic Glycolysis: Meeting the Metabolic Requirements of Cell Proliferation. Annu. Rev. Cell Dev. Biol. 2011, 27, 441–464. [Google Scholar] [CrossRef] [Green Version]
- Heiden, M.G.V.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [Green Version]
- Ramalho-Santos, J.; Amaral, S. Mitochondria and mammalian reproduction. Mol. Cell. Endocrinol. 2013, 379, 74–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, S.L.C.; Rodrigues, A.S.D.J.; Sousa, M.I.; Correia, M.; Perestrelo, T.; Ramalho-Santos, J. From gametogenesis and stem cells to cancer: Common metabolic themes. Hum. Reprod. Updat. 2014, 20, 924–943. [Google Scholar] [CrossRef] [Green Version]
- Lord, T.; Nixon, B. Metabolic Changes Accompanying Spermatogonial Stem Cell Differentiation. Dev. Cell 2020, 52, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Varuzhanyan, G.; Rojansky, R.; Sweredoski, M.J.; Graham, R.L.; Hess, S.; Ladinsky, M.S.; Chan, D.C. Mitochondrial fusion is required for spermatogonial differentiation and meiosis. eLife 2019, 8, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.-H.; Wang, R.; Wang, Y.; Kung, C.-P.; Weber, J.D.; Patti, G.J. Mitochondrial fusion supports increased oxidative phosphorylation during cell proliferation. eLife 2019, 8, 1–19. [Google Scholar] [CrossRef]
- Otani, H.; Tanaka, O.; Kasai, K.-I.; Yoshioka, T. Development of mitochondrial helical sheath in the middle piece of the mouse spermatid tail: Regular dispositions and synchronized changes. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 1988, 222, 26–33. [Google Scholar] [CrossRef]
- Ho, H.-C.; Wey, S. Three dimensional rendering of the mitochondrial sheath morphogenesis during mouse spermiogenesis. Microsc. Res. Tech. 2007, 70, 719–723. [Google Scholar] [CrossRef]
- Olson, G.E.; Winfrey, V.P. Mitochondria-cytoskeleton interactions in the sperm midpiece. J. Struct. Biol. 1990, 103, 13–22. [Google Scholar] [CrossRef]
- Zhang, Y.; Ou, Y.; Cheng, M.; Saadi, H.A.S.; Thundathil, J.C.; Van Der Hoorn, F.A. KLC3 is involved in sperm tail midpiece formation and sperm function. Dev. Biol. 2012, 366, 101–110. [Google Scholar] [CrossRef] [Green Version]
- Ursini, F. Dual Function of the Selenoprotein PHGPx during Sperm Maturation. Science 1999, 285, 1393–1396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piomboni, P.; Focarelli, R.; Stendardi, A.; Ferramosca, A.; Zara, V. The role of mitochondria in energy production for human sperm motility. Int. J. Androl. 2011, 35, 109–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Menzies, K.J.; Auwerx, J. The role of mitochondria in stem cell fate and aging. Development 2018, 145, dev143420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koppers, A.J.; De Iuliis, G.N.; Finnie, J.M.; Aitken, R.J.; McLaughlin, E. Significance of Mitochondrial Reactive Oxygen Species in the Generation of Oxidative Stress in Spermatozoa. J. Clin. Endocrinol. Metab. 2008, 93, 3199–3207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kothari, S.; Thompson, A.; Agarwal, A.; Du Plessis, S.S. Free radicals: Their beneficial and detrimental effects on sperm function. Indian J. Exp. Biol. 2010, 48, 425–435. [Google Scholar] [PubMed]
- Dutta, S.; Majzoub, A.; Agarwal, A. Oxidative stress and sperm function: A systematic review on evaluation and management. Arab. J. Urol. 2019, 17, 87–97. [Google Scholar] [CrossRef] [Green Version]
- Du Plessis, S.S.; Agarwal, A.; Mohanty, G.; Van Der Linde, M. Oxidative phosphorylation versus glycolysis: What fuel do spermatozoa use? Asian J. Androl. 2014, 17, 230–235. [Google Scholar] [CrossRef]
