The Adverse Impact of Pregestational Prediabetes Contributes to HELLP Syndrome Development
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Drugs and Chemicals
2.2. Animals
Experimental Design
2.3. Induction of PD
2.3.1. Criteria for PD
2.3.2. Experimental Diet
2.3.3. Diet Composition
2.4. Mating
2.5. Induction of PE
2.6. Plasma and Tissue Collection
2.7. Biochemical Tests
2.7.1. Lipid Profile
2.7.2. Antioxidant Profile
2.7.3. Lipid Peroxidation
2.7.4. Liver Inflammatory Markers
2.7.5. Liver Enzymes
2.7.6. Hematological Analysis
2.8. Statistical Analysis
3. Results
3.1. Liver Weight and Relative Liver Weights
3.2. Non-Fasting Blood Glucose
3.3. Liver Triglyceride (TG) Concentration
3.4. Oxidative Stress Marker
3.5. Inflammatory Markers (IL-6 and TNFα)
3.6. Plasma Liver Enzymes: Albumin and Bilirubin Concentrations
3.7. Hematological Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2015 Maternal Mortality Collaborators. Global, regional, and national levels of maternal mortality, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1775–1812. [Google Scholar] [CrossRef]
- Boucheron, P.; Lailler, G.; Moutengou, E.; Regnault, N.; Gabet, A.; Deneux-Tharaux, C.; Kretz, S.; Grave, C.; Mounier-Vehier, C.; Tsatsaris, V.; et al. Hypertensive disorders of pregnancy and onset of chronic hypertension in France: The nationwide CONCEPTION study. Eur. Heart J. 2022, 43, 3352–3361. [Google Scholar] [CrossRef] [PubMed]
- Ludidi, A.; Siboto, A.; Nkosi, A.; Xulu, N.D.; Khathi, A.; Sibiya, N.H.; Ngubane, P.S. High-fat-, high-carb-diet-induced prediabetes preconception in Sprague-Dawley rats as a risk factor for the development of preeclampsia: Assessing changes in placental metabolic insults. Front. Nutr. 2023, 10, 1241785. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Tang, K.; Magee, L.A.; von Dadelszen, P.; Ekeroma, A.; Li, X.; Zhang, E.; Bhutta, Z.A. A global view of hypertensive disorders and diabetes mellitus during pregnancy. Nat. Rev. Endocrinol. 2022, 18, 760–775. [Google Scholar] [CrossRef] [PubMed]
- Poon, L.C.; Nguyen-Hoang, L.; Smith, G.N.; Bergman, L.; O’BRien, P.; Hod, M.; Okong, P.; Kapur, A.; Maxwell, C.V.; McIntyre, H.D.; et al. Hypertensive disorders of pregnancy and long-term cardiovascular health: FIGO Best Practice Advice. Int. J. Gynecol. Obstet. 2023, 160, 22–34. [Google Scholar] [CrossRef]
- Magee, L.A.; Nicolaides, K.H.; Von Dadelszen, P. Preeclampsia. N. Engl. J. Med. 2022, 386, 1817–1832. [Google Scholar] [CrossRef]
- Van Lieshout, L.; Koek, G.; Spaanderman, M.; Heimel, P.v.R. Placenta derived factors involved in the pathogenesis of the liver in the syndrome of haemolysis, elevated liver enzymes and low platelets (HELLP): A review. Pregnancy Hypertens. 2019, 18, 42–48. [Google Scholar] [CrossRef]
- Gardikioti, A.; Venou, T.-M.; Gavriilaki, E.; Vetsiou, E.; Mavrikou, I.; Dinas, K.; Daniilidis, A.; Vlachaki, E. Molecular advances in preeclampsia and HELLP syndrome. Int. J. Mol. Sci. 2022, 23, 3851. [Google Scholar] [CrossRef]
- Alese, M.O.; Moodley, J.; Naicker, T. Preeclampsia and HELLP syndrome, the role of the liver. J. Matern.-Fetal Neonatal Med. 2021, 34, 117–123. [Google Scholar] [CrossRef]
- Siboto, A.; Akinnuga, A.M.; Ismail, M.B.; Booysen, I.N.; Sibiya, N.H.; Ngubane, P.; Khathi, A. Investigating the Protective Effects of a Rhenium (V) Compound with Uracil-Derived Ligands on Liver Damage Associated with Prediabetes in Diet-Induced Prediabetic Rats. Diabetology 2022, 3, 524–538. [Google Scholar] [CrossRef]
- Luvuno, M.; Mabandla, M.; Khathi, A. Voluntary ingestion of a high-fat high-carbohydrate diet: A model for prediabetes. Ponte Int. Sci. Res. J. 2018, 74. [Google Scholar] [CrossRef]
