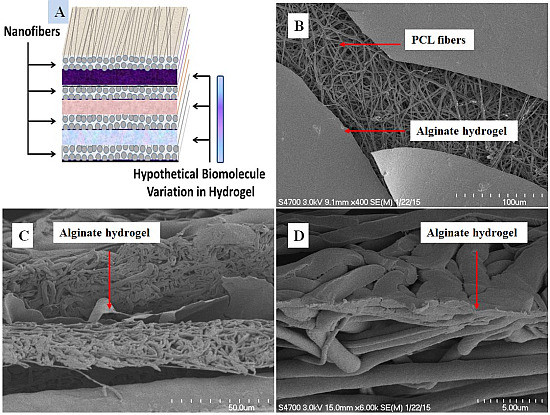
Fabrication and Evaluation of Multilayer Nanofiber-Hydrogel Meshes with a Controlled Release Property
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Electrospun Nanofiber Meshes
2.3. Fabrication of Multilayer Nanofiber-Hydrogel Meshes
2.4. Scanning Electron Microscopy (SEM)
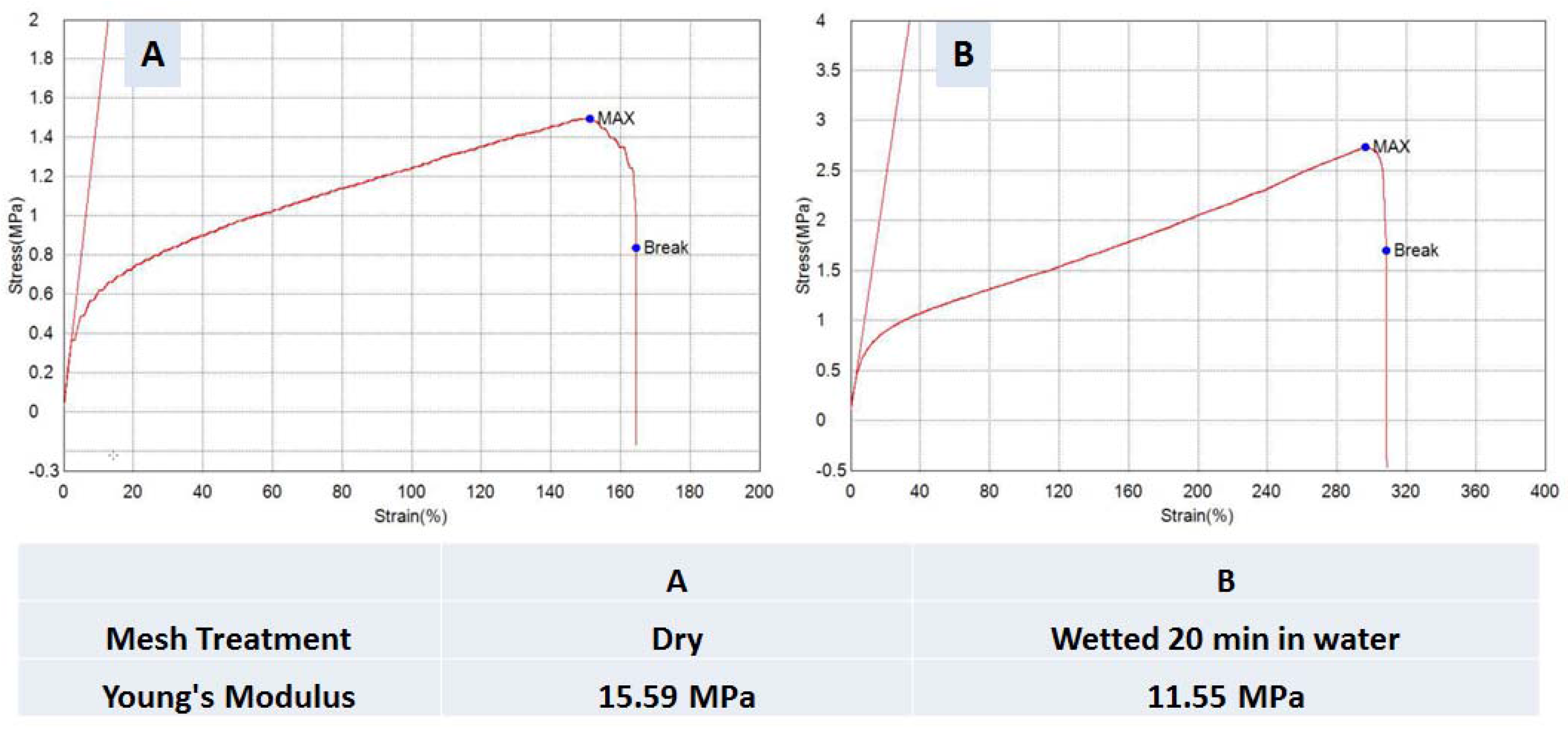