- Khosrowbeygi, A.; Zarghami, N. Fatty acid composition of human spermatozoa and seminal plasma levels of oxidative stress biomarkers in subfertile males. ProstaglandinsLeukot. Essent. Fat. Acids 2007, 77, 117–121. [Google Scholar] [CrossRef]
- De Lamirande, E.; Gagnon, C. Impact of reactive oxygen species on spermatozoa: A balancing act between beneficial and detrimental effects. Hum. Reprod. 1995, 10, 15–21. [Google Scholar] [CrossRef]
- Agarwal, A.; Virk, G.; Ong, C.; Du Plessis, S.S. Effect of Oxidative Stress on Male Reproduction. World J. Men’s Heal. 2014, 32, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Opuwari, C.S.; Henkel, R. An Update on Oxidative Damage to Spermatozoa and Oocytes. BioMed Res. Int. 2016, 2016, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Yan, M.; Cao, Z.; Li, X.; Zhang, Y.; Shi, J.; Feng, G.-H.; Peng, H.; Zhang, X.; Qian, J.; et al. Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science 2015, 351, 397–400. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Shi, J.; Rassoulzadegan, M.; Tuorto, F.; Chen, Q. Sperm RNA code programmes the metabolic health of offspring. Nat. Rev. Endocrinol. 2019, 15, 489–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nätt, D.; Kugelberg, U.; Casas, E.; Nedstrand, E.; Zalavary, S.; Henriksson, P.; Nijm, C.; Jäderquist, J.; Sandborg, J.; Flinke, E.; et al. Human sperm displays rapid responses to diet. PLoS Biol. 2019, 17, e3000559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, D.M.; Parker, R. Stressing Out over tRNA Cleavage. Cell 2009, 138, 215–219. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, K.; Maekawa, T.; Ly, N.H.; Fujita, S.-I.; Muratani, M.; Ando, M.; Katou, Y.; Araki, H.; Miura, F.; Shirahige, K.; et al. ATF7-Dependent Epigenetic Changes Are Required for the Intergenerational Effect of a Paternal Low-Protein Diet. Mol. Cell 2020, 78, 445–458. [Google Scholar] [CrossRef]
- Pepling, M.E.; Spradling, A.C. Female mouse germ cells form synchronously dividing cysts. Development 1998, 125, 3323–3328. [Google Scholar]
- Byskov, A.G. Differentiation of mammalian embryonic gonad. Physiol. Rev. 1986, 66, 71–117. [Google Scholar] [CrossRef]
- Snow, M.H.L.; Monk, M. Emergence and migration of mouse primordial germ cells. In Current Problems in Germ Cell Differerentiation; Cambridge University Press: Cambridge, UK, 1983; pp. 115–135. [Google Scholar]
- Evans, M.A. Effect of phenytoin on calcium disposition in pregnant and nonpregnant mice*1. Toxicol. Appl. Pharmacol. 1982, 63, 422–428. [Google Scholar] [CrossRef]
- Dumollard, R.; Campbell, K.; Halet, G.; Carroll, J.; Swann, K. Regulation of cytosolic and mitochondrial ATP levels in mouse eggs and zygotes. Dev. Biol. 2008, 316, 431–440. [Google Scholar] [CrossRef] [Green Version]
- De Bruin, J.; Dorland, M.; Spek, E.; Posthuma, G.; Van Haaften, M.; Looman, C.; Velde, E.T. Ultrastructure of the resting ovarian follicle pool in healthy young women. Biol. Reprod. 2002, 66, 1151–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cummins, J. The role of maternal mitochondria during oogenesis, fertilization and embryogenesis. Reprod. Biomed. Online 2002, 4, 176–182. [Google Scholar] [CrossRef]
- KofiArhin, S.; Lv, J.; Xi, H.; Jin, X. Energy requirements in mammalian oogenesis. Cell. Mol. Biol. 2018, 64, 12–19. [Google Scholar]