- Jackson, C.M.; Alexander, B.T.; Roach, L.; Haggerty, D.; Marbury, D.C.; Hutchens, Z.M.; Flynn, E.R.; Maric-Bilkan, C. Exposure to maternal overnutrition and a high-fat diet during early postnatal development increases susceptibility to renal and metabolic injury later in life. Am. J. Physiol.-Ren. Physiol. 2012, 302, F774–F783. [Google Scholar] [CrossRef]
- Qulu, L.; Daniels, W.M.; Mabandla, M.V. Exposure to prenatal stress has deleterious effects on hippocampal function in a febrile seizure rat model. Brain Res. 2015, 1624, 506–514. [Google Scholar] [CrossRef]
- Soobryan, N.; Murugesan, S.; Phoswa, W.; Gathiram, P.; Moodley, J.; Mackraj, I. The effects of sildenafil citrate on uterine angiogenic status and serum inflammatory markers in an L-NAME rat model of pre-eclampsia. Eur. J. Pharmacol. 2017, 795, 101–107. [Google Scholar] [CrossRef]
- Gamede, M.; Mabuza, L.; Ngubane, P.; Khathi, A. Preventing the onset of diabetes-induced chronic kidney disease during prediabetes: The effects of oleanolic acid on selected markers of chronic kidney disease in a diet-induced prediabetic rat model. Biomed. Pharmacother. 2021, 139, 111570. [Google Scholar] [CrossRef]
- Rimaitis, K.; Grauslyte, L.; Zavackiene, A.; Baliuliene, V.; Nadisauskiene, R.; Macas, A. Diagnosis of HELLP syndrome: A 10-year survey in a perinatology centre. Int. J. Environ. Res. Public Health 2019, 16, 109. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.; Ersan, F.; Ark, C.; Aydın, Ç.A. Partial HELLP syndrome: Maternal, perinatal, subsequent pregnancy and long-term maternal outcomes. J. Obstet. Gynaecol. Res. 2014, 40, 932–940. [Google Scholar] [CrossRef]
- Clinck, I.; Verelst Faro, R.; Vrijkotte, T.G.M.; Bartholomeus, T.T. Early pregnancy triglycerides and not fructosamine are associated with birth weight (with foetal sexual dimorphism). Eur. J. Clin. Investig. 2022, 53, e13896. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hamid, T.A.; AbdelLatif, D.; Ahmed, E.; Abdel-Rasheed, M.; A-Mageed, A. Relation between maternal and neonatal serum lipid profile and their impact on birth weight. Am. J. Perinatol. 2020, 39, 1112–1116. [Google Scholar] [CrossRef]
- Chen, K.-Y.; Lin, S.-Y.; Lee, C.-N.; Wu, H.-T.; Kuo, C.-H.; Kuo, H.-C.; Chuang, C.-C.; Kuo, C.-H.; Chen, S.-C.; Fan, K.-C.; et al. Maternal plasma lipids during pregnancy, insulin-like growth factor-1, and excess fetal growth. J. Clin. Endocrinol. Metab. 2021, 106, e3461–e3472. [Google Scholar] [CrossRef] [PubMed]
- Mocarzel, C.C.; Velarde, G.C.; Antunes, R.d.A.; de Sá, R.A.M.; Kurjak, A. Maternal obesity influences the endocrine cord blood profile of their offspring. J. Perinat. Med. 2020, 48, 242–248. [Google Scholar] [CrossRef]
- Haggai, M.C.; Sgayer, I.; Bornstein, J.; Odeh, M.; Lowenstein, L.; Wolf, M.F. Liver stiffness and steatosis in preeclampsia as shown by transient elastography–a prospective cohort study. Am. J. Obstet. Gynecol. 2022, 227, 515.e1–515.e9. [Google Scholar] [CrossRef]
- Gamede, M.; Mabuza, L.; Ngubane, P.; Khathi, A. Plant-derived oleanolic acid ameliorates markers associated with non-alcoholic fatty liver disease in a diet-induced pre-diabetes rat model. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, Ume 12, 1953–1962. [Google Scholar] [CrossRef]
- Hershman, M.; Mei, R.; Kushner, T. Implications of nonalcoholic fatty liver disease on pregnancy and maternal and child outcomes. Gastroenterol. Hepatol. 2019, 15, 221. [Google Scholar]
- Sarkar, M.; Grab, J.; Dodge, J.L.; Gunderson, E.P.; Rubin, J.; Irani, R.A.; Cedars, M.; Terrault, N. Non-alcoholic fatty liver disease in pregnancy is associated with adverse maternal and perinatal outcomes. J. Hepatol. 2020, 73, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.K.; Yates, E.; Lilly, K.; Dhanda, A.D. Oxidative stress in alcohol-related liver disease. World J. Hepatol. 2020, 12, 332. [Google Scholar] [CrossRef] [PubMed]