2.5. Tensile Testing
2.6. ATP Release Kinetics
2.7. Cytotoxicity Assessment
2.8. Statistical Analysis
3. Results and Discussion
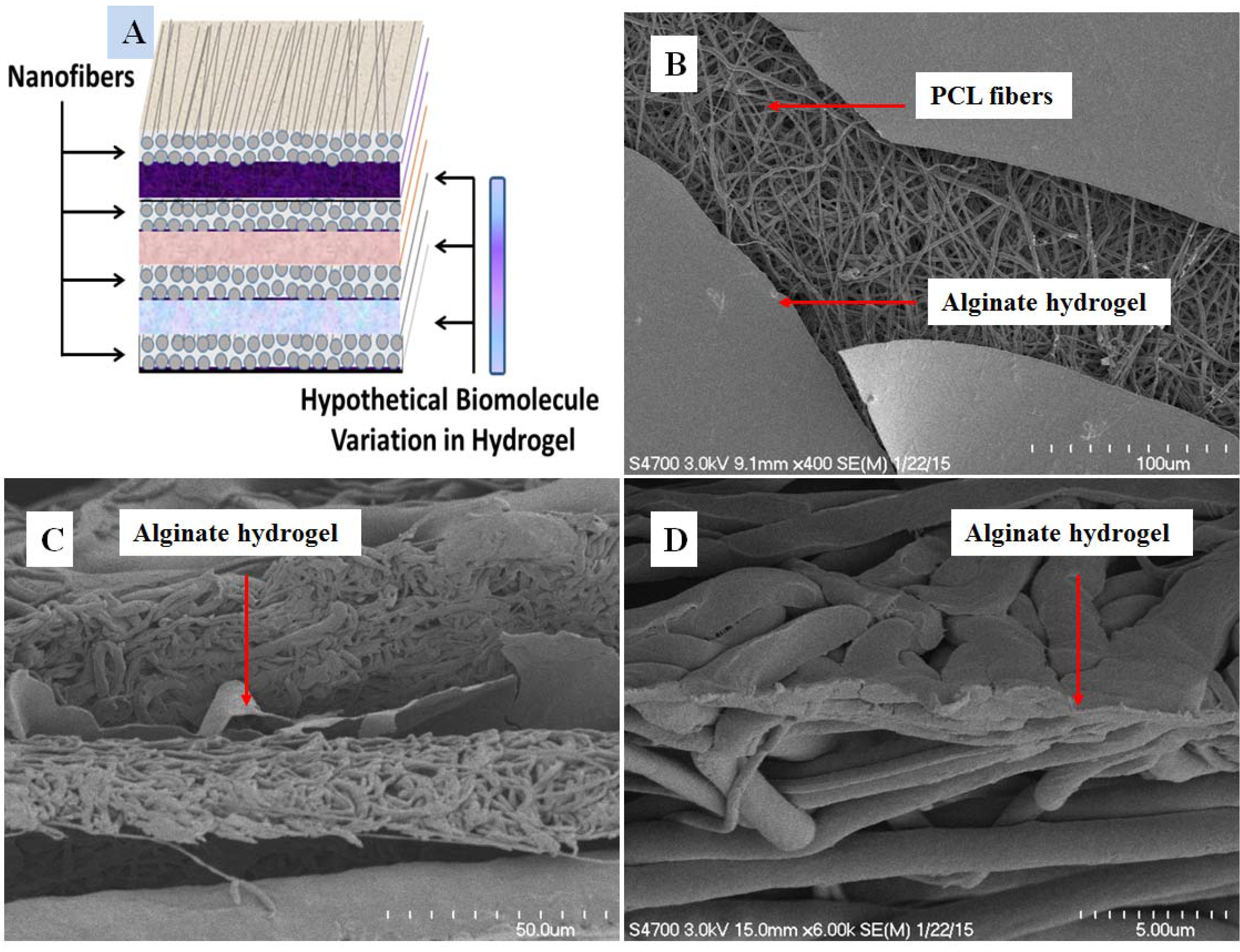
3.1. Scanning Electron Microscopy Characterization
3.2. Micro-Tensile Testing
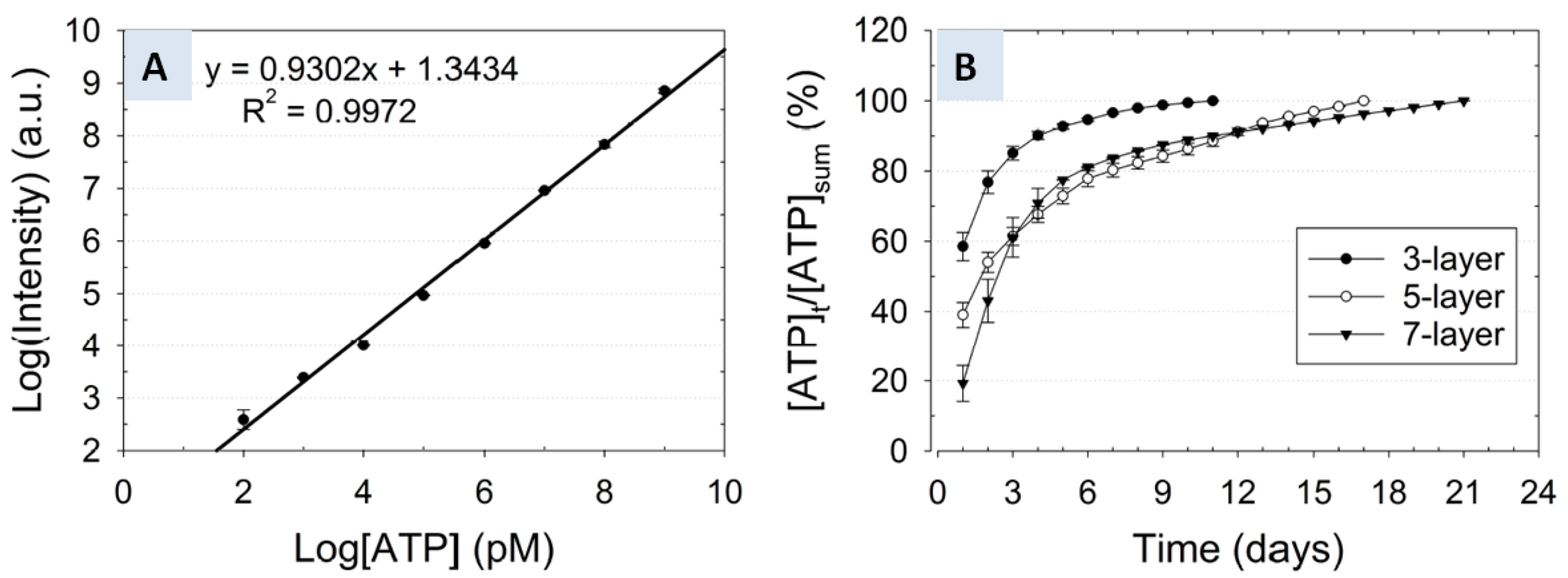
3.3. Correlation of Release Kinetics to the Number of Layers
 ); Cumulative release profile of ATP from a five-layer nanofiber-hydrogel mesh (
); Cumulative release profile of ATP from a five-layer nanofiber-hydrogel mesh (  ); Cumulative release profile of ATP from a seven-layer nanofiber-hydrogel mesh (
); Cumulative release profile of ATP from a seven-layer nanofiber-hydrogel mesh (  ).