- Zhang, M.; Bener, M.B.; Jiang, Z.; Wang, T.; Esencan, E.; Iii, R.S.; Horvath, T.; Seli, E. Mitofusin 1 is required for female fertility and to maintain ovarian follicular reserve. Cell Death Dis. 2019, 10, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Bener, M.B.; Jiang, Z.; Wang, T.; Esencan, E.; Scott, R.; Horvath, T.; Seli, E. Mitofusin 2 plays a role in oocyte and follicle development, and is required to maintain ovarian follicular reserve during reproductive aging. Aging 2019, 11, 3919–3938. [Google Scholar] [CrossRef]
- Laloraya, M.M.; Kumar, G.P.; Kumar, P.G. Histochemical study of superoxide dismutase in the ovary of the rat during the oestrous cycle. Reproduction 1989, 86, 583–587. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; He, G.; Chen, M.; Tao, Z.; Xu, W.; Liu, X. The Role of Antioxidant Enzymes in the Ovaries. Oxidative Med. Cell. Longev. 2017, 2017, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Matos, L.; Stevenson, D.; Gomes, F.; Silva-Carvalho, J.; Almeida, H. Superoxide dismutase expression in human cumulus oophorus cells. Mol. Hum. Reprod. 2009, 15, 411–419. [Google Scholar] [CrossRef] [Green Version]
- Harris, S.E.; Leese, H.J.; Gosden, R.G.; Picton, H.M. Pyruvate and oxygen consumption throughout the growth and development of murine oocytes. Mol. Reprod. Dev. 2009, 76, 231–238. [Google Scholar] [CrossRef]
- Sturmey, R.; Leese, H.; Sturmey, R.G. Energy metabolism in pig oocytes and early embryos. Reproduction 2003, 126, 197–204. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, A.; Gupta, S.; Sharma, R. Role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 2005, 3, 28. [Google Scholar] [CrossRef] [Green Version]
- Gardiner, C.S. Status of glutathione during oxidant-induced oxidative stress in the preimplantation mouse embryo. Biol. Reprod. 1994, 51, 1307–1314. [Google Scholar] [CrossRef] [Green Version]
- El Mouatassim, S.; Guerin, P.; Menezo, Y. Mammalian oviduct and protection against free oxygen radicals: Expression of genes encoding antioxidant enzymes in human and mouse. Eur. J. Obstet. Gynecol. Reprod. Biol. 2000, 89, 1–6. [Google Scholar] [CrossRef]
- Tsai-Turton, M.; Luderer, U. Opposing Effects of Glutathione Depletion and Follicle-Stimulating Hormone on Reactive Oxygen Species and Apoptosis in Cultured Preovulatory Rat Follicles. Endocrinology 2006, 147, 1224–1236. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, M.; Chaube, S.K. Moderate increase of reactive oxygen species triggers meiotic resumption in rat follicular oocytes. J. Obstet. Gynaecol. Res. 2016, 42, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Lane, M.; Gardner, D.K. Fertilization and early embryology: Selection of viable mouse blastocysts prior to transfer using a metabolic criterion. Hum. Reprod. 1996, 11, 1975–1978. [Google Scholar] [CrossRef] [Green Version]
- Lopes, A.; Lane, M.; Thompson, J.G. Oxygen consumption and ROS production are increased at the time of fertilization and cell cleavage in bovine zygotes. Hum. Reprod. 2010, 25, 2762–2773. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Li, Y. Adenosine triphosphate content in human unfertilized oocytes, undivided zygotes and embryos unsuitable for transfer or cryopreservation. J. Int. Med Res. 2012, 40, 734–739. [Google Scholar] [CrossRef]
- Santos, T.A.; El Shourbagy, S.; John, J.C.S. Mitochondrial content reflects oocyte variability and fertilization outcome. Fertil. Steril. 2006, 85, 584–591. [Google Scholar] [CrossRef] [Green Version]
- St. John, J.C.; Facucho-Oliveira, J.; Jiang, Y.; Kelly, R.; Salah, R. Mitochondrial DNA transmission, replication and inheritance: A journey from the gamete through the embryo and into offspring and embryonic stem cells. Hum. Reprod. Updat. 2010, 16, 488–509. [Google Scholar] [CrossRef] [Green Version]