- Arroyave-Ospina, J.C.; Wu, Z.; Geng, Y.; Moshage, H. Role of oxidative stress in the pathogenesis of non-alcoholic fatty liver disease: Implications for prevention and therapy. Antioxidants 2021, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- Farzanegi, P.; Dana, A.; Ebrahimpoor, Z.; Asadi, M.; Azarbayjani, M.A. Mechanisms of beneficial effects of exercise training on non-alcoholic fatty liver disease (NAFLD): Roles of oxidative stress and inflammation. Eur. J. Sport Sci. 2019, 19, 994–1003. [Google Scholar] [CrossRef]
- Wang, M.; Li, L.; Xu, Y.; Du, J.; Ling, C. Roles of hepatic stellate cells in NAFLD: From the perspective of inflammation and fibrosis. Front. Pharmacol. 2022, 13, 958428. [Google Scholar] [CrossRef]
- Ziolkowska, S.; Binienda, A.; Jabłkowski, M.; Szemraj, J.; Czarny, P. The interplay between insulin resistance, inflammation, oxidative stress, base excision repair and metabolic syndrome in nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2021, 22, 11128. [Google Scholar] [CrossRef]
- Utzschneider, K.M.; Kahn, S.E.; Polidori, D.C. Hepatic insulin extraction in NAFLD is related to insulin resistance rather than liver fat content. J. Clin. Endocrinol. Metab. 2019, 104, 1855–1865. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Kim, W.R.; Poterucha, J.J. Evaluation of elevated liver enzymes. Clin. Liver Dis. 2012, 16, 183–198. [Google Scholar] [CrossRef]
- Brady, C.W. Liver disease in pregnancy: What’s new. Hepatol. Commun. 2020, 4, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Perez Botero, J.; Reese, J.A.; George, J.N.; McIntosh, J.J. Severe thrombocytopenia and microangiopathic hemolytic anemia in pregnancy: A guide for the consulting hematologist. Am. J. Hematol. 2021, 96, 1655–1665. [Google Scholar] [CrossRef] [PubMed]
- Ang, S.X.; Chen, C.-P.; Sun, F.-J.; Chen, C.-Y. Comparison of maternal and neonatal outcomes between acute fatty liver of pregnancy and hemolysis, elevated liver enzymes and low platelets syndrome: A retrospective cohort study. BMC Pregnancy Childbirth 2021, 21, 293. [Google Scholar] [CrossRef]
- Oe, Y.; Ko, M.; Fushima, T.; Sato, E.; Karumanchi, S.A.; Sato, H.; Sugawara, J.; Ito, S.; Takahashi, N. Hepatic dysfunction and thrombocytopenia induced by excess sFlt1 in mice lacking endothelial nitric oxide synthase. Sci. Rep. 2018, 8, 102. [Google Scholar] [CrossRef]
- Pernow, J.; Mahdi, A.; Yang, J.; Zhou, Z. Red blood cell dysfunction: A new player in cardiovascular disease. Cardiovasc. Res. 2019, 115, 1596–1605. [Google Scholar] [CrossRef]
- Randeria, S.N.; Thomson, G.J.A.; Nell, T.A.; Roberts, T.; Pretorius, E. Inflammatory cytokines in type 2 diabetes mellitus as facilitators of hypercoagulation and abnormal clot formation. Cardiovasc. Diabetol. 2019, 18, 1–15. [Google Scholar] [CrossRef]
- Nada, A.M. Red cell distribution width in type 2 diabetic patients. Diabetes Metab. Syndr. Obes. Targets Ther. 2015, 8, 525–533. [Google Scholar] [CrossRef]
- Mzimela, N.; Ngubane, P.; Khathi, A. Research Article The Haemolytic Changes During Progression of Pre-Diabetes to Type 2 Diabetes in a High-Fat High-Carbohydrate Diet-Induced Pre-Diabetic Rat Model. Pak. J. Nutr. 2021, 20, 55–63. [Google Scholar] [CrossRef]

 p ˂ 0.05 by comparison with normal pregnant group. α p < 0 05 by comparison to pregnant preeclamptic group.
p ˂ 0.05 by comparison with normal pregnant group. α p < 0 05 by comparison to pregnant preeclamptic group.
 p ˂ 0.05 by comparison with normal pregnant group. α p < 0 05 by comparison to pregnant preeclamptic group.
