CVD-Grown CNTs on Basalt Fiber Surfaces for Multifunctional Composite Interphases
Abstract
:1. Introduction
- (i)
- basalt fibers with different chemical composition,
- (ii)
- alkali attacked basalt fibers, and
- (iii)
- sized basalt fibers
2. Materials and Methods
- CVD01: includes treatment from stage I to VI at Ti = 800 °C
- CVD02: includes treatment from stage I to VI, but without stage IV
- CVD03: includes treatment from stage I to VI, at Ti = 700 °C
3. Results
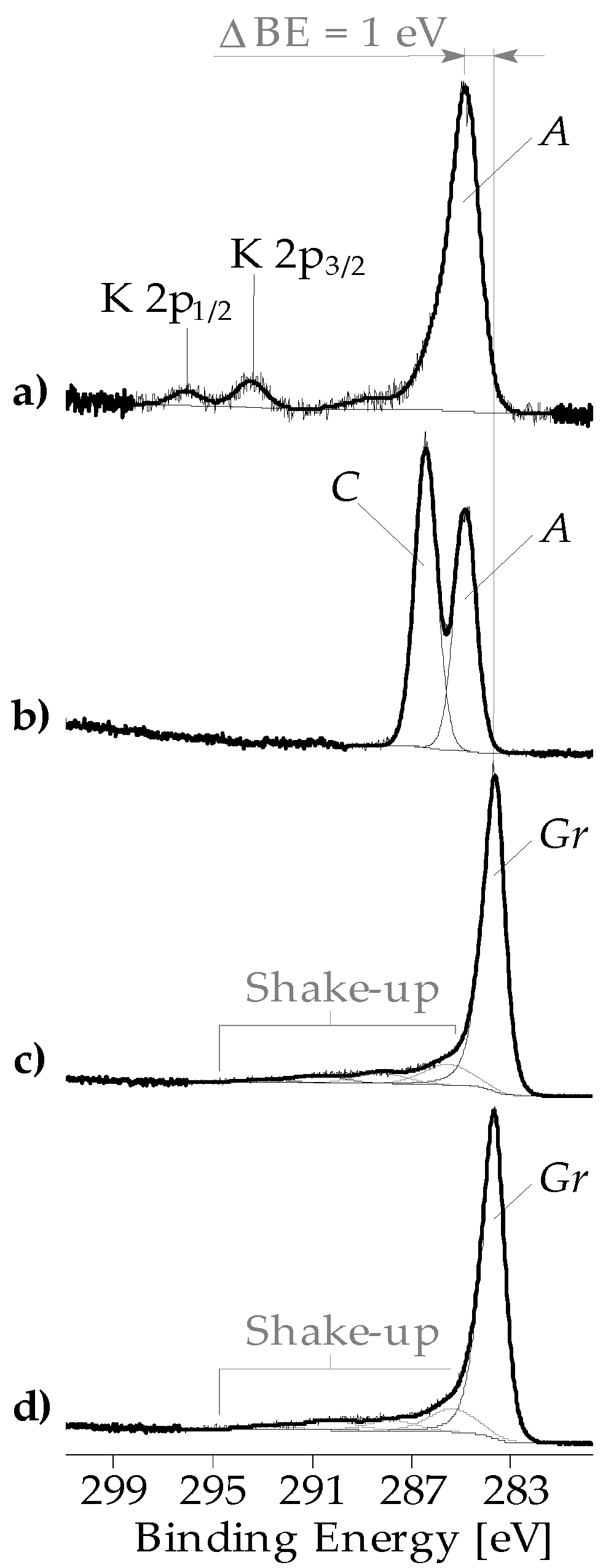
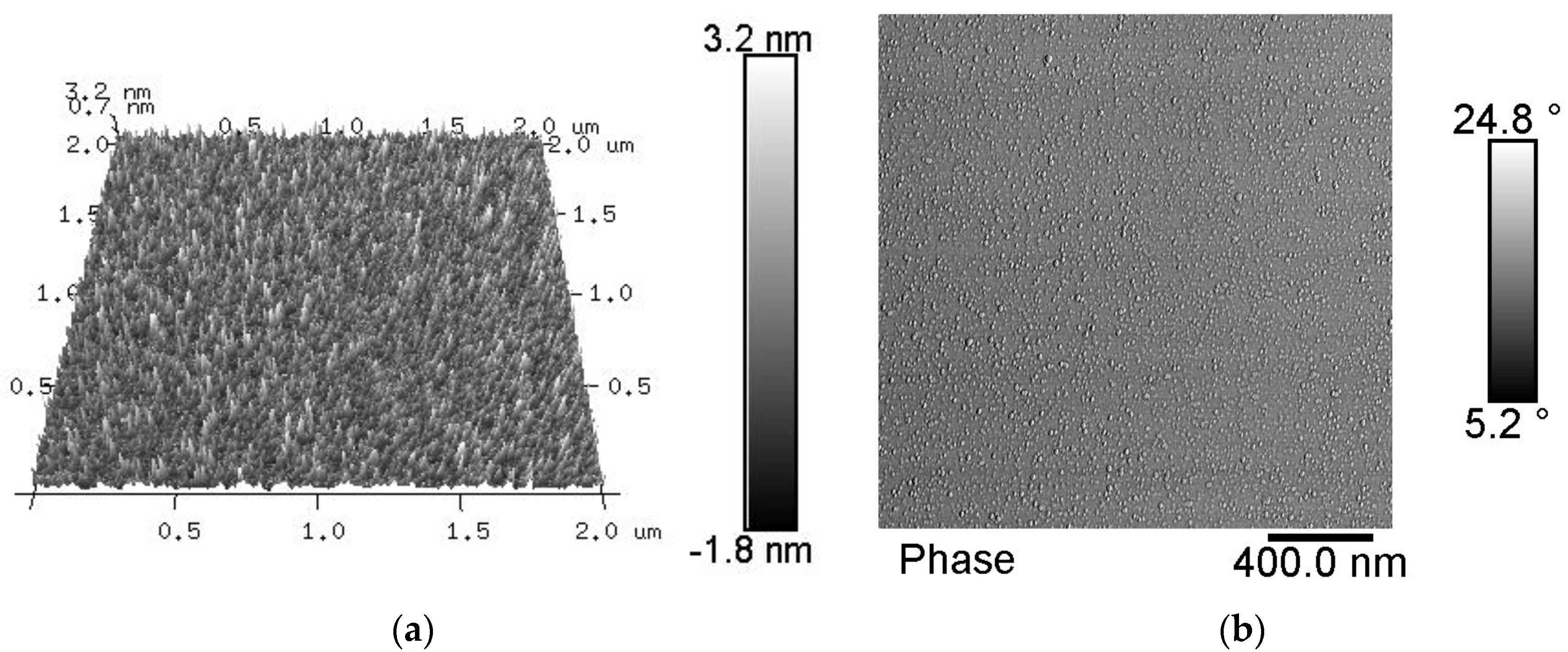
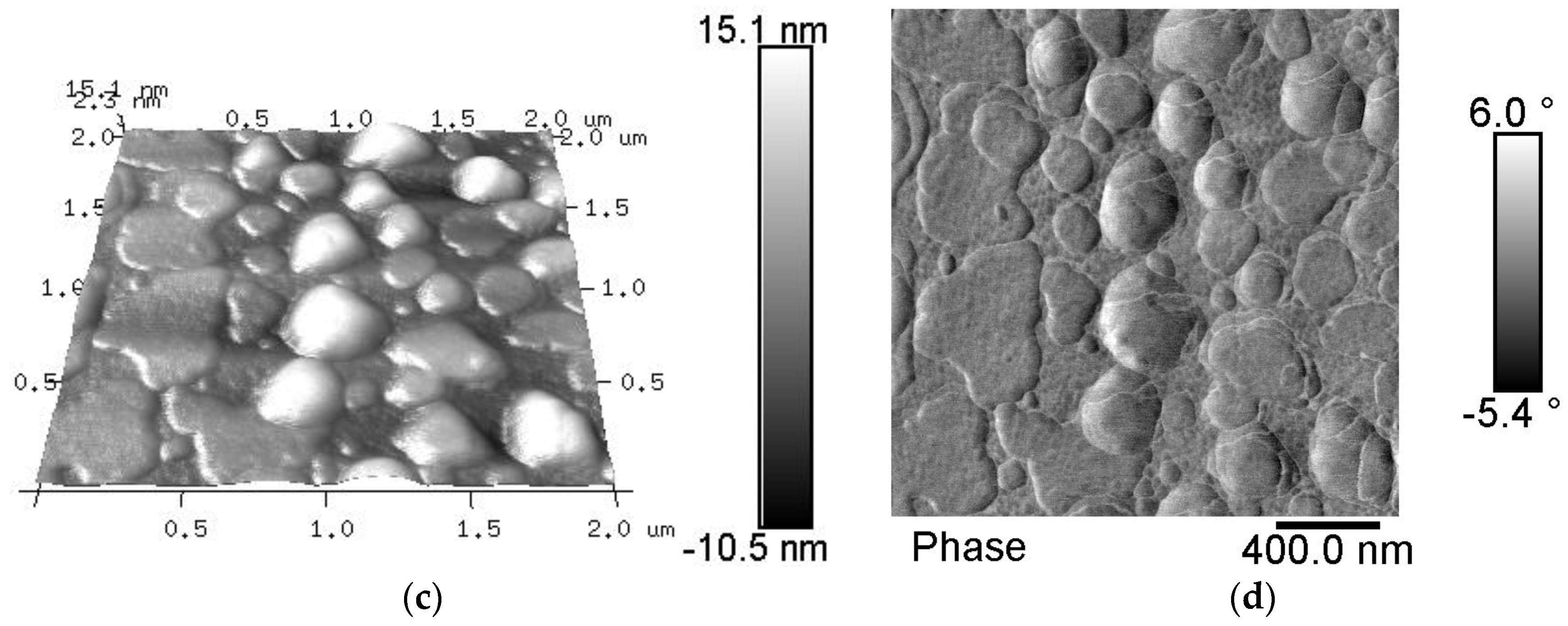
3.1. Chemical Composition of the Fiber Surfaces Before and After CVD Treatment
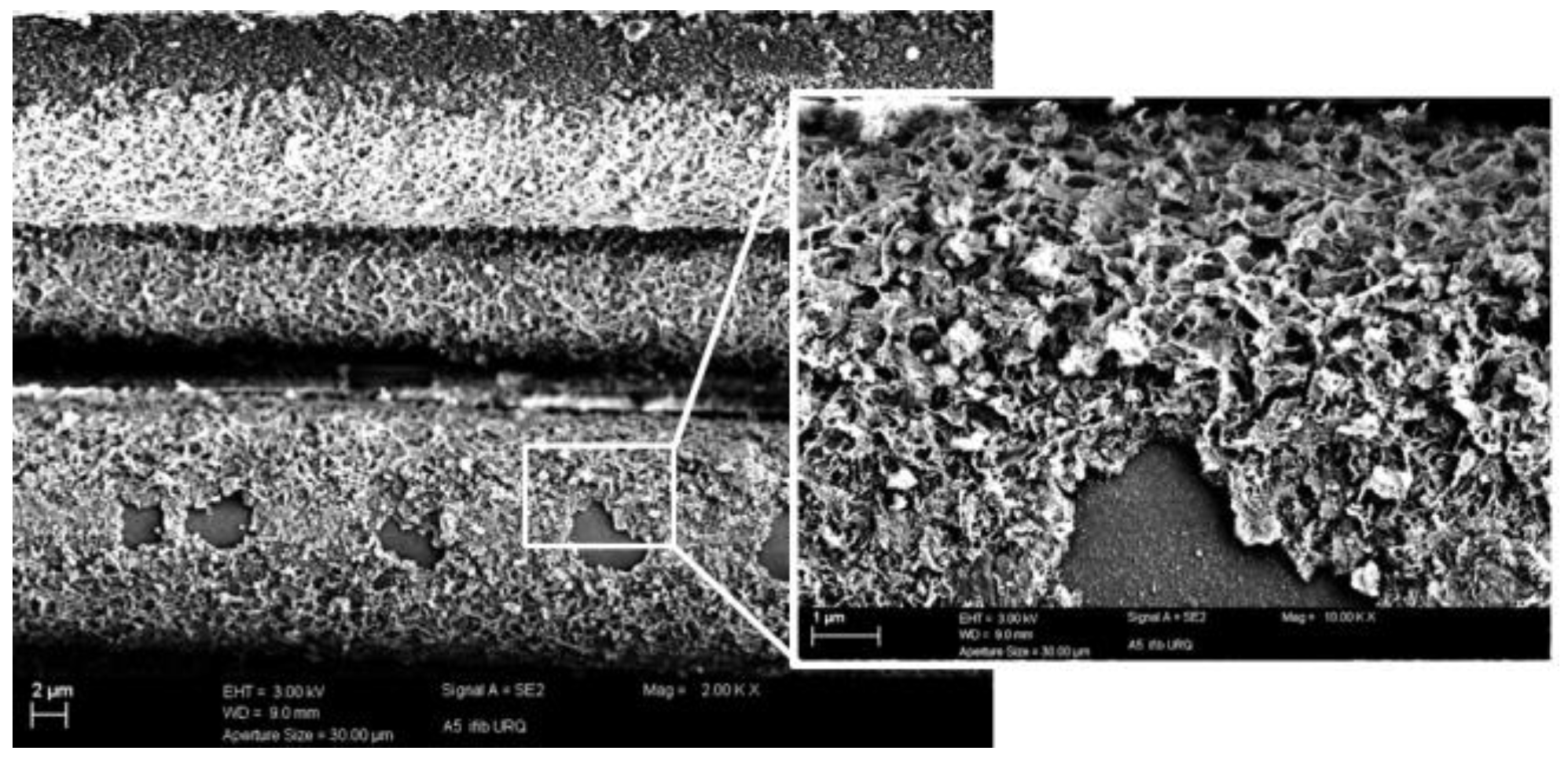
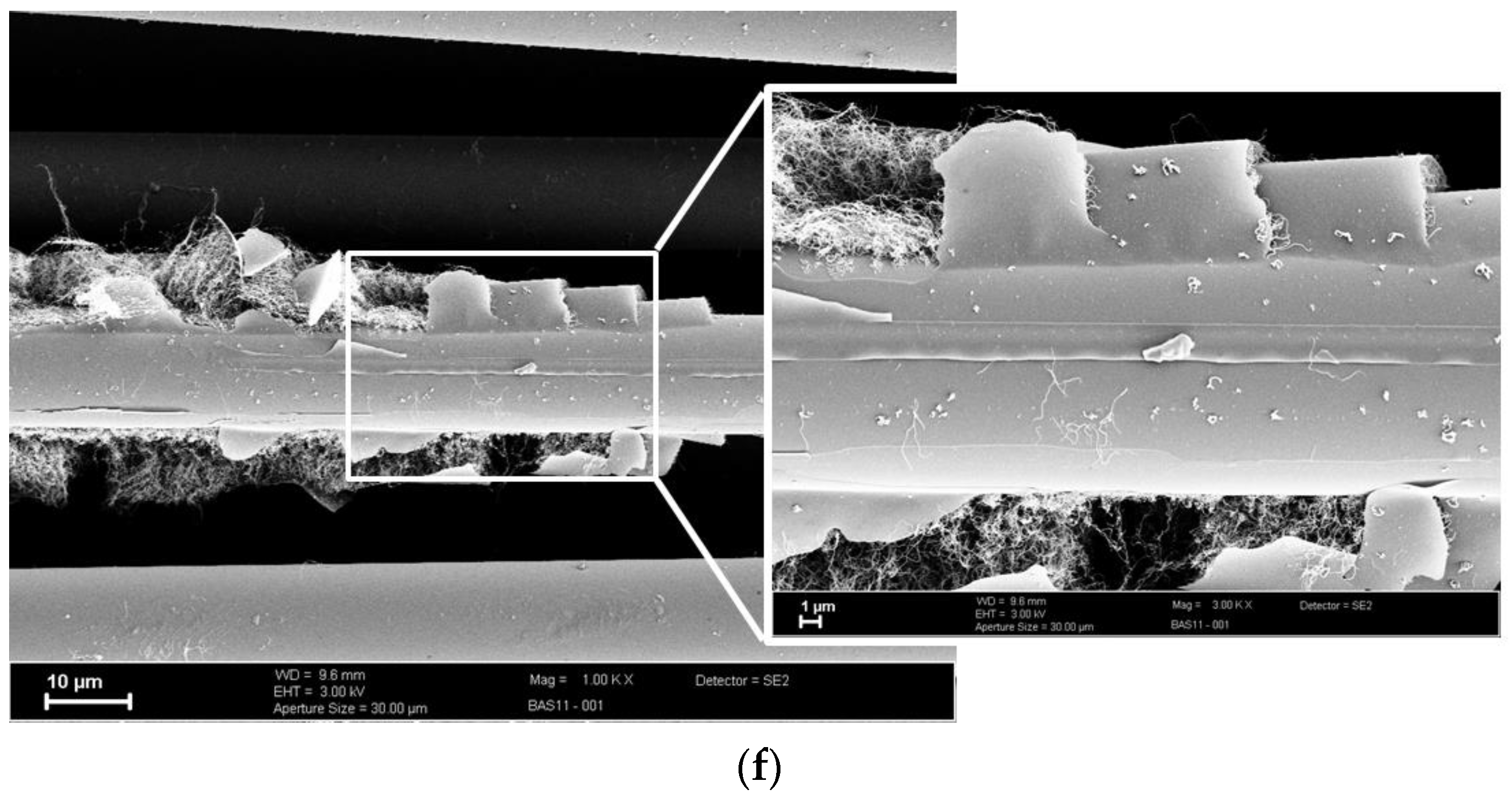
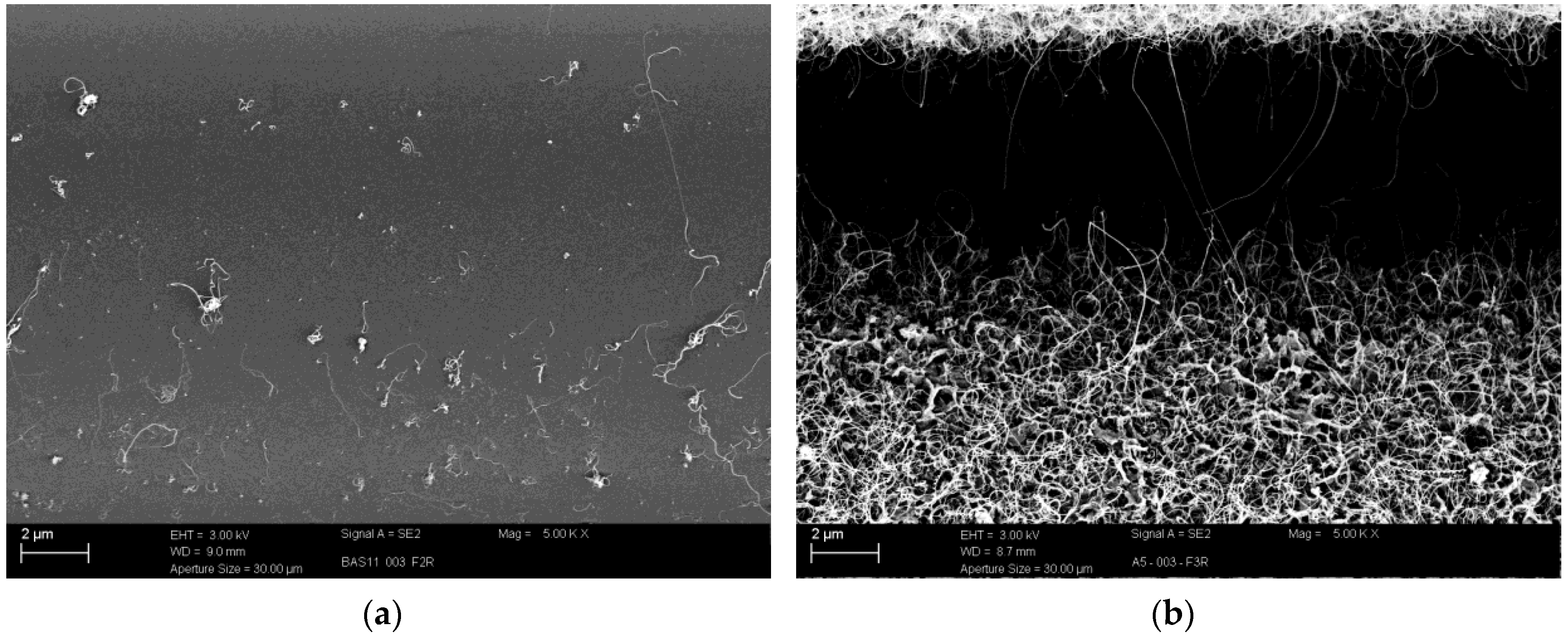
3.2. CNT Growth onto Basalt Fibers after CVD Treatment at 800 °C and at 700 °C
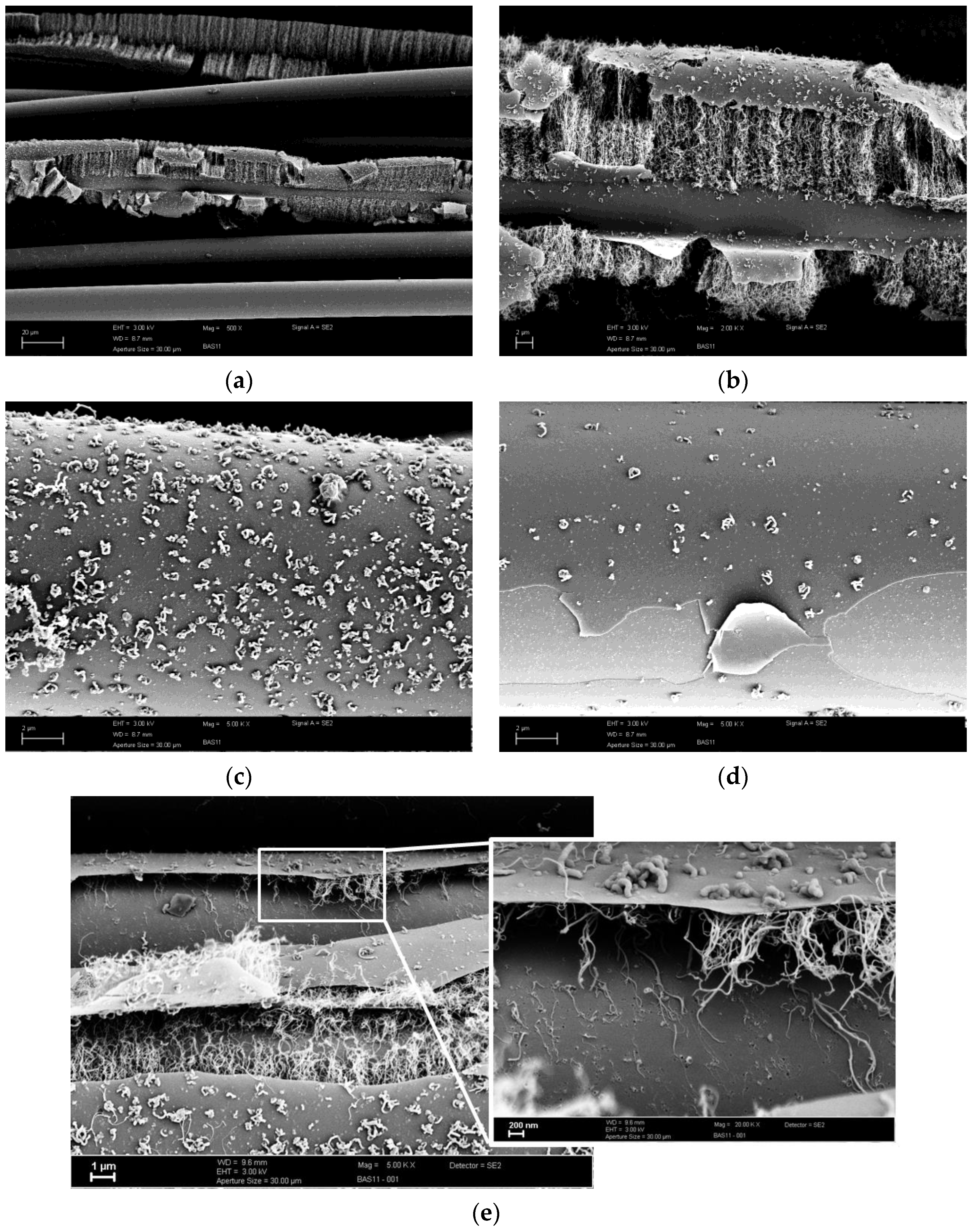
3.3. CVD Treated Fiber Surfaces without C2H2 Flow