- Tsunoda, S.; Kimura, N.; Fujii, J. Oxidative stress and redox regulation of gametogenesis, fertilization, and embryonic development. Reprod. Med. Biol. 2013, 13, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Morales, H.; Tilquin, P.; Rees, J.; Massip, A.; Dessy, F.; Van Langendonckt, A. Pyruvate prevents peroxide-induced injury of in vitro preimplantation bovine embryos. Mol. Reprod. Dev. 1999, 52, 149–157. [Google Scholar] [CrossRef]
- Fischer, B.; Bavister, B.D. Oxygen tension in the oviduct and uterus of rhesus monkeys, hamsters and rabbits. Reproduction 1993, 99, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Bailey, P.S.J.; Nathan, J.A. Metabolic Regulation of Hypoxia-Inducible Transcription Factors: The Role of Small Molecule Metabolites and Iron. Biomedicines 2018, 6, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wheaton, W.W.; Chandel, N.S. Hypoxia. 2. Hypoxia regulates cellular metabolism. Am. J. Physiol. Physiol. 2010, 300, C385–C393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuhrmann, D.C.; Brüne, B. Mitochondrial composition and function under the control of hypoxia. Redox Biol. 2017, 12, 208–215. [Google Scholar] [CrossRef]
- Leese, H.J. Quiet please, do not disturb: A hypothesis of embryo metabolism and viability. BioEssays 2002, 24, 845–849. [Google Scholar] [CrossRef]
- Cecchino, G.N.; Garcia-Velasco, J.A. Mitochondrial DNA copy number as a predictor of embryo viability. Fertil. Steril. 2019, 111, 205–211. [Google Scholar] [CrossRef]
- Otten, A.B.; Smeets, B. Evolutionary defined role of the mitochondrial DNA in fertility, disease and ageing. Hum. Reprod. Updat. 2015, 21, 671–689. [Google Scholar] [CrossRef] [Green Version]
- Anderson, A.P.; Luo, X.; Russell, W.; Yin, Y.W. Oxidative damage diminishes mitochondrial DNA polymerase replication fidelity. Nucleic Acids Res. 2019, 48, 817–829. [Google Scholar] [CrossRef]
- Park, J.S.; Sharma, L.K.; Li, H.; Xiang, R.; Holstein, D.; Wu, J.; Lechleiter, J.; Naylor, S.L.; Deng, J.J.; Lu, J.; et al. A heteroplasmic, not homoplasmic, mitochondrial DNA mutation promotes tumorigenesis via alteration in reactive oxygen species generation and apoptosis. Hum. Mol. Genet. 2009, 18, 1578–1589. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K.; Takenaga, K.; Akimoto, M.; Koshikawa, N.; Yamaguchi, A.; Imanishi, H.; Nakada, K.; Honma, Y.; Hayashi, J.-I. “ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis”-a critical commentary. Free Radic. Biol. Med. 2008, 45, 1217–1219. [Google Scholar]
- Rana, M.; De Coo, I.; Diaz, F.; Smeets, H.; Moraes, C.T. An out-of-frame cytochrome b gene deletion from a patient with parkinsonism is associated with impaired complex III assembly and an increase in free radical production. Ann. Neurol. 2000, 48, 774–781. [Google Scholar] [CrossRef]
- Arnold, R.S.; Sun, Q.; Sun, C.Q.; Richards, J.C.; O’Hearn, S.; Osunkoya, A.O.; Wallace, U.C.; Petros, J.A. An Inherited Heteroplasmic Mutation in Mitochondrial Gene COI in a Patient with Prostate Cancer Alters Reactive Oxygen, Reactive Nitrogen and Proliferation. BioMed Res. Int. 2012, 2013, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hahn, A.; Zuryn, S. Hahn Mitochondrial Genome (mtDNA) Mutations that Generate Reactive Oxygen Species. Antioxidants 2019, 8, 392. [Google Scholar] [CrossRef] [Green Version]
- Sun, N.; Youle, R.J.; Finkel, T. The Mitochondrial Basis of Aging. Mol. Cell 2016, 61, 654–666. [Google Scholar] [CrossRef] [Green Version]
- Edgar, D.; Trifunovic, A. The mtDNA mutator mouse: Dissecting mitochondrial involvement in aging. Aging 2009, 1, 1028–1032. [Google Scholar] [CrossRef] [Green Version]