p ˂ 0.05 by comparison with normal pregnant group. α p < 0 05 by comparison to pregnant preeclamptic group.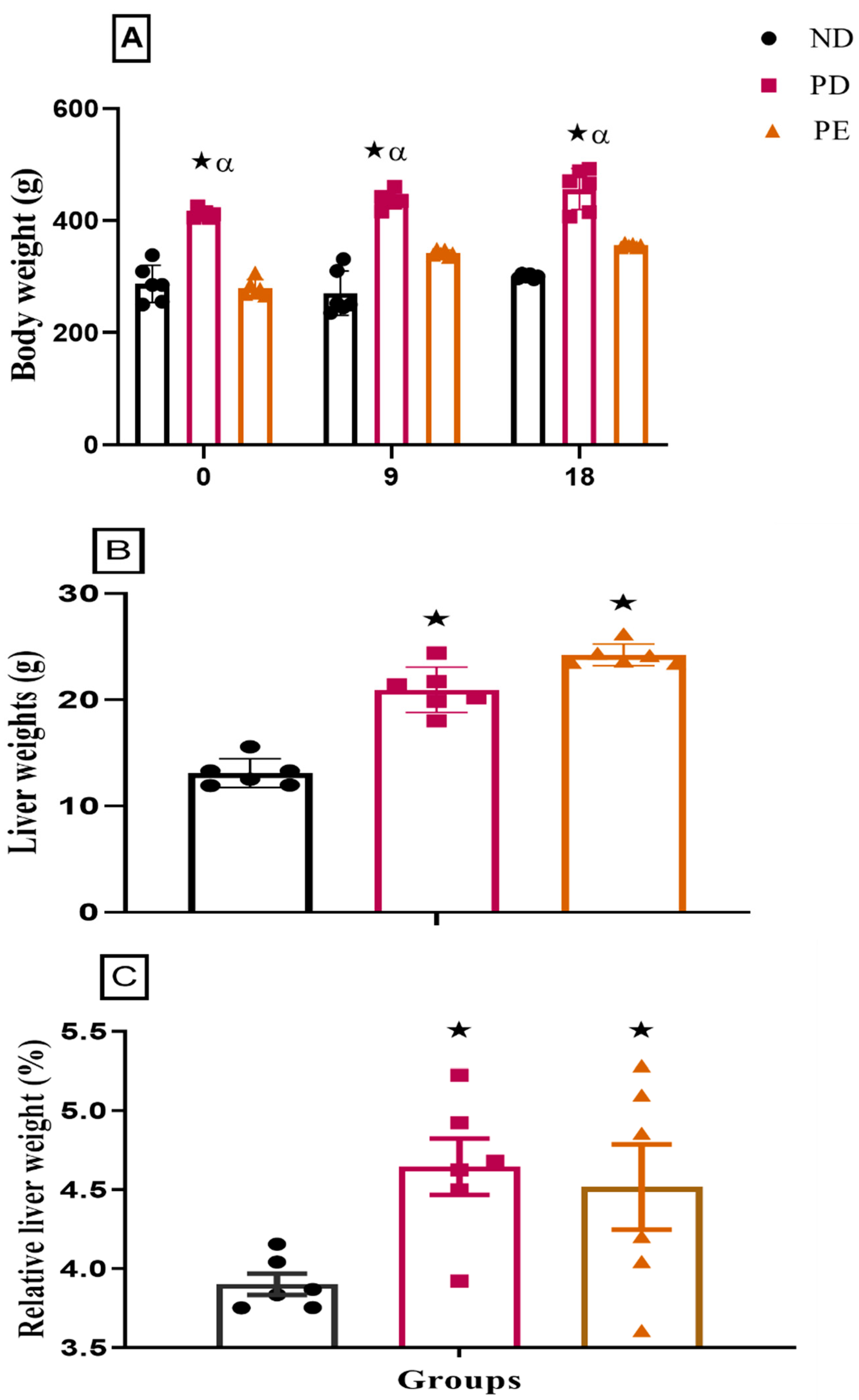
 p ˂ 0.05 by comparison with normal pregnant group. α p < 0 05 by comparison to pregnant preeclamptic group.
p ˂ 0.05 by comparison with normal pregnant group. α p < 0 05 by comparison to pregnant preeclamptic group.
 p ˂ 0.05 by comparison with normal pregnant group. α p < 0 05 by comparison to pregnant preeclamptic group.
p ˂ 0.05 by comparison with normal pregnant group. α p < 0 05 by comparison to pregnant preeclamptic group.
 p ˂ 0.05 by comparison with normal pregnant group.
p ˂ 0.05 by comparison with normal pregnant group.
 p ˂ 0.05 by comparison with normal pregnant group.