).
 ); Cumulative release profile of ATP from a five-layer nanofiber-hydrogel mesh (
); Cumulative release profile of ATP from a five-layer nanofiber-hydrogel mesh (  ); Cumulative release profile of ATP from a seven-layer nanofiber-hydrogel mesh (
); Cumulative release profile of ATP from a seven-layer nanofiber-hydrogel mesh (  ).
).
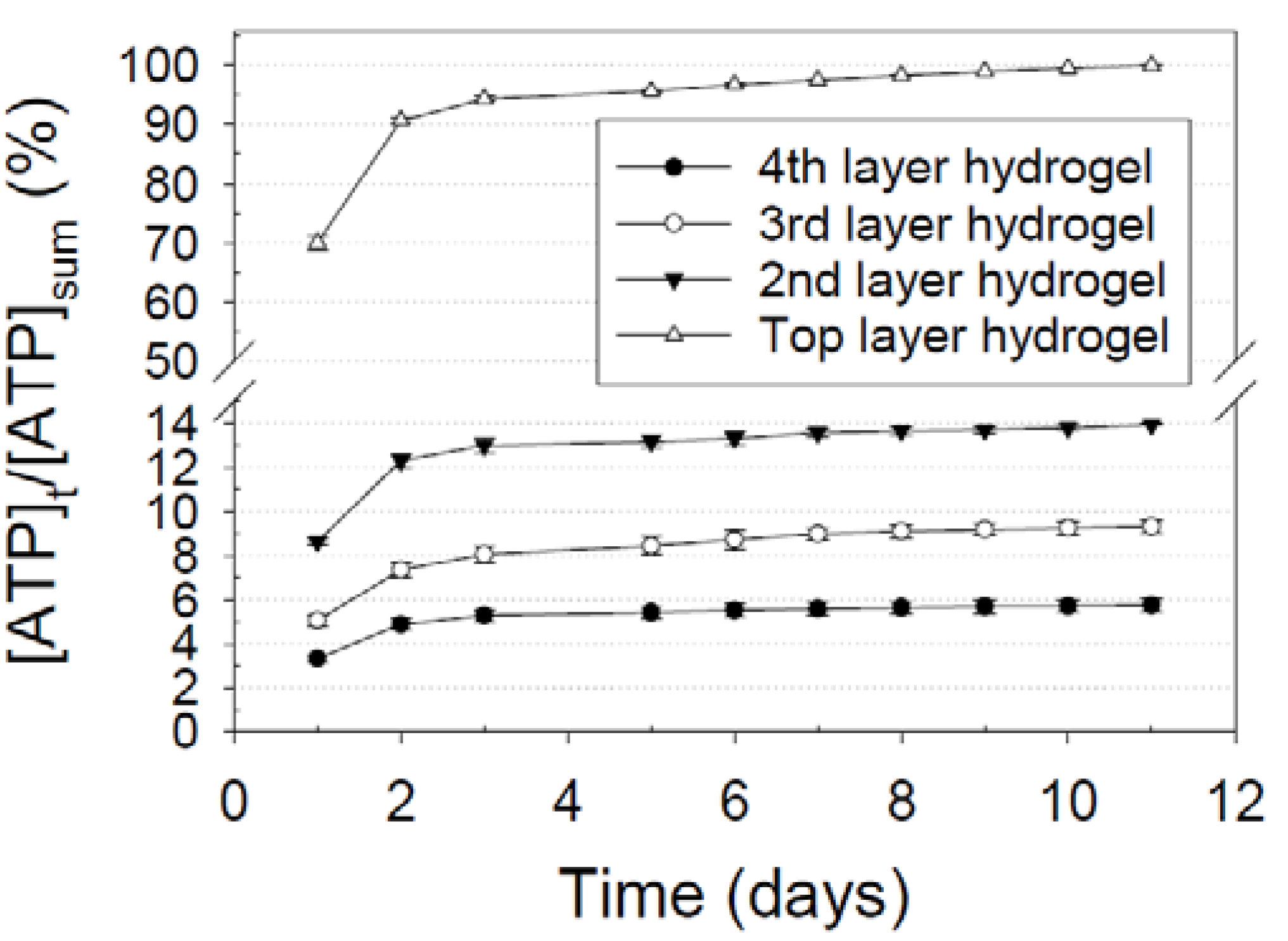
3.4. Differential Release Kinetics for Designated Layers
 ); Release from the second hydrogel layer from the top surface of an eight-layer nanofiber-hydrogel mesh (
); Release from the second hydrogel layer from the top surface of an eight-layer nanofiber-hydrogel mesh (  ); Release from the third hydrogel layer from the top surface of an eight-layer nanofiber-hydrogel mesh (
); Release from the third hydrogel layer from the top surface of an eight-layer nanofiber-hydrogel mesh (  ); Release from the fourth hydrogel layer from the top surface (or the hydrogel layer attached to the bottom nanofiber layer) of an eight-layer nanofiber-hydrogel mesh (
); Release from the fourth hydrogel layer from the top surface (or the hydrogel layer attached to the bottom nanofiber layer) of an eight-layer nanofiber-hydrogel mesh (  ).
).
 ); Release from the second hydrogel layer from the top surface of an eight-layer nanofiber-hydrogel mesh (
); Release from the second hydrogel layer from the top surface of an eight-layer nanofiber-hydrogel mesh (  ); Release from the third hydrogel layer from the top surface of an eight-layer nanofiber-hydrogel mesh (
); Release from the third hydrogel layer from the top surface of an eight-layer nanofiber-hydrogel mesh (  ); Release from the fourth hydrogel layer from the top surface (or the hydrogel layer attached to the bottom nanofiber layer) of an eight-layer nanofiber-hydrogel mesh (
); Release from the fourth hydrogel layer from the top surface (or the hydrogel layer attached to the bottom nanofiber layer) of an eight-layer nanofiber-hydrogel mesh (  ).
).