- Leadsham, J.E.; Sanders, G.; Giannaki, S.; Bastow, E.L.; Hutton, R.; Naeimi, W.R.; Breitenbach, M.; Gourlay, C.W. Loss of Cytochrome c Oxidase Promotes RAS-Dependent ROS Production from the ER Resident NADPH Oxidase, Yno1p, in Yeast. Cell Metab. 2013, 18, 279–286. [Google Scholar] [CrossRef] [Green Version]
- Cimadomo, D.; Fabozzi, G.; Vaiarelli, A.; Ubaldi, N.; Ubaldi, F.M.; Rienzi, L. Impact of Maternal Age on Oocyte and Embryo Competence. Front. Endocrinol. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Han, M.; Li, X.; Wang, H.; Ma, M.; Zhang, S.; Guo, Y.; Wang, S.; Wang, Y.; Duan, N.; et al. Age-related changes in the mitochondria of human mural granulosa cells. Hum. Reprod. 2017, 32, 2465–2473. [Google Scholar] [CrossRef]
- Müller-Höcker, J.; Schafer, S.; Weis, S.; Münscher, C.; Strowitzki, T. Morphological-cytochemical and molecular genetic analyses of mitochondria in isolated human oocytes in the reproductive age. Mol. Hum. Reprod. 1996, 2, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Wilding, M.; Dale, B.; Marino, M.; Di Matteo, L.; Alviggi, C.; Pisaturo, M.L.; Lombardi, L.; De Placido, G. Mitochondrial aggregation patterns and activity in human oocytes and preimplantation embryos. Hum. Reprod. 2001, 16, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Eichenlaub-Ritter, U. Oocyte ageing and its cellular basis. Int. J. Dev. Biol. 2012, 56, 841–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.-J.; Liu, X.; Chen, L.; Zhang, S.; Zhang, X.; Hao, C.; Miao, Y.-L. Advanced maternal age alters expression of maternal effect genes that are essential for human oocyte quality. Aging 2020, 12, 3950–3961. [Google Scholar] [CrossRef]
- Alonso-Alvarez, C.; Canelo, T.; Romero-Haro, A. Ángela The Oxidative Cost of Reproduction: Theoretical Questions and Alternative Mechanisms. Bioscience 2017, 67, 258–270. [Google Scholar] [CrossRef]
- Chao, H.-T.; Lee, S.-Y.; Lee, H.-M.; Liao, T.-L.; Wei, Y.-H.; Kao, S.-H. Repeated Ovarian Stimulations Induce Oxidative Damage and Mitochondrial DNA Mutations in Mouse Ovaries. Ann. New York Acad. Sci. 2005, 1042, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, K.; Sato, E.F.; Kasahara, E.; Jikumaru, M.; Hiramoto, K.; Tabata, H.; Katsuragi, M.; Odo, S.; Utsumi, K.; Inoue, M. Effect of oxidative stress during repeated ovulation on the structure and functions of the ovary, oocytes, and their mitochondria. Free. Radic. Biol. Med. 2010, 49, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.-Y.; Chen, H.-W.; Tzeng, C.Y. Low oxygen tension increases mitochondrial membrane potential and enhances expression of antioxidant genes and implantation protein of mouse blastocyst cultured in vitro. J. Ovarian Res. 2017, 10, 47. [Google Scholar] [CrossRef]
- Belli, M.; Zhang, L.; Liu, X.; Donjacour, A.; Ruggeri, E.; Palmerini, M.G.; Nottola, S.A.; Macchiarelli, G.; Rinaudo, P. Oxygen concentration alters mitochondrial structure and function in in vitro fertilized preimplantation mouse embryos. Hum. Reprod. 2019, 34, 601–611. [Google Scholar] [CrossRef] [Green Version]
- Tarahomi, M.; Vaz, F.M.; Van Straalen, J.P.; Schrauwen, F.A.P.; Van Wely, M.; Hamer, G.; Repping, S.; Mastenbroek, S. The composition of human preimplantation embryo culture media and their stability during storage and culture. Hum. Reprod. 2019, 34, 1450–1461. [Google Scholar] [CrossRef]