p ˂ 0.05 by comparison with normal pregnant group.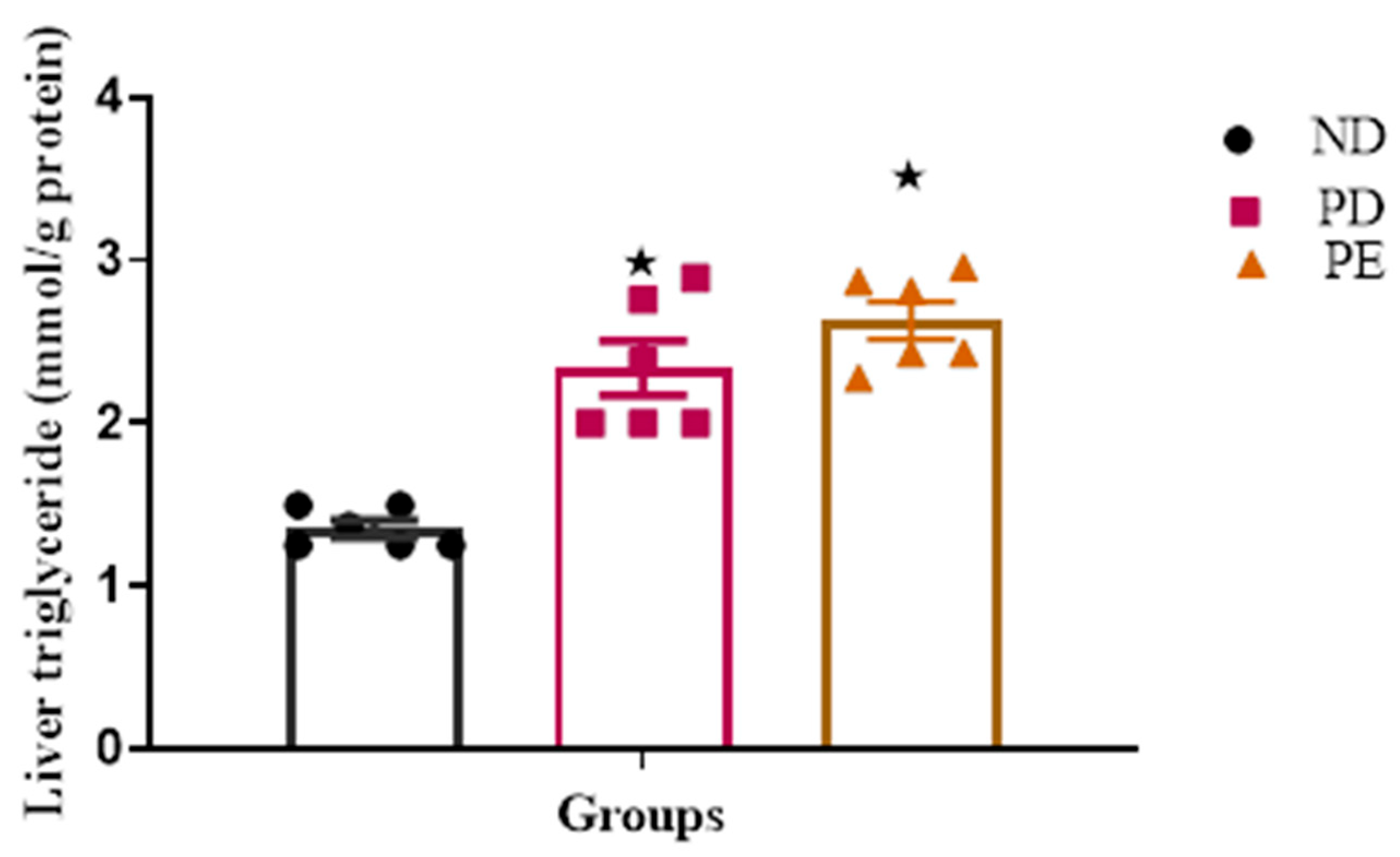
 p ˂ 0.05 by comparison with the normal pregnant group. α p < 0.05 by comparison to the pregnant preeclamptic group.
p ˂ 0.05 by comparison with the normal pregnant group. α p < 0.05 by comparison to the pregnant preeclamptic group.
 p ˂ 0.05 by comparison with the normal pregnant group. α p < 0.05 by comparison to the pregnant preeclamptic group.