3.5. Cytotoxicity Assessment of Multilayer Nanofiber-Hydrogel Meshes
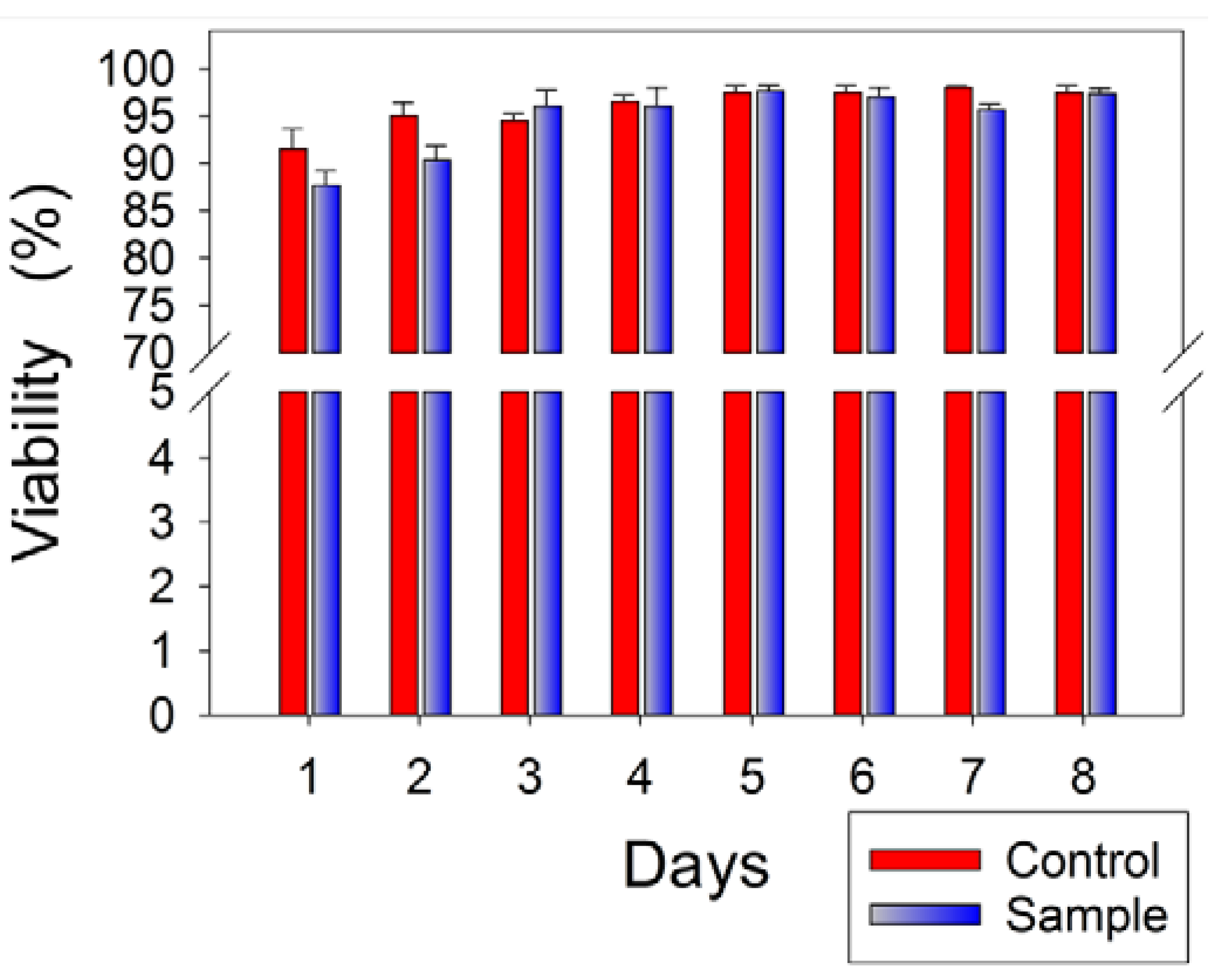
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pham, Q.P.; Sharma, U.; Mikos, A.G. Electrospinning of polymeric nanofibers for tissue engineering applications: A review. Tissue Eng. 2006, 12, 1197–1211. [Google Scholar] [CrossRef] [PubMed]
- Vasita, R.; Katti, D.S. Nanofibers and their applications in tissue engineering. Int. J. Nanomedicine 2006, 1, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Synth. Biomim. Polym. 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Park, H.; Karajanagi, S.; Wolak, K.; Aanestad, J.; Daheron, L.; Kobler, J.B.; Lopez-Guerra, G.; Heaton, J.T.; Langer, R.S.; Zeitels, S.M. Three-Dimensional Hydrogel Model Using Adipose-Derived Stem Cells for Vocal Fold Augmentation. Tissue Eng. Part A 2010, 16, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; MacEwan, M.R.; Li, X.; Sakiyama-Elbert, S.E.; Xia, Y. Neurite Outgrowth on Nanofiber Scaffolds with Different Orders, Structures, and Surface Properties. ACS Nano 2009, 3, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-J.; Tuli, R.; Okafor, C.; Derfoul, A.; Danielson, K.G.; Hall, D.J.; Tuan, R.S. A three-dimensional nanofibrous scaffold for cartilage tissue engineering using human mesenchymal stem cells. Biomaterials 2005, 26, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Casper, M.E.; Fitzsimmons, J.S.; Stone, J.J.; Meza, A.O.; Huang, Y.; Ruesink, T.J.; O’Driscoll, S.W.; Reinholz, G.G. Tissue engineering of cartilage using poly-ε-caprolactone nanofiber scaffolds seeded in vivo with periosteal cells. Osteoarthr. Cartil. 2010, 18, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, H.; Shin, Y.M.; Terai, H.; Vacanti, J.P. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials 2003, 24, 2077–2082. [Google Scholar] [CrossRef]
- Liu, H.; Ding, X.; Zhou, G.; Li, P.; Wei, X.; Fan, Y. Electrospinning of Nanofibers for Tissue Engineering Applications. J. Nanomater. 2013, 2013, 1–11. [Google Scholar] [CrossRef]
- Chaturvedi, T.P. Doxycycline Poly E-Caprolactone Nanofibers in Patients with Chronic Periodontitis—A Clinical Evaluation. J. Clin. Diagn. Res. 2013, 7, 2339–2342. [Google Scholar] [CrossRef] [PubMed]
- Kenawy, E.-R.; Abdel-Hay, F.I.; El-Newehy, M.H.; Wnek, G.E. Processing of polymer nanofibers through electrospinning as drug delivery systems. Mater. Chem. Phys. 2009, 113, 296–302. [Google Scholar] [CrossRef]
- Borjigin, M.; Strouse, B.; Niamat, R.A.; Bialk, P.; Eskridge, C.; Xie, J.; Kmiec, E.B. Proliferation of Genetically Modified Human Cells on Electrospun Nanofiber Scaffolds. Mol. Ther. Nucleic Acids 2012, 1, e59. [Google Scholar] [CrossRef] [PubMed]