- Gardner, D.K.; Lane, M.; Calderon, I.; Leeton, J. Environment of the preimplantation human embryo in vivo: Metabolite analysis of oviduct and uterine fluids and metabolism of cumulus cells; Supported by IVF America Inc., Greenwich, Connecticut and Monash IVF Pty. Ltd., Melbourne, Victoria, Australia. Fertil. Steril. 1996, 65, 349–353. [Google Scholar] [CrossRef]
- Lee, T.-H.; Lee, M.-S.; Liu, C.-H.; Tsao, H.-M.; Huang, C.-C.; Yang, Y.-S. The Association between Microenvironmental Reactive Oxygen Species and Embryo Development in Assisted Reproduction Technology Cycles. Reprod. Sci. 2012, 19, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Zera, A.J.; Harshman, L.G. The physiology of life history trade-offs in animals. Annu. Rev. Ecolocy Syst. 2001, 32, 95–126. [Google Scholar] [CrossRef] [Green Version]
- Ziomkiewicz-Wichary, A.; Sancilio, A.; Galbarczyk, A.; Klimek, M.; Jasienska, G.; Bribiescas, R.G. Evidence for the Cost of Reproduction in Humans: High Lifetime Reproductive Effort Is Associated with Greater Oxidative Stress in Post-Menopausal Women. PLoS ONE 2016, 11, e0145753. [Google Scholar] [CrossRef] [Green Version]
- Gago-Dominguez, M. Role of Lipid Peroxidation in the Epidemiology and Prevention of Breast Cancer. Cancer Epidemiol. Biomark. Prev. 2005, 14, 2829–2839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mihu, D.; Sabău, L.; Costin, N.; Ciortea, R.; Măluţan, A.; Mihu, C.M. Implications of maternal systemic oxidative stress in normal pregnancy and in pregnancy complicated by preeclampsia. J. Matern. Neonatal. Med. 2011, 25, 944–951. [Google Scholar] [CrossRef]
- Speakman, J.; Garratt, M. Oxidative stress as a cost of reproduction: Beyond the simplistic trade-off model. BioEssays 2013, 36, 93–106. [Google Scholar] [CrossRef]
- Drummond, G.R.; Sobey, C.G. Endothelial NADPH oxidases: Which NOX to target in vascular disease? Trends Endocrinol. Metab. 2014, 25, 452–463. [Google Scholar] [CrossRef]
- Jana, S.K.; Babu, N.; Chattopadhyay, R.; Chakravarty, B.; Chaudhury, K.; Karuputhula, N. Upper control limit of reactive oxygen species in follicular fluid beyond which viable embryo formation is not favorable. Reprod. Toxicol. 2010, 29, 447–451. [Google Scholar] [CrossRef]
- Oyawoye, O.; Gadir, A.A.; Garner, A.; Constantinovici, N.; Perrett, C.; Hardiman, P. Antioxidants and reactive oxygen species in follicular fluid of women undergoing IVF: Relationship to outcome. Hum. Reprod. 2003, 18, 2270–2274. [Google Scholar] [CrossRef]
- Pasqualotto, E.B.; Lara, L.V.; Salvador, M.; Sobreiro, B.P.; Borges, E., Jr.; Pasqualotto, F.F. The role of enzymatic antioxidants detected in the follicular fluid and semen of infertile couples undergoing assisted reproduction. Hum. Fertil. 2009, 12, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Nishihara, T.; Matsumoto, K.; Hosoi, Y.; Morimoto, Y. Evaluation of antioxidant status and oxidative stress markers in follicular fluid for human in vitro fertilization outcome. Reprod. Med. Biol. 2018, 17, 481–486. [Google Scholar] [CrossRef] [Green Version]
- Majzoub, A.; Arafa, M.; Mahdi, M.; Agarwal, A.; Al Said, S.; Al-Emadi, I.; El Ansari, W.; Alattar, A.; Al Rumaihi, K.; ElBardisi, H. Oxidation-reduction potential and sperm DNA fragmentation, and their associations with sperm morphological anomalies amongst fertile and infertile men. Arab. J. Urol. 2018, 16, 87–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, T.; Kobori, Y.; Terai, K.; Inoue, Y.; Osaka, A.; Yoshikawa, N.; Shimomura, Y.; Suzuki, K.; Minami, T.; Iwahata, T.; et al. Seminal oxidation–reduction potential and sperm DNA fragmentation index increase among infertile men with varicocele. Hum. Fertil. 