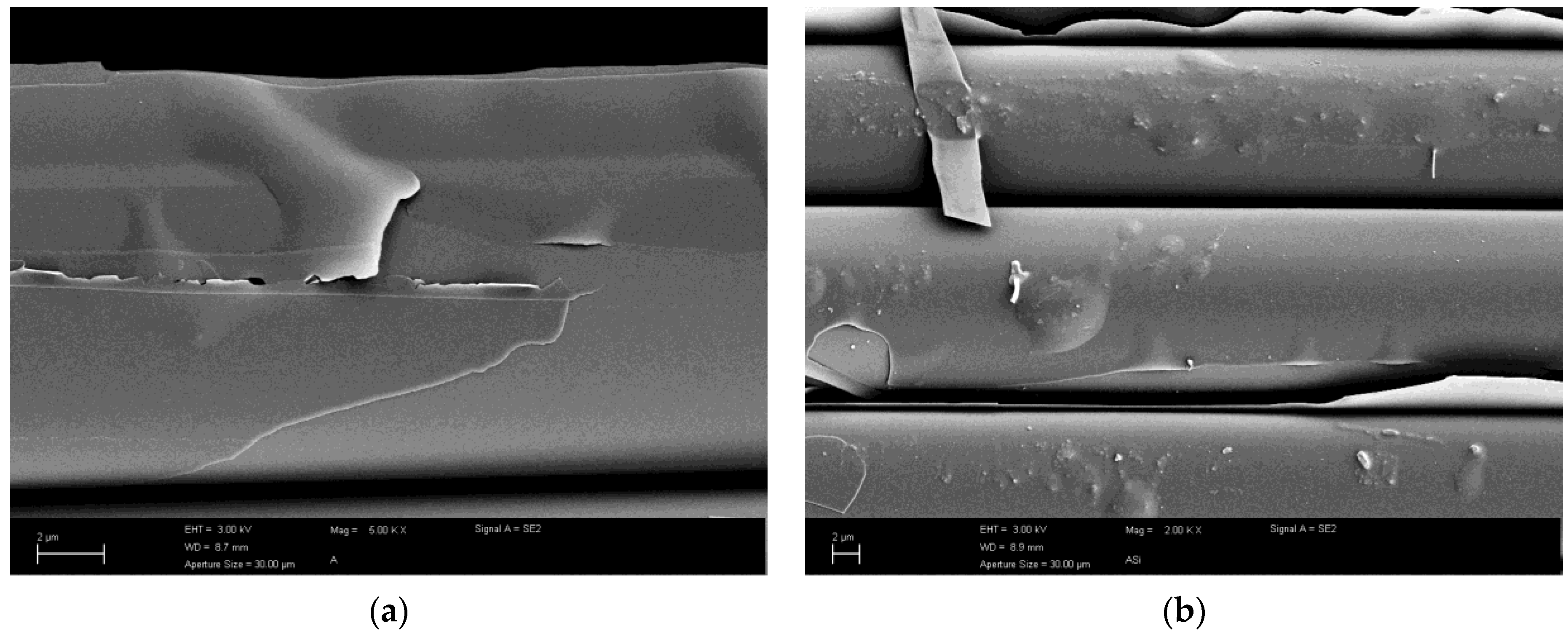
3.4. Alkali Attacked Surfaces and Initial Sized Surface
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Thomason, J.L.; Yang, L.; Meier, R. The properties of glass fibres after conditioning at composite recycling temperatures. Compos. A Appl. Sci. Manuf. 2014, 61, 201–208. [Google Scholar] [CrossRef]
- Yang, L.; Sáez, E.R.; Nagel, U.; Thomason, J.L. Can thermally degraded glass fibre be regenerated for closed-loop recycling of thermosetting composites? Compos. A Appl. Sci. Manuf. 2015, 72, 167–174. [Google Scholar] [CrossRef]
- Förster, T.; Mäder, E. Defect healing efficiency of fibre sizings. To be submitted.
- Maples, H.A.; Wakefield, S.; Robinson, P.; Bismarck, A. High performance carbon fibre reinforced epoxy composites with controllable stiffness. Compos. Sci. Technol. 2014, 105, 134–143. [Google Scholar] [CrossRef]