- Borjigin, M.; Eskridge, C.; Niamat, R.; Strouse, B.; Bialk, P.; Kmiec, E.B. Electrospun fiber membranes enable proliferation of genetically modified cells. Int. J. Nanomedicine 2013, 8, 855–864. [Google Scholar] [PubMed]
- Tønnesen, H.H.; Karlsen, J. Alginate in drug delivery systems. Drug Dev. Ind. Pharm. 2002, 28, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. Hydrogels for biomedical applications. Recent Dev. Hydrogels 2002, 54, 3–12. [Google Scholar] [CrossRef]
- Jeong, S.I.; Jeon, O.; Krebs, M.D.; Hill, M.C.; Alsberg, E. Biodegradable photo-crosslinked alginate nanofibre scaffolds with tuneable physical properties, cell adhesivity and growth factor release. Eur. Cell. Mater. 2012, 24, 331–343. [Google Scholar] [PubMed]
- Gombotz, W.R.; Wee, S.F. Protein release from alginate matrices. Adv. Drug Deliv. Rev. 2012, 64, 194–205. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Hu, Y.; Li, Y.; Zhao, P.; Zhu, K.; Chen, W. A facile technique to prepare biodegradable coaxial electrospun nanofibers for controlled release of bioactive agents. J. Controlled Release 2005, 108, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Z.; Wang, X.; Feng, Y.; Li, J.; Lim, C.T.; Ramakrishna, S. Coaxial Electrospinning of (Fluorescein Isothiocyanate-Conjugated Bovine Serum Albumin)-Encapsulated Poly(ε-caprolactone) Nanofibers for Sustained Release. Biomacromolecules 2006, 7, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Dash, T.K.; Konkimalla, V.B. Poly-є-caprolactone based formulations for drug delivery and tissue engineering: A review. J. Control. Release 2012, 158, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Brazel, C.S. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J. Control. Release 2001, 73, 121–136. [Google Scholar] [CrossRef]
- Keller, G. Embryonic stem cell differentiation: Emergence of a new era in biology and medicine. Genes Dev. 2005, 19, 1129–1155. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Huangfu, D. Human pluripotent stem cells: An emerging model in developmental biology. Development 2013, 140, 705–717. [Google Scholar] [CrossRef] [PubMed]