2020, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Arafa, M.; Chandrakumar, R.; Majzoub, A.; Alsaid, S.; ElBardisi, H. A multicenter study to evaluate oxidative stress by oxidation-reduction potential, a reliable and reproducible method. Androlology 2017, 5, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Micheli, L.; Collodel, G.; Cerretani, D.; Menchiari, A.; Noto, D.; Signorini, C.; Moretti, E. Relationships between Ghrelin and Obestatin with MDA, Proinflammatory Cytokines, GSH/GSSG Ratio, Catalase Activity, and Semen Parameters in Infertile Patients with Leukocytospermia and Varicocele. Oxidative Med. Cell. Longev. 2019, 2019, 7261842. [Google Scholar] [CrossRef] [PubMed]
- Moretti, E.; Micheli, L.; Noto, D.; Fiaschi, A.I.; Menchiari, A.; Cerretani, D. Resistin in Human Seminal Plasma: Relationship with Lipid Peroxidation, CAT Activity, GSH/GSSG Ratio, and Semen Parameters. Oxidative Med. Cell. Longev. 2019, 2019, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Otasevic, V.; Kalezic, A.; Macanovic, B.; Jankovic, A.; Stancic, A.; Garalejic, E.; Korac, A.; Korac, B. Evaluation of the antioxidative enzymes in the seminal plasma of infertile men: Contribution to classic semen quality analysis. Syst. Biol. Reprod. Med. 2019, 65, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Pizarro, B.M.; Cordeiro, A.; Reginatto, M.W.; Campos, S.P.C.; Mancebo, A.C.; Areas, P.C.F.; Antunes, R.A.; Souza, M.D.C.B.; Oliveira, K.J.; Bloise, F.F.; et al. Estradiol and progesterone levels are related to redox status in the follicular fluid during in vitro fertilization. J. Endocr. Soc. 2020, 4, 1–18. [Google Scholar] [CrossRef]
- Lan, K.-C.; Lin, Y.-C.; Chang, Y.-C.; Lin, H.-J.; Tsai, Y.-R.; Kang, H.-Y. Limited relationships between reactive oxygen species levels in culture media and zygote and embryo development. J. Assist. Reprod. Genet. 2018, 36, 325–334. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, E.M.; Giorgi, V.S.I.; Rodrigues, J.K.; De Andrade, A.Z.; Junior, A.A.J.; Navarro, P.A.D.A.S. Systemic oxidative stress as a possible mechanism underlying the pathogenesis of mild endometriosis-related infertility. Reprod. Biomed. Online 2019, 39, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Becatti, M.; Fucci, R.; Mannucci, A.; Barygina, V.; Pozzebon, A.; Criscuoli, L.; Giachini, C.; Bertocci, F.; Picone, R.; Emmi, G.; et al. A Biochemical Approach to Detect Oxidative Stress in Infertile Women Undergoing Assisted Reproductive Technology Procedures. Int. J. Mol. Sci. 2018, 19, 592. [Google Scholar] [CrossRef] [Green Version]
- Littarru, G.P.; Tiano, L. Clinical aspects of coenzyme Q10: An update. Nutrients 2010, 26, 250–254. [Google Scholar] [CrossRef]
- Balercia, G.; Buldreghini, E.; Vignini, A.; Tiano, L.; Paggi, F.; Amoroso, S.; LaMonica, G.R.; Boscaro, M.; Lenzi, A.; Littarru, G. Coenzyme Q10 treatment in infertile men with idiopathic asthenozoospermia: A placebo-controlled, double-blind randomized trial. Fertil. Steril. 2009, 91, 1785–1792. [Google Scholar] [CrossRef] [PubMed]
- Tirabassi, G.; Vignini, A.; Tiano, L.; Buldreghini, E.; Brugè, F.; Silvestri, S.; Orlando, P.; D’Aniello, A.; Mazzanti, L.; Lenzi, A.; et al. Protective effects of coenzyme Q10 and aspartic acid on oxidative stress and DNA damage in subjects affected by idiopathic asthenozoospermia. Endocrinology 2014, 49, 549–552. [Google Scholar] [CrossRef]
- Ben-Meir, A.; Burstein, E.; Borrego-Alvarez, A.; Chong, J.; Wong, E.; Yavorska, T.; Naranian, T.; Chi, M.; Wang, Y.; Bentov, Y.; et al. Coenzyme Q10 restores oocyte mitochondrial function and fertility during reproductive aging. Aging Cell 2015, 14, 887–895. [Google Scholar] [CrossRef]