- Bismarck, A.; Blaker, J.; Anthony, D.; Qian, H.; Maples, H.; Robinson, P.; Shaffer, M.; Greenhalgh, E. Development of novel composites through fibre and interface/interphase modification. IOP Conf. Ser. Mater. Sci. Eng. 2016, 139, 12001. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Zhuang, R.; Mäder, E.; Heinrich, G.; Gao, S. Single MWNT-Glass Fiber as Strain Sensor and Switch. Adv. Mater. 2011, 23, 3392–3397. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Zhuang, R.-C.; Zhang, J.; Liu, J.-W.; Mäder, E. Glass Fibers with Carbon Nanotube Networks as Multifunctional Sensors. Adv. Funct. Mater. 2010, 20, 1885–1893. [Google Scholar] [CrossRef]
- Zhang, J.; Zhuang, R.; Liu, J.; Mäder, E.; Heinrich, G.; Gao, S. Functional interphases with multi-walled carbon nanotubes in glass fibre/epoxy composites. Carbon 2010, 48, 2273–2281. [Google Scholar] [CrossRef]
- Bekyarova, E.; Thostenson, E.T.; Yu, A.; Kim, H.; Gao, J.; Tang, J.; Hahn, H.T.; Chou, T.-W.; Itkis, M.E.; Haddon, R.C. Multiscale carbon nanotube-carbon fiber reinforcement for advanced epoxy composites. Langmuir 2007, 23, 3970–3974. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.T.; Rhee, K.Y.; Lee, J.H.; Hui, D.; Lau, A.K.T. Property enhancement of a carbon fiber/epoxy composite by using carbon nanotubes. Compos. B Eng. 2011, 42, 1257–1261. [Google Scholar] [CrossRef]
- Lee, J.H.; Rhee, K.Y.; Park, S.J. The tensile and thermal properties of modified CNT-reinforced basalt/epoxy composites. Mater. Sci. Eng. A 2010, 527, 6838–6843. [Google Scholar] [CrossRef]
- Kim, M.T.; Rhee, K.Y.; Park, S.J.; Hui, D. Effects of silane-modified carbon nanotubes on flexural and fracture behaviors of carbon nanotube-modified epoxy/basalt composites. Compos. B Eng. 2012, 43, 2298–2302. [Google Scholar] [CrossRef]
- Lee, S.-O.; Choi, S.-H.; Kwon, S.H.; Rhee, K.-Y.; Park, S.-J. Modification of surface functionality of multi-walled carbon nanotubes on fracture toughness of basalt fiber-reinforced composites. Compos. B Eng. 2015, 79, 47–52. [Google Scholar] [CrossRef]
- Teo, K.B.; Singh, C.; Chhowalla, M.; Milne, W.I. Catalytic synthesis of carbon nanotubes and nanofibers. Encycl. Nanosci. Nanotechnol. 2003, 10, 1–22. [Google Scholar]
- Yan, Y.; Miao, J.; Yang, Z.; Xiao, F.-X.; Yang, H.B.; Liu, B.; Yang, Y. Carbon nanotube catalysts: Recent advances in synthesis, characterization and applications. Chem. Soc. Rev. 2015, 44, 3295–3346. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, N.; Mishra, P.; Joshi, B.; Islam, S.S. Precise control over physical characteristics of Carbon Nanotubes by differential variation of Argon flow rate during Chemical Vapor Deposition processing: A systematic study on growth kinetics. Mater. Sci. Semicond. Process. 2015, 35, 207–215. [Google Scholar] [CrossRef]
- De Greef, N.; Zhang, L.; Magrez, A.; Forró, L.; Locquet, J.-P.; Verpoest, I.; Seo, J.W. Direct growth of carbon nanotubes on carbon fibers: Effect of the CVD parameters on the degradation of mechanical properties of carbon fibers. Diam. Relat. Mater. 2015, 51, 39–48. [Google Scholar] [CrossRef]