- Lotz, S.; Goderie, S.; Tokas, N.; Hirsch, S.E.; Ahmad, F.; Corneo, B.; Le, S.; Banerjee, A.; Kane, R.S.; Stern, J.H.; Temple, S.; Fasano, C.A. Sustained Levels of FGF2 Maintain Undifferentiated Stem Cell Cultures with Biweekly Feeding. PLoS ONE 2013, 8, e56289. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Dan, Y.; Yang, S.; Liu, G.; Shao, Z.; Yang, C.; Xiao, B.; Liu, X.; Wu, S.; Zhang, T.; et al. Controlled chondrogenesis from adipose-derived stem cells by recombinant transforming growth factor-beta3 fusion protein in peptide scaffolds. Acta Biomater. 2015, 11, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Van Lune, H.; Wiel, J. Method for Detecting ATP. US 6503723 B1, 7 January 2003. Available online: http://www.google.com/patents/US6503723 (accessed on 5 May 2015). [Google Scholar]
- Bölgen, N.; Menceloğlu, Y.Z.; Acatay, K.; Vargel, I.; Pişkin, E. In vitro and in vivo degradation of non-woven materials made of poly (ε-caprolactone) nanofibers prepared by electrospinning under different conditions. J. Biomater. Sci. Polym. Ed. 2005, 16, 1537–1555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollister, S.J. Porous scaffold design for tissue engineering. Nat. Mater. 2005, 4, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.P.; Leong, K.W. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur. Spine J. 2008, 17, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.H. Electrospun PCL nanofibers with anisotropic mechanical properties as a biomedical scaffold. Biomed. Mater. 2008, 3. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.; Niehaus, A.; Nichols, S.; Lee, D.; Koepsel, J.; Anderson, D.; Lannutti, J. Electrospun PCL in Vitro: A Microstructural Basis for Mechanical Property Changes. J. Biomater. Sci. Polym. Ed. 2009, 20, 467–481. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.; Yang, H.; Ko, F.; Ayranci, C.; Basu, S. Tensile Stress-Strain Response of Small-diameter Electrospun Fibers. Agil. Technol. 2012. Available online: http://cp.literature.agilent.com/itweb/pdf/5991-0178EN.pdf (accessed on 28 May 2015).
- Cao, X.; Kwek, K.; Chan, J.K.Y.; Chan, C.K.H.; Lim, M. Electrospun nanofibers as a bioadhesive platform for capturing adherent leukemia cells. J. Biomed. Mater. Res. A 2014, 102, 523–531. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, R.; Niamat, R.A.; Sansbury, B.; Borjigin, M. Fabrication and Evaluation of Multilayer Nanofiber-Hydrogel Meshes with a Controlled Release Property. Fibers 2015, 3, 296-308. https://doi.org/10.3390/fib3030296
Wu R, Niamat RA, Sansbury B, Borjigin M. Fabrication and Evaluation of Multilayer Nanofiber-Hydrogel Meshes with a Controlled Release Property. Fibers. 2015; 3(3):296-308. https://doi.org/10.3390/fib3030296
Chicago/Turabian StyleWu, Rigumula, Rohina A. Niamat, Brett Sansbury, and Mandula Borjigin. 2015. "Fabrication and Evaluation of Multilayer Nanofiber-Hydrogel Meshes with a Controlled Release Property" Fibers 3, no. 3: 296-308. https://doi.org/10.3390/fib3030296