p ˂ 0.05 by comparison with the normal pregnant group. α p < 0.05 by comparison to the pregnant preeclamptic group.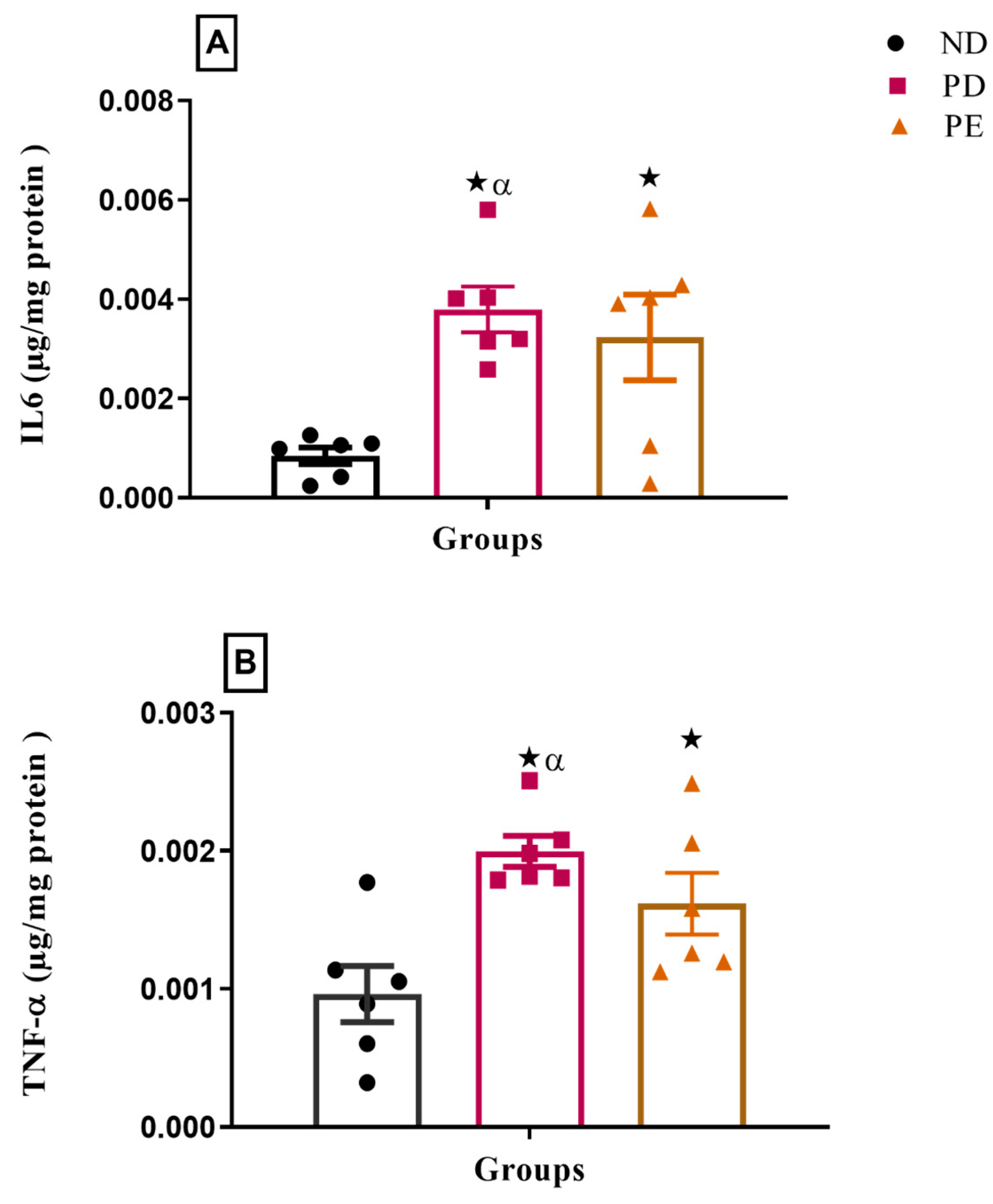
 p ˂ 0.05 by comparison with normal pregnant group.
p ˂ 0.05 by comparison with normal pregnant group.
 p ˂ 0.05 by comparison with normal pregnant group.
p ˂ 0.05 by comparison with normal pregnant group.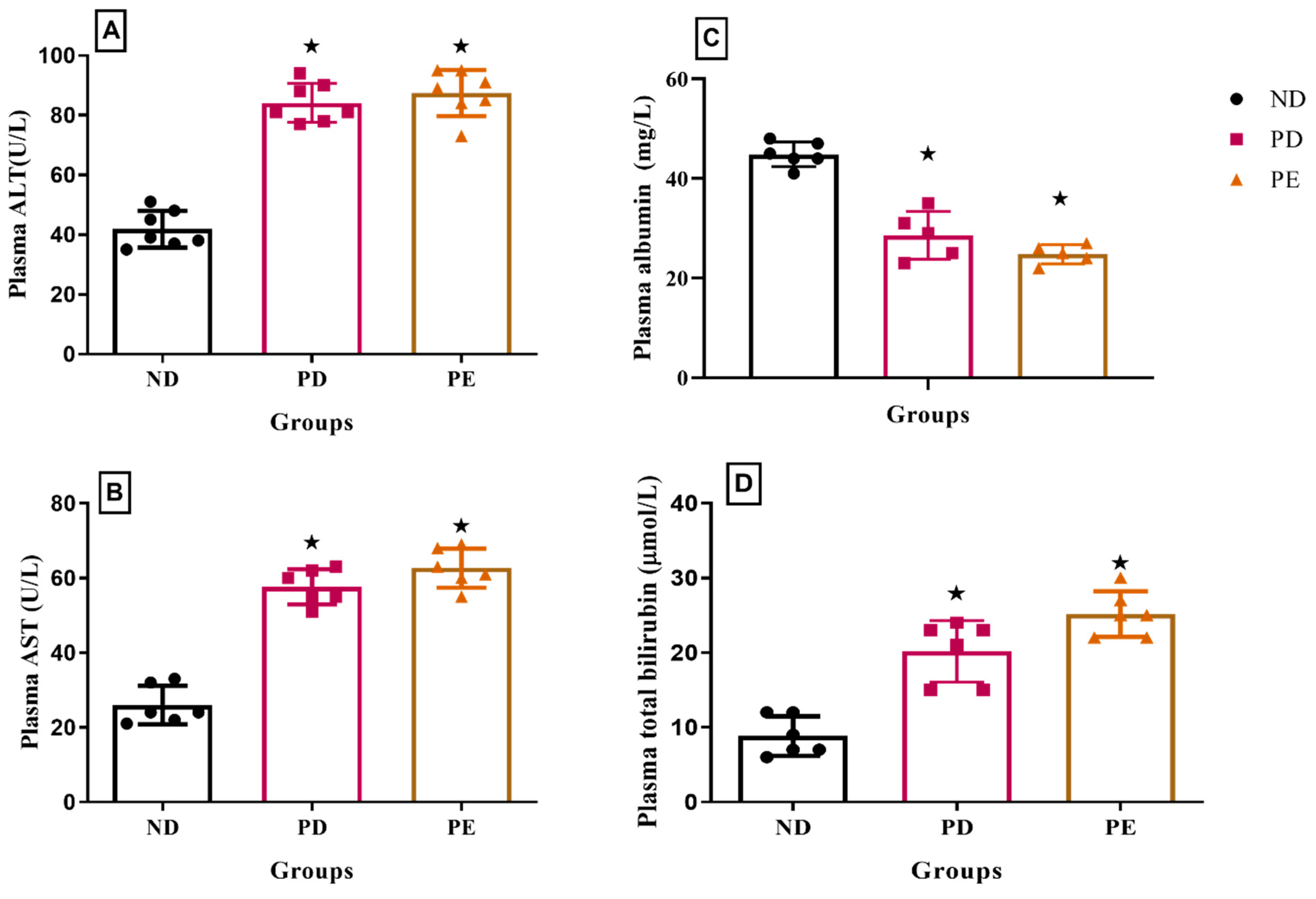
 p ˂ 0.05 by comparison with normal pregnant group. α p < 0.05 by comparison to pregnant preeclamptic group.
p ˂ 0.05 by comparison with normal pregnant group. α p < 0.05 by comparison to pregnant preeclamptic group.
 p ˂ 0.05 by comparison with normal pregnant group. α p < 0.05 by comparison to pregnant preeclamptic group.
p ˂ 0.05 by comparison with normal pregnant group. α p < 0.05 by comparison to pregnant preeclamptic group.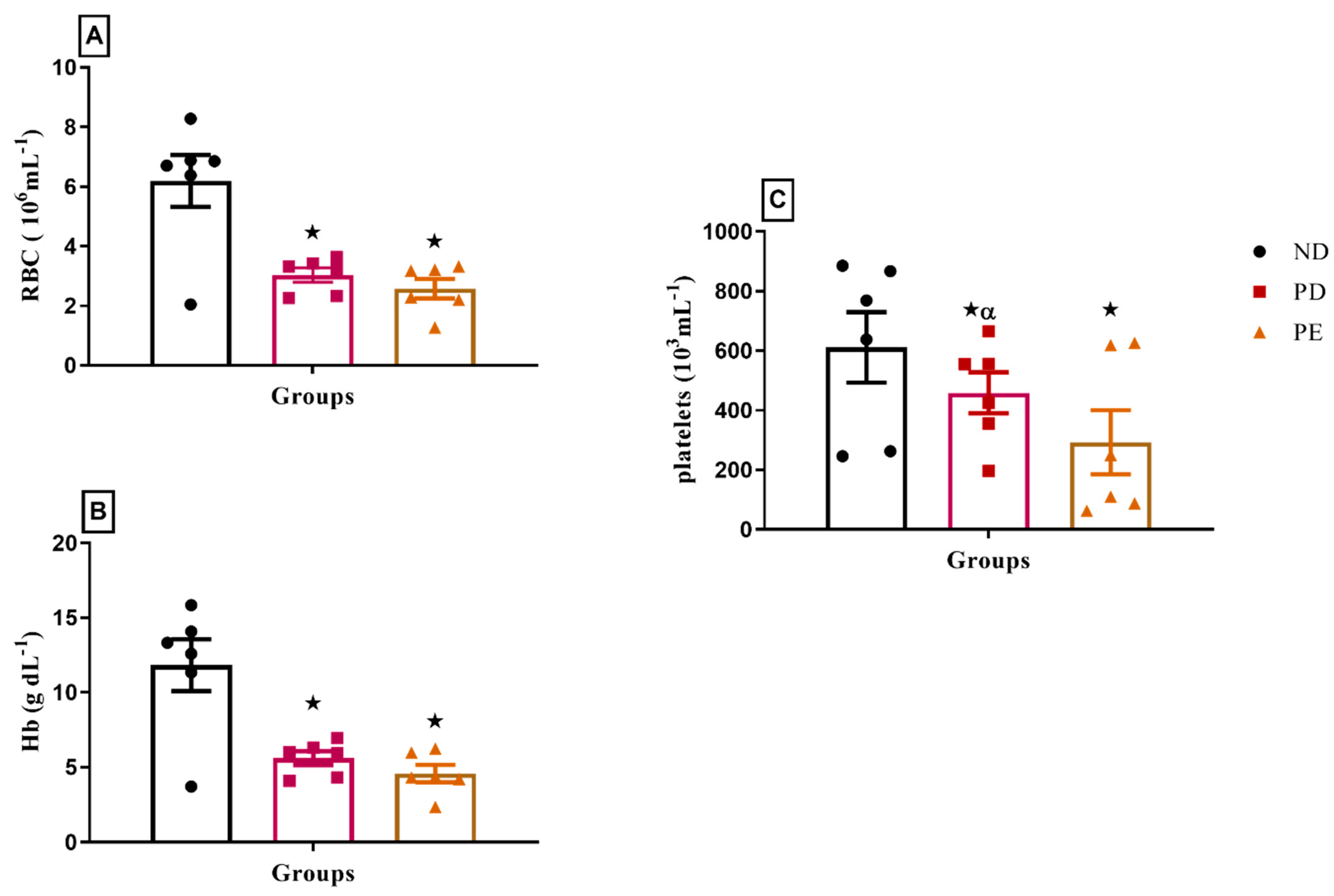
| Nutrient | Units | Actual | Nutrient | Units | Actual |