- Tripathi, N.; Mishra, P.; Joshi, B.; Harsh; Islam, S.S. Catalyst free, excellent quality and narrow diameter of CNT growth on Al2O3 by a thermal CVD technique. Physica E 2014, 62, 43–47. [Google Scholar] [CrossRef]
- Li, R.; Lachman, N.; Florin, P.; Wagner, H.D.; Wardle, B.L. Hierarchical carbon nanotube carbon fiber unidirectional composites with preserved tensile and interfacial properties. Compos. Sci. Technol. 2015, 117, 139–145. [Google Scholar] [CrossRef]
- Huang, S.; Cai, Q.; Chen, J.; Qian, Y.; Zhang, L. Metal-catalyst-free growth of single-walled carbon nanotubes on substrates. J. Am. Chem. Soc. 2009, 131, 2094–2095. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.A.; Tali, B.A. Synthesis of carbon nanotubes by catalytic chemical vapour deposition: A review on carbon sources, catalysts and substrates. Mater. Sci. Semicond. Process. 2016, 41, 67–82. [Google Scholar] [CrossRef]
- Rahaman, A.; Kar, K.K. Carbon nanomaterials grown on E-glass fibers and their application in composite. Compos. Sci. Technol. 2014, 101, 1–10. [Google Scholar] [CrossRef]
- Wood, C.D.; Palmeri, M.J.; Putz, K.W.; Ho, G.; Barto, R.; Brinson, L.C. Nanoscale structure and local mechanical properties of fiber-reinforced composites containing MWCNT-grafted hybrid glass fibers. Compos. Sci. Technol. 2012, 72, 1705–1710. [Google Scholar] [CrossRef]
- Karger-Kocsis, J.; Mahmood, H.; Pegoretti, A. Recent advances in fiber/matrix interphase engineering for polymer composites. Prog. Mater. Sci. 2015, 73, 1–43. [Google Scholar] [CrossRef] [Green Version]
- Förster, T.; Mäder, E.; Jesson, D.A.; Watts, J.F. Surface analyses of basalt fibres: Tailoring the interphase of “green” fibre reinforced composites. In Proceedings of the 19th International Conference on Composite Materials (ICCM19), Montreal, QC, Canada, 28 July–2 August 2013.