- Marei, W.F.; Bosch, L.V.D.; Pintelon, I.; Mohey-Elsaeed, O.; Bols, P.E.J.; Leroy, J.L.M.R. Mitochondria-targeted therapy rescues development and quality of embryos derived from oocytes matured under oxidative stress conditions: A bovine in vitro model. Hum. Reprod. 2019, 34, 1984–1998. [Google Scholar] [CrossRef]
- Escribano-Lopez, I.; Diaz-Morales, N.; Rovira-Llopis, S.; De Marañon, A.M.; Orden, S.; Alvarez, A.; Bañuls, C.; Rocha, M.; Murphy, M.P.; Hernandez-Mijares, A.; et al. The mitochondria-targeted antioxidant MitoQ modulates oxidative stress, inflammation and leukocyte-endothelium interactions in leukocytes isolated from type 2 diabetic patients. Redox Biol. 2016, 10, 200–205. [Google Scholar] [CrossRef] [Green Version]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol Improves Mitochondrial Function and Protects against Metabolic Disease by Activating SIRT1 and PGC-1α. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef]
- Espino, J.; Bejarano, I.; Ortiz, Á; Lozano, G.M.; Garcia, J.F.; Pariente, J.A.; Rodríguez, A.B. Melatonin as a potential tool against oxidative damage and apoptosis in ejaculated human spermatozoa. Fertil. Steril. 2010, 94, 1915–1917. [Google Scholar] [CrossRef]
- Cheuqueman, C.; Arias, M.E.; Risopatrón, J.; Felmer, R.; Alvarez, J.; Mogas, T.; Sánchez, R. Supplementation of IVF medium with melatonin: Effect on sperm functionality andin vitroproduced bovine embryos. Andrology 2014, 47, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Li, X.X.; Yang, X.G.; Lu, Y.Q.; Lu, S.S.; Zhang, M.; Yao, H.L.; Meng, L.J.; Lu, K.H. Protective Effects of Melatonin against Oxidative Stress in Flow Cytometry-sorted Buffalo Sperm. Reprod. Domest. Anim. 2011, 47, 299–307. [Google Scholar] [CrossRef] [PubMed]
| Cell Type | ASSAY | Method | Results | Ref. |
|---|---|---|---|---|
| Sperm | ORP | MiOXSYS | Higher values in infertile men | [116] |
| SDF | Halosperm | [117] | ||
| Sperm | ORP | MiOXSYS | Higher values in infertile men | [118] |
| Sperm | GSH/GSSG | enzymatic | Altered in varicocele and leukocytospermia | [119] |
| Catalase | [120] | |||
| Sperm | GSH-Px | enzymatic | Correlate with sperm parameters | [121] |
| Follicular fluid | HPSC | Levels of estradiol and progesterone are related to the redox status | [122] | |
| Embryo | General Redox species | luminol | No association presence of ROS in culture media with embryo quality | [123] |
| Serum samples endometriosis | GSH/GSSG MDA TAC 8OHdG | Oxidative stress markers were good predictors of clinical pregnancy and live births after ICSI in women with stage I or II endometriosis. | [124] | |
| Blood and Follicular fluid | ROS TAC | Flow cytometry Fluorometric assay | Oxidative stress markers were good predictors of ART outcomes. | [125] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almansa-Ordonez, A.; Bellido, R.; Vassena, R.; Barragan, M.; Zambelli, F. Oxidative Stress in Reproduction: A Mitochondrial Perspective. Biology 2020, 9, 269. https://doi.org/10.3390/biology9090269
Almansa-Ordonez A, Bellido R, Vassena R, Barragan M, Zambelli F. Oxidative Stress in Reproduction: A Mitochondrial Perspective. Biology. 2020; 9(9):269. https://doi.org/10.3390/biology9090269
Chicago/Turabian StyleAlmansa-Ordonez, Alexandra, Raquel Bellido, Rita Vassena, Montserrat Barragan, and Filippo Zambelli. 2020. "Oxidative Stress in Reproduction: A Mitochondrial Perspective" Biology 9, no. 9: 269. https://doi.org/10.3390/biology9090269