|---|---|---|---|---|---|
| Dry Matter | g/kg | 919.93 | Available Total Sulfur Amino Acids (ASTSAA) | g/kg | 6.79 |
| Metabolizable Energy | MJ/kg | 15.86 | Asvaline | g/kg | 5.80 |
| Crude Protein | g/kg | 151.27 | Fat | g/kg | 250.46 |
| Threonine | g/kg | 4.51 | Carbohydrate | g/kg | 427.29 |
| Isoleucine | g/kg | 5.24 | Fiber | g/kg | 22.08 |
| Lysine | g/kg | 6.54 | Ash | g/kg | 26.31 |
| Methionine | g/kg | 4.86 | Available Phosphorus (Avl-P) | g/kg | 1.66 |
| Tryptophan | g/kg | 1.30 | Calcium | g/kg | 5.47 |
| Astatine | g/kg | 3.30 | Total Phosphorus | g/kg | 3.60 |
| Groups | MDA (nmol/g Protein) | SOD (pg/mg Protein) |
|---|---|---|
| ND | 0.025 ± 0.001 | 2.077 ± 0.172 |
| PD | 0.053 ± 0.005 *α | 1.134 ± 0.231 *α |
| PE | 0.049 ± 0.004 * | 0.528 ± 0.144 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siboto, A.; Ludidi, A.; Xulu, N.; Nkosi, A.; Sibiya, N.; Khathi, A.; Ngubane, P.S. The Adverse Impact of Pregestational Prediabetes Contributes to HELLP Syndrome Development. Biology 2025, 14, 1707. https://doi.org/10.3390/biology14121707
Siboto A, Ludidi A, Xulu N, Nkosi A, Sibiya N, Khathi A, Ngubane PS. The Adverse Impact of Pregestational Prediabetes Contributes to HELLP Syndrome Development. Biology. 2025; 14(12):1707. https://doi.org/10.3390/biology14121707
Chicago/Turabian StyleSiboto, Anelisiwe, Asiphaphola Ludidi, Nombuso Xulu, Ayanda Nkosi, Ntethelelo Sibiya, Andile Khathi, and Phikelelani Siphosethu Ngubane. 2025. "The Adverse Impact of Pregestational Prediabetes Contributes to HELLP Syndrome Development" Biology 14, no. 12: 1707. https://doi.org/10.3390/biology14121707
APA StyleSiboto, A., Ludidi, A., Xulu, N., Nkosi, A., Sibiya, N., Khathi, A., & Ngubane, P. S. (2025). The Adverse Impact of Pregestational Prediabetes Contributes to HELLP Syndrome Development. Biology, 14(12), 1707. https://doi.org/10.3390/biology14121707