- Smedskjaer, M.M.; Yue, Y.; Deubener, J.; Gunnlaugsson, H.P.; Mørup, S. Modifying glass surfaces via internal diffusion. J. Non-Cryst. Solids 2010, 356, 290–298. [Google Scholar] [CrossRef]
- Smedskjaer, M.M.; Yue, Y.; Deubener, J.; Mørup, S. Impact of cationic diffusion on properties of iron-bearing glass fibres. Phys. Chem. Glas. J. Glasses Sci. Technol. B 2010, 51, 271–280. [Google Scholar]
- Smedskjaer, M.M.; Yue, Y.Z. Inward cationic diffusion in glass. J. Non-Cryst. Solids 2009, 355, 908–912. [Google Scholar] [CrossRef]
- Yue, Y.; Korsgaard, M.; Kirkegaard, L.F.; Heide, G. Formation of a Nanocrystalline Layer on the Surface of Stone Wool Fibers. J. Am. Ceram. Soc. 2009, 92, 62–67. [Google Scholar] [CrossRef]
- Förster, T.; Plonka, R.; Scheffler, C.; Mäder, E.; Brameshuber, W. Challenges for fibre and interphase design of basalt fibre reinforced concrete. In International RILEM Conference on Material Science; RILEM Publications SARL: Bagneux, France, 2010; pp. 57–66. [Google Scholar]
- Förster, T.; Sommer, G.S.; Mäder, E.; Scheffler, C. Surface, interphase and tensile properties of unsized, sized and heat treated basalt fibres. IOP Conf. Ser. Mater. Sci. Eng. 2016, 139, 12019. [Google Scholar] [CrossRef]
- Rybin, V.A.; Utkin, А.V.; Baklanova, N.I. Corrosion of uncoated and oxide-coated basalt fibre in different alkaline media. Corros. Sci. 2016, 102, 503–509. [Google Scholar] [CrossRef]
- Novais, R.M.; Simon, F.; Paiva, M.C.; Covas, J.A. The influence of carbon nanotube functionalization route on the efficiency of dispersion in polypropylene by twin-screw extrusion. Compos. Appl. Sci. Manuf. 2012, 43, 2189–2198. [Google Scholar] [CrossRef]

| Fiber | BAS11 | BAS16(A) | BAS16(A1) | BAS16(A5) | BAS16(A-Si) |
|---|---|---|---|---|---|
| Sizing | no | no | no | no | yes |
| Alkali treatment | no | no | 1 h | 5 h | no |
| Stage | Temperature (°C) | Time (min) | Gases | Flow Rate (sccm) |
|---|---|---|---|---|
| I | AT to 300 °C | 30 | Ar | 200 |
| II | 300 to Ti | 50 | Ar+H2 | 200/14 |
| III | Ti | 30 | Ar+H2 | 200/14 |
| IV | Ti | 30 | Ar+H2+C2H2 | 200/14/37 |
| V | Ti to 700 °C | - | Ar | 200 |
| VI | 700 °C to AT | - | Ar | <200 |
| Elemental Ratio | BAS11 Initial | BAS11 CVD02 | BAS16(A) Initial | BAS16(A) CVD02 | BAS16(A5) NaOH Treated |
|---|---|---|---|---|---|
| (C):(O) | 0.85 | 0.67 | 0.64 | 0.33 | 0.37 |
| (Na):(O) | 0.02 | 0.05 | 0.02 | 0.04 | 0.03 |
| (Mg):(O) | 0.01 | - | 0.03 | - | 0.01 |
| (Al):(O) | 0.10 | 0.13 | 0.12 | 0.14 | 0.05 |
| (Si):(O) | 0.35 | 0.40 | 0.32 | 0.39 | 0.29 |
| (K):(O) | - | 0.03 | 0.01 | 0.03 | - |
| (Ca):(O) | 0.02 | 0.01 | traces | 0.02 | 0.07 |
| (Ti):(O) | traces | 0.01 | traces | traces | traces |
| (Mn):(O) | traces | - | - | - | - |
| (Fe):(O) | 0.01 | - | 0.03 | - | 0.02 |
| (C):(Si) | 2.42 | 1.68 | 2.00 | 0.85 | 1.28 |
| Fiber | CVD | Single Fiber Tensile Test | ||||||
|---|---|---|---|---|---|---|---|---|
| Ti | Carbon Source | CNT | σm | σ0 | m | R | σm, residual | |
| (°C) | (MPa) | (MPa) | (-) | (-) | (%) | |||
| BAS11–initial | no | no | no | 1698 ± 718 | 1932 | 2.3 | 0.96 | |
| BAS11–CVD02 | 800 | no | no | 863 ± 157 | 927 | 6.5 | 0.98 | 51 |
| BAS11–CVD03 | 700 | C2H2 | yes | 1023 ± 276 | 1123 | 4.3 | 0.99 | 60 |
| BAS16(A)–initial | no | no | no | 1762 ± 594 | 1973 | 3.2 | 0.98 | |
| BAS16(A)–CVD02 | 800 | no | no | 1034 ± 216 | 1115 | 6.0 | 0.89 | 59 |
| BAS16(A)–CVD03 | 700 | C2H2 | no | 1190 ± 375 | 1315 | 4.0 | 0.87 | 68 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Förster, T.; Hao, B.; Mäder, E.; Simon, F.; Wölfel, E.; Ma, P.-C. CVD-Grown CNTs on Basalt Fiber Surfaces for Multifunctional Composite Interphases. Fibers 2016, 4, 28. https://doi.org/10.3390/fib4040028
Förster T, Hao B, Mäder E, Simon F, Wölfel E, Ma P-C. CVD-Grown CNTs on Basalt Fiber Surfaces for Multifunctional Composite Interphases. Fibers. 2016; 4(4):28. https://doi.org/10.3390/fib4040028
Chicago/Turabian StyleFörster, Theresa, Bin Hao, Edith Mäder, Frank Simon, Enrico Wölfel, and Peng-Cheng Ma. 2016. "CVD-Grown CNTs on Basalt Fiber Surfaces for Multifunctional Composite Interphases" Fibers 4, no. 4: 28. https://doi.org/10.3390/fib4040028






